Abstract
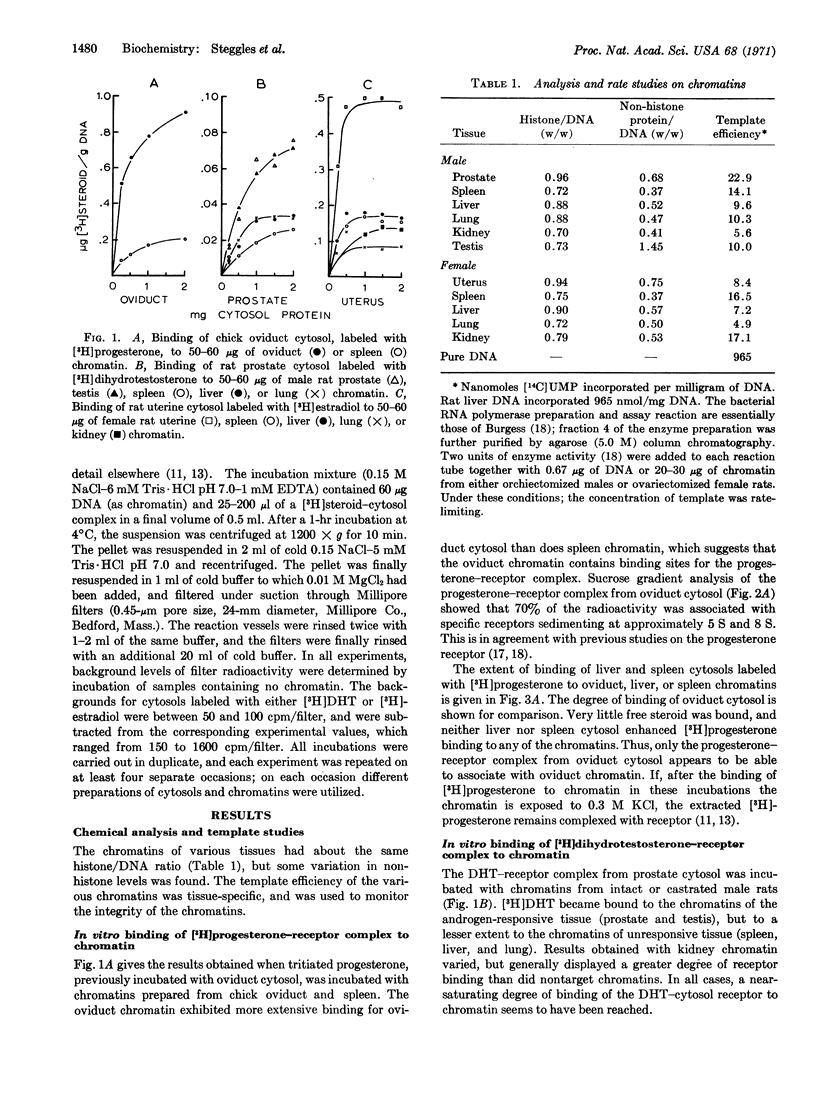
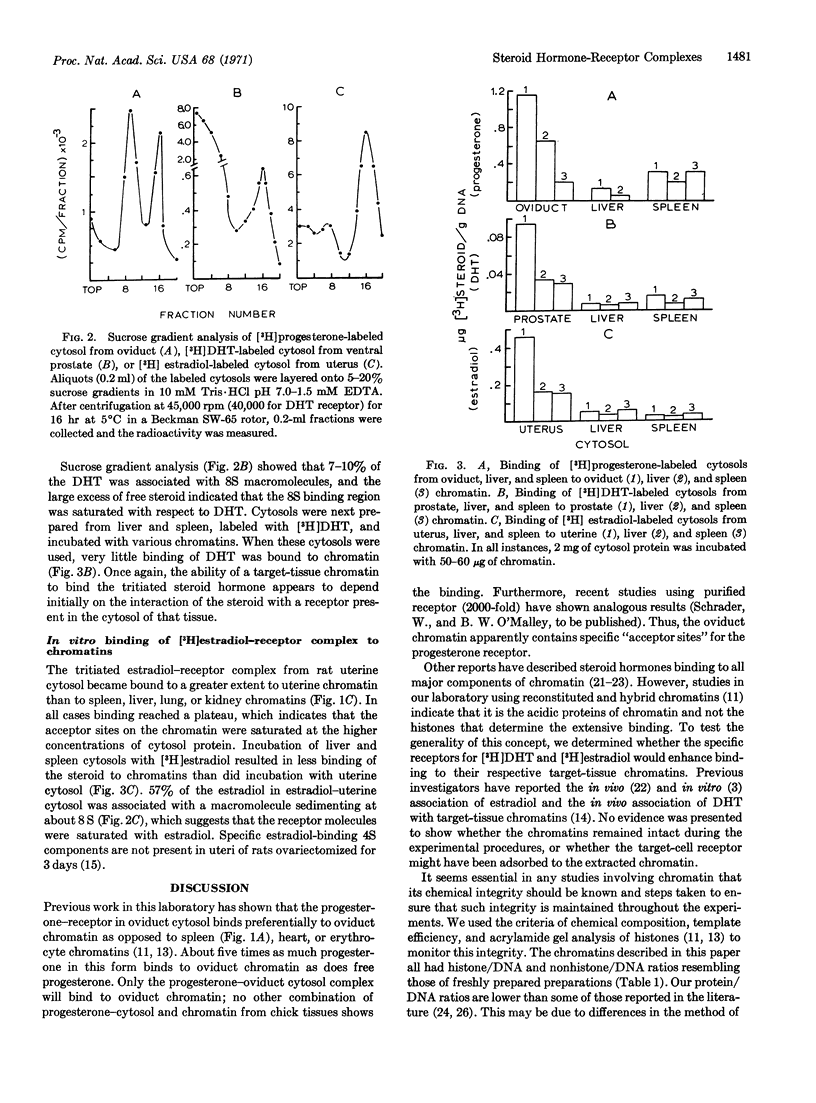
Cytoplasmic fractions containing steroid hormone receptor were prepared from rat prostate and uterine tissues, incubated first with [3H]dihydrotestosterone or [3H]estradiol, and then with their respective target and non-target tissue chromatins. Only prostate and testis chromatin bound the dihydrotestosterone-receptor complex from prostate cytosol extensively. Similarly, uterine chromatin bound more estradiol-receptor complex from uterus than did liver, spleen, or lung chromatin. Complexes between dihydrotestosterone or estradiol with cytosols prepared from liver and spleen bound less extensively, and similarly, to all chromatins. Analogous results are described for the [3H]progesterone-receptor complex from chick oviduct cytosol binding to oviduct chromatin. These studies suggest that the chromatin of all steroid hormone target tissues may contain “acceptor sites” for their respective hormone-receptor complexes, and are thus programmed to receive the complex as it is transferred into the nucleus from the cytoplasm of the cell.
Keywords: dihydrotestosterone-prostate, estradiol-uterus, progesterone-oviduct
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Elgin S. C., Bonner J. Limited heterogeneity of the major nonhistone chromosomal proteins. Biochemistry. 1970 Oct 27;9(22):4440–4447. doi: 10.1021/bi00824a027. [DOI] [PubMed] [Google Scholar]
- Fang S., Liao S. Androgen receptors. Steroid- and tissue-specific retention of a 17 beta-hydroxy-5 alpha-androstan-3-one-protein complex by the cell nuclei of ventral prostate. J Biol Chem. 1971 Jan 10;246(1):16–24. [PubMed] [Google Scholar]
- Fang S., Liao S. Androgen receptors. Steroid- and tissue-specific retention of a 17 beta-hydroxy-5 alpha-androstan-3-one-protein complex by the cell nuclei of ventral prostate. J Biol Chem. 1971 Jan 10;246(1):16–24. [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. J., Gordon J. An attempt to isolate an oestradiol receptor from nuclei by adsorption on oestradiol-17-beta. J Endocrinol. 1968 Feb;40(2):195–204. doi: 10.1677/joe.0.0400195. [DOI] [PubMed] [Google Scholar]
- King R. J., Gordon J., Steggles A. W. The properties of a nuclear acidic protein fraction that binds [6,7-3H]oestradiol-17beta. Biochem J. 1969 Sep;114(3):649–657. doi: 10.1042/bj1140649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mainwaring W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969 Dec;45(4):531–541. doi: 10.1677/joe.0.0450531. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. The binding of (1,2-3H)testosterone within nuclei of the rat prostate. J Endocrinol. 1969 Jul;44(3):323–333. doi: 10.1677/joe.0.0440323. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Chalkley G. R. Some properties of a nuclear binding site of estradiol. J Mol Biol. 1967 Aug 14;27(3):431–441. doi: 10.1016/0022-2836(67)90049-6. [DOI] [PubMed] [Google Scholar]
- Monder C., Walker M. C. Interactions between corticosteroids and histones. Biochemistry. 1970 Jun 9;9(12):2489–2497. doi: 10.1021/bi00814a015. [DOI] [PubMed] [Google Scholar]
- Musliner T. A., Chader G. J., Villee C. A. Studies on estradiol receptors of the rat uterus. Nuclear uptake in vitro. Biochemistry. 1970 Oct 27;9(22):4448–4453. doi: 10.1021/bi00824a028. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Sherman M. R., Toft D. O. Progesterone "receptors" in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A. 1970 Oct;67(2):501–508. doi: 10.1073/pnas.67.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Toft D. O., Sherman M. R. Progesterone-binding components of chick oviduct. II. Nuclear components. J Biol Chem. 1971 Feb 25;246(4):1117–1122. [PubMed] [Google Scholar]
- Sherman M. R., Corvol P. L., O'Malley B. W. Progesterone-binding components of chick oviduct. I. Preliminary characterization of cytoplasmic components. J Biol Chem. 1970 Nov 25;245(22):6085–6096. [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. II. Specificity of RNA synthesis. Biochim Biophys Acta. 1971 Jan 1;228(1):212–222. doi: 10.1016/0005-2787(71)90561-2. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., King R. J. The use of protamine to study [6,7-3H] oestradiol-17-beta binding in rat uterus. Biochem J. 1970 Aug;118(5):695–701. doi: 10.1042/bj1180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steggles A. W., Spelsberg T. C., O'Malley B. W. Tissue specific binding in vitro of progesterone-receptor to the chromatins of chick tissues. Biochem Biophys Res Commun. 1971 Apr 2;43(1):20–27. doi: 10.1016/s0006-291x(71)80079-7. [DOI] [PubMed] [Google Scholar]
- Swaneck G. E., Chu L. L., Edelman I. S. Stereospecific binding of aldosterone to renal chromatin. J Biol Chem. 1970 Oct 25;245(20):5382–5389. [PubMed] [Google Scholar]


