Von Willebrand factor (VWF) tethers platelets to injured subendothelium through binding sites for collagen and platelet glycoprotein Ib (GPIb). The collagen binding site has been localized to the VWF A1 and A3 domains [1]. This interaction is measured in vitro by the VWF collagen binding assay, or VWF:CB [2,3]. VWD guidelines recently published by the NHLBI suggest restricting use of the VWF:CB to subjects who have abnormal initial screening results with VWF antigen (VWF:Ag) and VWF ristocetin cofactor activity (VWF:RCo) [4]. The VWF:RCo assay measures VWF-platelet interactions, as induced by the antibiotic ristocetin, and therefore a defect exclusive to the VWF-collagen axis could potentially be missed by omitting the VWF:CB assay.
We report here on a subject with type 1 VWD who was discovered to have abnormal collagen binding and a mutation in the VWF A3 domain. The index case and family members were enrolled in the Zimmerman Program for the Molecular and Clinical Biology of VWF after informed consent was obtained. VWF:Ag, VWF:RCo, VWF:CB, multimer distribution, and blood type analysis were performed in the clinical hemostasis laboratory of the BloodCenter of Wisconsin as previously described [5].
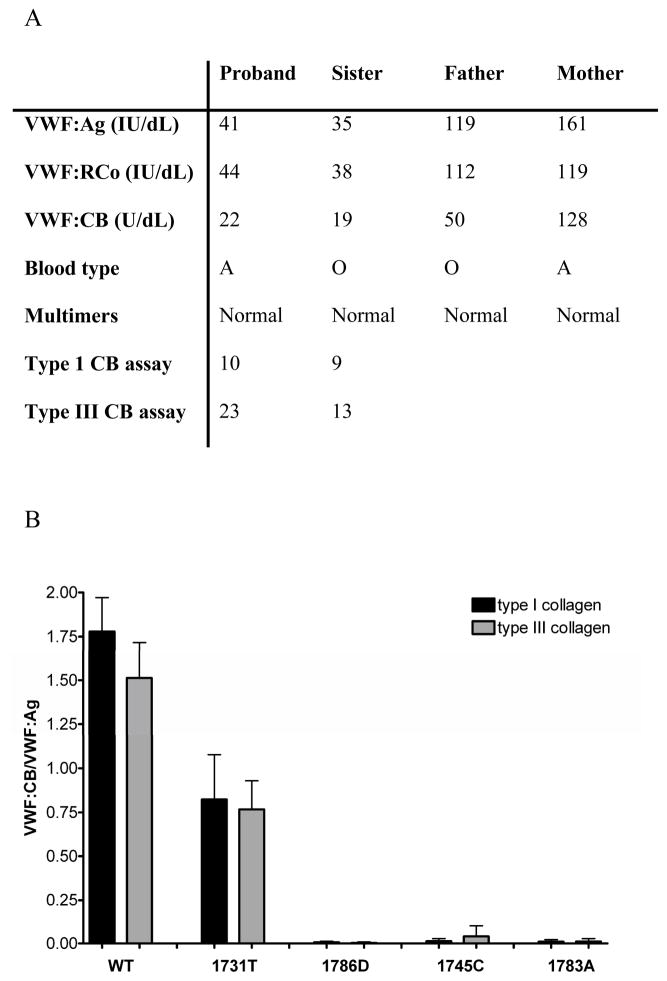
This subject had VWF:Ag of 41 IU/dL and VWF:RCo 44 IU/dL with normal multimer distribution, but VWF:CB was only 22 U/dL (figure 1A). Her history was significant for epistaxis requiring cautery and hemorrhage following tonsillectomy as a child. She also had prolonged bleeding following a dilation and curettage procedure. Her bleeding score, calculated using the scoring system from the European MCMDM-1 VWD study, was elevated at 8 [6]. DNA sequencing of the full length VWF coding sequence, performed on an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA), showed this subject was heterozygous for a mutation in exon 31 (5356C>G) which led to substitution of aspartic acid for the wild-type histidine at amino acid 1786 (H1786D).
Figure 1.
Panel A shows VWF levels for the H1786D proband and family members. VWF:Ag, VWF:RCo, and VWF:CB listed for each subject were performed in the clinical laboratory along with blood type and multimer analysis. The VWF:CB used type III collagen. In addition, plasma samples from the proband and sibling were tested in the research laboratory using collagen binding assays for type I and type III collagen. Results are the mean of 3 separate assays. Panel B shows collagen binding for the recombinant VWF constructs. Binding to type I collagen is shown in black and binding to type III collagen is shown in grey for wild-type (WT), 1731T, 1786D, 1745C, and 1783A VWF. Error bars represent 1 SD for a minimum of 3 separate assays.
Recombinant VWF containing the 1786D mutation was synthesized via site-directed mutagenesis using the Stratagene QuikChange kit (La Jolla, CA) and expressed in HEK293T cells. Additional constructs were synthesized to incorporate three previously reported mutations in the collagen binding domain, 1731T [7], 1745C, and 1783A [8]. For the research laboratory collagen binding assays, ELISA plates were coated with either type I human placental collagen (Sigma, St. Louis, MO) at 5 μg/mL or type III human placental collagen (Southern Biotech) at 1 μg/mL, diluted in carbonate coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, 3 mM sodium azide), and incubated at 4°C overnight. Either plasma or recombinant VWF diluted in phosphate-buffered saline with 1% BSA was added to each well and incubated at room temperature for 1 hour. VWF bound to collagen was detected using a biotin-conjugated polyclonal antibody to VWF (Dako, Carpinteria, CA) also diluted in phosphate-buffered saline with 1% BSA.
Both the 1786D and the 1731T constructs had normal expression compared to wild-type (WT) VWD. Multimer analysis showed a full spectrum of multimers, with no decrease in high molecular weight multimers for the A3 domain mutants. Collagen binding studies were performed in our research laboratory using recombinant WT, 1786D, and 1731T VWF. Results are expressed as a ratio of VWF:CB to VWF:Ag. The previously described 1731T mutation showed a reduction in binding to both type I and type III collagen, at 45% and 50% of WT. In contrast, the 1786D mutant showed barely detectable binding to type I and type III collagen, at <1% of wild-type (figure 1B). Similar lack of binding was seen with the 1745C and 1783A constructs.
The profound defect in collagen binding shown with the 1786D construct is supported by studies of the VWF A3 domain crystal structure, which shows H1786 at one of the interfaces with collagen [9]. Replacement of this histidine with alanine also abolished VWF-collagen binding [10]. The S1731T mutation previously reported by Ribba and colleagues led to a decrease in VWF:CB [7]. A recent report by Riddell and colleagues details two A3 domain mutations, W1745C and S1783A, both with a significant decrease in VWF:CB [8]. Three additional A3 domain mutations, Q1734H, I1741T, and Q1762R, have also been reported to be associated with decreased collagen binding although the affected subjects did not display profound bleeding symptoms [11].
Both the VWF A1 and A3 domains are capable of binding collagen [1]. The major binding site is thought to be in the A3 domain [12]. The in vitro collagen binding assays with 1786D VWF did not demonstrate any evidence of compensatory A1 domain binding. It is possible that in vivo the A1 domain binding site can adequately compensate for the H1786D mutation in the A3 domain when VWF is activated under flow conditions, which may decrease the symptoms seen in the patients with this defect. Previous work with recombinant H1786A VWF supports this hypothesis, as it has been demonstrated that under flow conditions, binding to collagen can occur through the VWF A1 domain [13]. It is also possible that the severity of the in vitro phenotype is partially alleviated by the presence of some normal VWF in the heterozygous patients since multimeric VWF would likely contain some monomers with the intact collagen binding site.
The H1786D mutation fits best into the current VWD classification system as a type 2M variant, with abnormal VWF function despite normal multimer distribution. Not all 2M mutations, however, have abnormal collagen binding. Previous work from our laboratory demonstrated the A1 domain mutations F1369I and I1425F had normal binding to type III collagen, while an 11 amino acid deletion mutant, Δ1392–1492, did appear to affect collagen binding [14]. These patients, however, all presented with decreased VWF:RCo/VWF:Ag ratios, prompting their inclusion as 2M mutations. The H1786D mutation described here has a different effect on VWF function than those mutations considered as classic 2M. Defects in collagen binding may ultimately require an alternate classification scheme to distinguish them from platelet-binding defects. This case also suggests that the VWF:CB assay may have utility in diagnosis of variant VWD.
Acknowledgments
The authors would like to acknowledge the Zimmerman Program investigators, staff, and subjects who participated in this study. Additional sequencing analysis was performed by P. Morateck and C. Perry. Initial identification of the H1786D index case was thanks to the testing performed through Esoterix laboratories under the direction of Dorothy Adcock. At her urging, further evaluation of the proband and family was initiated.
This work was supported by the National Institutes of Health program project grant HL081588. Support for VHF was also provided by a Mentored Research Award from the Hemophilia and Thrombosis Research Society and a Career Development Award from the National Hemophilia Foundation. Support for RRM was also provided through National Institutes of Health grants HL33721 and HL044612.
References
- 1.Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. Isolation and characterization of two domains of human von willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987;262:13835–41. [PubMed] [Google Scholar]
- 2.Brown JE, Bosak JO. An ELISA test for the binding of von willebrand antigen to collagen. Thromb Res. 1986;43:303–11. doi: 10.1016/0049-3848(86)90150-7. [DOI] [PubMed] [Google Scholar]
- 3.Favaloro EJ. Von willebrand factor collagen-binding (activity) assay in the diagnosis of von willebrand disease: A 15-year journey. Semin Thromb Hemost. 2002;28:191–202. doi: 10.1055/s-2002-27821. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, Rick ME, Sadler JE, Weinstein M, Yawn BP. Von willebrand disease (VWD): Evidence-based diagnosis and management guidelines, the national heart, lung, and blood institute (NHLBI) expert panel report (USA) Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 5.Flood VH, Friedman KD, Gill JC, Morateck PA, Wren JS, Scott JP, Montgomery RR. Limitations of the ristocetin cofactor assay in measurement of von willebrand factor function. J Thromb Haemost. 2009;7:1832–9. doi: 10.1111/j.1538-7836.2009.03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Fressinaud E, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, Peake I. A quantitative analysis of bleeding symptoms in type 1 von willebrand disease: Results from a multicenter european study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 7.Ribba AS, Loisel I, Lavergne JM, Juhan-Vague I, Obert B, Cherel G, Meyer D, Girma JP. Ser968Thr mutation within the A3 domain of von willebrand factor (VWF) in two related patients leads to a defective binding of VWF to collagen. Thromb Haemost. 2001;86:848–54. [PubMed] [Google Scholar]
- 8.Riddell AF, Gomez K, Millar CM, Mellars G, Gill S, Brown SA, Sutherland M, Laffan MA, McKinnon TA. Characterization of W1745C and S1783A: 2 novel mutations causing defective collagen binding in the A3 domain of von willebrand factor. Blood. 2009;114:3489–96. doi: 10.1182/blood-2008-10-184317. [DOI] [PubMed] [Google Scholar]
- 9.Romijn RA, Bouma B, Wuyster W, Gros P, Kroon J, Sixma JJ, Huizinga EG. Identification of the collagen-binding site of the von willebrand factor A3-domain. J Biol Chem. 2001;276:9985–91. doi: 10.1074/jbc.M006548200. [DOI] [PubMed] [Google Scholar]
- 10.Romijn RA, Westein E, Bouma B, Schiphorst ME, Sixma JJ, Lenting PJ, Huizinga EG. Mapping the collagen-binding site in the von willebrand factor-A3 domain. J Biol Chem. 2003;278:15035–9. doi: 10.1074/jbc.M208977200. [DOI] [PubMed] [Google Scholar]
- 11.Schneppenheim R, Obser T, Drewke E, Gross-Wieltsch U, Oyen F, Sutor A, Wermes C, Budde U. Isolated molecular defects of von willebrand factor binding to collagen do not correlate with bleeding symptoms. Blood. 2001;98:165. [Google Scholar]
- 12.Cruz MA, Yuan H, Lee JR, Wise RJ, Handin RI. Interaction of the von willebrand factor (vWF) with collagen. localization of the primary collagen-binding site by analysis of recombinant vWF a domain polypeptides. J Biol Chem. 1995;270:10822–7. doi: 10.1074/jbc.270.18.10822. [DOI] [PubMed] [Google Scholar]
- 13.Bonnefoy A, Romijn RA, Vandervoort PA, VAN Rompaey I, Vermylen J, Hoylaerts MF. Von willebrand factor A1 domain can adequately substitute for A3 domain in recruitment of flowing platelets to collagen. J Thromb Haemost. 2006;4:2151–61. doi: 10.1111/j.1538-7836.2006.02111.x. [DOI] [PubMed] [Google Scholar]
- 14.Hillery CA, Mancuso DJ, Evan Sadler J, Ponder JW, Jozwiak MA, Christopherson PA, Cox Gill J, Paul Scott J, Montgomery RR. Type 2M von willebrand disease: F606I and I662F mutations in the glycoprotein ib binding domain selectively impair ristocetin-but not botrocetin- mediated binding of von willebrand factor to platelets. Blood. 1998;91:1572–81. [PubMed] [Google Scholar]