Highlights
-
•
Immune responses invoke antibodies binding many epitopes and employing many mechanisms.
-
•
Antibody cocktails are more potent, less immunogenic, and reduce escape variant risk.
-
•
Recombinant polyclonal antibody therapeutics combine up to 26 unique antibodies.
-
•
Immune repertoire characterization facilitates selection of synergistic antibodies.
-
•
Novel manufacturing and regulatory processes make recombinant polyclonals possible.
Abstract
Antibody therapeutics are one of the fastest growing classes of pharmaceuticals, with an annual US market over $20 billion, developed to treat a variety of diseases including cancer, auto-immune and infectious diseases. Most are currently administered as a single molecule to treat a single disease; however there is mounting evidence that cocktails of multiple antibodies, each with a unique binding specificity and protective mechanism, may improve clinical efficacy. Here, we review progress in the development of oligoclonal combinations of antibodies to treat disease, focusing on identification of synergistic antibodies. We then discuss the application of modern antibody engineering technologies to produce highly potent antibody preparations, including oligoclonal antibody cocktails and truly recombinant polyclonal antibodies. Specific examples illustrating the synergy conferred by multiple antibodies will be provided for diseases caused by botulinum toxin, cancer and immune thrombocytopenia. The bioprocessing and regulatory options for these preparations will be discussed.
Current Opinion in Chemical Engineering 2013, 2:405–415
This review comes from a themed issue on Biotechnology and bioprocess engineering
Edited by Wei-Shou Hu and James C Liao
For a complete overview see the Issue and the Editorial
Available online 25th September 2013
2211-3398/$ – see front matter, © 2013 Elsevier Ltd. All rights reserved.
Introduction
Antibodies now represent the largest fraction of the biologics market, currently >$20 billion annually, with 28 antibodies commercially available to treat cancer, inflammatory and infectious diseases [1]. Their appeal is due in large part to good safety profiles, clear development paths and robust manufacturing processes. Improvements in cell culture engineering have led to yields of >10 g/L [2], while protein engineering has allowed tailoring of key characteristics such as ligand binding affinity, in vivo half-life and immunogenicity [3]. Nevertheless, engineering of a single antibody does not always result in improved clinical efficacy, as was seen with Motavizumab, a highly engineered Palivizumab variant with 70-fold improved affinity for the respiratory syncytial virus F protein that conferred no additional clinical benefit [4].
In contrast to therapeutics, the native immune response does not generate a single antibody in response to disease, but instead a complex polyclonal response, comprised of multiple antibodies binding multiple epitopes with the ability to mediate a variety of effector functions. This begs the question, if the natural response is not monoclonal, why are our therapies?
Polyclonal anti-serum was first used as an immunotherapy in 1891 by Emil von Behring and Shibasaburo Kitasato and is still used to treat numerous diseases, including those caused by viruses, venoms and toxins. Targeting multiple epitopes offers broad strain protection and, unlike a monotherapy, is less likely to provide selective pressure for escape mutants already present in the population [5]. Development of resistance to hyperimmune immunoglobulin by a previously sensitive pathogen has, to our knowledge, never been described, while escape variants to individual antibodies are readily generated in lab settings [6]. As blood-derived products, intravenous immunoglobulins (IVIG) have limited availability, carry the risk of blood-borne disease transmission and batch-to-batch variability [5]. More critically, even in high-titer immunoglobulin preparations isolated from immunized volunteers, only a small fraction of antibodies bind the target of interest and of these, only a fraction will exert the desired effect (Figure 1 ). This results in a low specific activity and relatively high doses to observe a clinical effect.
Figure 1.
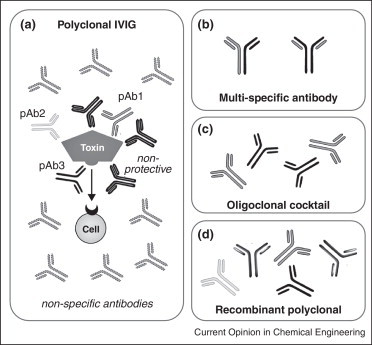
Polyclonal antibody therapeutics. (a) While a traditional IVIG product contains a large number of antibodies binding a variety of antigens, only a fraction bind the antigen of interest (e.g., a bacterial toxin) and of those, only a fraction are clinically relevant protective antibodies (pAb, e.g., those that competitively inhibit a toxin-receptor interaction, block toxin endocytosis or catalysis). IVIG thus typically requires large doses for efficacy. (b) Multi-specific antibodies yield a single molecular entity able to bind two distinct epitopes, thereby combining the ease of monoclonal antibody manufacturing with broader antigen specificity. (c) Oligoclonal antibody cocktails are a combination of several monoclonal antibodies, each grown, purified and characterized in parallel before combining. (d) Recombinant polyclonal antibodies are comprise of multiple (two to 26) molecular entities, each binding clinically relevant epitopes. These are produced and purified en masse from a single cell line (Crucell/Merus) or a polyclonal master cell bank (Symphogen).
In recent years, evidence has accumulated supporting the idea that mixtures of antibodies binding multiple non-overlapping epitopes combine the benefits of an engineered monoclonal antibody with the broad-spectrum activity of a polyclonal therapeutic. Moreover, since the component antibodies can be produced individually, they can be selected on the basis of affinity, epitope specificity and protective mechanism to ensure that each member of the mixture contributes to the overall pharmacological effect. Here, we review recent progress towards development of oligoclonal antibody cocktails and truly recombinant polyclonal antibody therapeutics, including manufacturing and regulatory approaches.
Oligoclonal antibody cocktails to treat cancer
In the simplest case, monoclonal antibodies already approved for use in humans are being tested in new combinations with other approved or investigational monoclonal antibodies (Table 1 ) [7]. For instance, disregulation of the epidermal growth factor receptor (EGFR) is involved in many cancers. Two approved anti-EGFR monoclonal antibodies (Cetuximab and Panitumumab) bind EGFR domain III, blocking ligand–receptor activation and downstream signaling. Recent studies have demonstrated that combinations of antibodies binding non-overlapping epitopes are more effective than a single antibody at inhibiting tumor growth by triggering EGFR internalization and degradation [8]. Antibody combinations may also access novel mechanisms, such as complement-dependent cytotoxicity and potentiate otherwise non-protective antibodies [9]. A recently approved biclonal combination consisting of the monoclonal antibodies Trastuzumab and Pertuzumab, which target different epitopes on the HER-2 growth factor receptor, was effective in reducing progression rates in breast cancer patients who had shown progression with Trastuzumab treatment alone [10, 11]. In a remarkable feat of protein engineering, a single antibody binding site was engineered to bind both VEGF and HER2 with high affinity (K ds of 3 and 0.2 nm, respectively) and enhanced in vivo efficacy, creating a new type of bispecific antibody in which each binding site can bind one of two distinct antigens [12]. This approach confers the advantages of bivalency to bi-specific antibodies.
Table 1.
Oligoclonal and polyclonal antibody therapeutics in development
| Name | Disease | Antibodies | Developer | Clinical phase | Reference |
|---|---|---|---|---|---|
| Antibody cocktails: combining approved drug products | |||||
| Pertuzumab (anti-HER2) + Trastuzumab (anti-HER2) + chemotherapy | First-line treatment of HER2+ metastatic breast cancer | 2 | Genentech/Roche | Approved 2012 | NCT01120184, NCT00567190 [10, 11] |
| Epratuzumab (anti-CD22) + Rituximab (anti-CD20) + chemotherapy | Diffuse large B cell lymphoma | 2 | North Central Cancer Treatment Group | II complete | NCT00301821 [81] |
| Veltuzumab (anti-CD20) + Epratuzumab (anti-CD22) | B-cell acute lymphoblastic leukemia | 2 | Immunomedics | I/II | NCT01279707 |
| Veltuzumab (anti-CD20) + Milatuzumab (anti-CD74) | B-cell non-Hodgkin's lymphoma | 2 | Immunomedics | I/II (suspended) | NCT00989586 |
| 4E10 + 2F5 + 2G12 (gp120 + gp41) | HIV | 3 | Rockefeller University | I/II | NCT00219986 [32] |
| Antibody cocktails: new drug product | |||||
| MK3415A (CDA-1 + CDB-1) | Clostridium difficile infection | 2 | Merck/Medarex/MBL | Phase III | NCT01241552 [28] |
| Sym004 (non-overlapping epitopes on EGFR domain III) | Squamous cell cancer of the head and neck, solid tumors | 2 | Symphogen, Merck KGaA | Phase II | NCT01417936, NCT01117428 [13] |
| XTL-001 (HepeX-B) | Hepatitis B virus | 2 | Cubist | Phase IIb terminated | [30] |
| XOMA 3AB | Botulinum toxin A | 3 | Xoma/UCSF | Phase I | [20•] www.xoma.com |
| MM-151 | Non-overlapping epitopes on EGFR | 3 | Adimab | Phase I | www.adimab.com |
| – | H5N1 influenza | 2 | – | Preclinical | [82] |
| Anti-anthrax toxin (PA + LF) | Anthrax | 2 | – | Preclinical | [83] |
| Recombinant polyclonal antibodies | |||||
| Rozrolimupab (Sym001; anti-RhD) | Immune thrombocytopenic purpura (ITP) | 25 | Symphogen | II completed | NCT00718692 [40••] |
| CL184 | Rabies virus glycoprotein | 2 | Crucell Holland BV/Sanofi | II (fast-track) | NCT01228383 [26, 27•] |
| Sym013 EGFR, HER2, HER3 | Multiple cancer indications | 6 | Symphogen | Phase I | [84] |
| Sym002 | Smallpox (vaccinia) | 26 | Symphogen | Preclinical | [42••] |
| – | Methicillin-resistant S. aureus | 5 | Excelimmune | Preclinical | [44] |
To create the novel anti-EGFR cocktail Sym004, Pedersen et al. [13, 14••] systematically tested combinations of two or three antibodies from a pool of 88 murine anti-EGFR antibodies for tumor inhibition activity. The most potent mixture, a 1:1 combination of antibodies 992 and 1024, showed maximal tumor inhibition, which correlated with EGFR downregulation. The antibodies bind non-overlapping epitopes on EGFR domain III; indeed antibody 992 exhibited synergistic growth inhibition with all antibodies tested that bind non-overlapping epitopes. Suggesting this may be a common strategy, Spangler et al. examined pairwise combinations of six murine anti-EGFR antibodies, with the pair most potently reducing surface EGFR levels binding non-overlapping domain III epitopes, leading to reduced receptor recycling [15]. Treatment with 50 mg/kg of Sym004 led to complete tumor regression in all mice, while Cetuximab alone or the individual antibodies exhibited only partial suppression. Sym004 performed well in preclinical studies and is currently in Phase II trials [16].
Oligoclonal antibody cocktails to treat infectious diseases
Development of antibody therapeutics to treat infectious diseases has lagged behind those for cancer, with only a single monoclonal antibody available (Palivizumab, MedImmune), in part due to the difficulties associated with multiple antigens and naturally occurring strain variation. These therapeutics may be more viable as combinations of antibodies binding potently neutralizing epitopes and triggering appropriate effector functions. Since polyclonal antibodies were first used to treat toxin-mediated diseases, early efforts to deconvolute the polyclonal response into a mixture of specific monoclonal antibodies centered on these pathogens. One study showed that antibodies elicited by murine immunization bound 20 distinct epitopes on the tetanus toxoid heavy chain [17], while in humans 17 unique epitopes were observed [18]. Quaternary combinations of the murine antibodies were 200-fold more protective in a mouse model than the individual antibodies [17]. In another study, two human anti-tetanus toxin antibodies provided similar protection as a polyclonal preparation at >100-fold lower dose (0.7 mg versus 100 mg) [19].
Similar studies with botulinum toxin A (BoNT/A) showed that while no single antibody significantly neutralized the toxin, a combination of three antibodies binding non-overlapping epitopes on the heavy chain exhibited a potency 90 times greater than human hyperimmune globulin and 20 000-fold greater than any single antibody [20•]. Analysis of the detailed pharmacokinetics of BoNT/A in the presence of a similar set of antibodies identified therapeutic windows for passive immunization postintoxication [21••]. The antibodies are thought to block receptor binding and toxin internalization, followed by Fc-dependent hepatic clearance of antibody-toxin complexes [22]. Engineered, human versions of these antibodies are in development as Xoma 3AB, an equimolar mixture of three antibodies, which is over 500-times more potent in mouse potency tests than the equine IVIG. While this product can treat only one of the seven BoNT serotypes (encompassing sub-types A1, A2 and A3) [22], analogous cocktails are in development for other serotypes. In another protein engineering feat, individual antibodies have been engineered to bind the same epitope as presented by multiple sub-types or even multiple serotypes with high affinity and specificity and could potentially be combined to create a single cocktail with broader strain coverage [23•, 24••].
A second cocktail in development neutralizes rabies virus, for which high titer human or equine polyclonal immunoglobulin remains the standard of care. Crucell Holland BV identified two human antibodies, CR57 and CR4098 from immunized humans that bind non-overlapping epitopes on the rabies glycoprotein with high affinity (2.4 and 4.5 nm, respectively) [25]. While most antibodies neutralizing rabies virus bind conformational epitopes in antigenic site II, CR57 binds a linear epitope in site I (residues 226–231) and CR4098 binds a conformational epitope within site III. Each monoclonal antibody alone was shown to neutralize 24 of 26 street isolates, similar to rabies IVIG. However, the binary combination neutralized all 26 strains and was at least as potent as IVIG in a hamster model [26]. Critically, escape variants with mutations in the CR57 epitope are neutralized by CR4098 and vice versa, while in vitro selection for variants escaping both antibodies was unsuccessful [6]. This binary cocktail is safe in humans [27•], can be administered in conjunction with the rabies vaccine and is now in Phase II clinical trials as CL184.
Additional antibody cocktails are in development to treat infectious diseases, although their effectiveness has yet to be demonstrated in clinical trials (Table 1). Merck Medarex has recently presented promising Phase II clinical data showing that a biclonal therapeutic targeting C. difficile toxins A and B was able to lower the incidence of recurrence in patients when administered in combination with vancomycin or metronidazole [28]. These antibodies are currently in Phase III trials with liscensee Merck as individual monoclonals and the biclonal combination (NCT01241552). Crucell is pursuing a biclonal cocktail against SARS coronavirus [29]. Preclinical data have also been presented demonstrating the effectiveness of biclonals against Hepatitis C virus [30, 31] in vitro and in animal models. A triclonal mixture of broadly neutralizing anti-HIV antibodies binding the fusogenic gp120 (2G12) and conformational epitopes within the membrane proximal region of gp41 (4E10, 2F5) has been assessed in a Phase II trial, with six of fourteen volunteers showing partial or complete control of viremia [32, 33]. A binary mixture of antibodies binding the non-overlapping CD4 binding site (VRC01) and a glycan-dependent epitope in the V1V2 region of the viral spike trimer (PG9) demonstrated neutralization of 98% of 208 HIV strains in vitro [34].
Recombinant polyclonal antibodies
Biclonal and triclonal antibodies function synergistically to neutralize a target pathogen and can target more than one epitope, yet they are still vulnerable to mutations of their target epitopes. Jim Marks and his group at UCSF have observed, targeting BoNT/A, that a minimum of three antibodies is required for effective neutralization, and that through point mutation or epitope shift of any of these three antibodies could cause the antibody cocktail to become ineffective, events that are less likely to occur for this particular target antigen but could be a hazard for more mutable pathogens [20•]. Thus, in many cases more complex polyclonal mixtures may be required to confer adequate protection. While not all activities may be relevant for each clinical case, in aggregate the drug would enable protection against a complex disease, while reducing the potential immunogenicity of each individual component [35].
The rhesus D antigen (RhD) was selected for development as Sym001 (Rozrolimumpab), the first in class recombinant polyclonal therapeutic by Symphogen. Currently, plasma-derived polyclonal antibodies with a high titer against the rhesus D antigen (RhIG) antigen are standard care to treat immune thrombocytopenia (ITP), to prevent maternal RhD-sensitization when an RhD− woman carries an RhD+ fetus and to treat hemolytic disease in the newborn. In ITP, platelets are opsonized by auto-antibodies and destroyed by Fc-mediated phagocytosis. IVIG and RhIG are speculated to protect against ITP by opsonizing red blood cells, which then bind macrophage FcγRIIIa receptors, outcompeting antibody-bound platelets. This notion is supported by evidence that red blood cell clearance correlates with anti-RhD coverage [36•]. Since RhIG is currently purified from the serum of immunized male volunteers, experiences periodic supply shortages and recognizes a single antigen, it has been the target of efforts to develop recombinant antibody therapies.
Analysis of the RhD-responsive antibody repertoire in alloimmunized women revealed considerable conservation, including a strong preference for the VH 3-33 gene segment among eight women analyzed, with five sharing identical VDJ usage and CDR H3-length [37]. In spite of this apparent conservation, efficacious monoclonal antibodies or cocktails have yet to be identified. Godeau et al. observed a transient response in one of seven patients at 47–95 μg/kg dosages, with further dose increases restricted by toxicity [38]. A biclonal mixture of the Brad5 and Brad3 antibodies, binding the same loop 6/7 epitope and using IgG1 and IgG3 isotypes respectively, showed efficacy similar to RhIG but only at a three-fold higher dose [36•]. A careful analysis of protection conferred by combinations of six antibodies, suggested that the specific epitope recognized and antibody isotype are key variables [39].
An explanation for RhIG but not monoclonal efficacy at 50–75 μg/kg doses may be the antigenic nature of RhD: the epitopes are small and overlapping with >100 observed mutations. Thus, subtle epitope specificity may be required to efficiently engage RhD and FcγRIIIa simultaneously, with different individuals having different requirements. To provide coverage for these possibilities, Sym001 was designed as a combination of 25 individual anti-RhD antibodies, binding 99% of observed RhD variants. These antibodies were derived from the lymphocytes of eight RhD− women with high anti-RhD titers [37]. The product was recently tested in a Phase I/II dose-escalation study from 75 to 300 μg/kg with 61 subjects. Overall, 34% (21 patients) showed response at day 7, while at the 300 μg/kg dose, 62% (eight of 13) responded. This is a similar response rate as seen with RhIG, but requires an approximately fivefold dose increase [40••].
Following the trail-blazing path led by Sym001, several additional recombinant polyclonal therapeutics are in development. Symphogen has performed preclinical tests on a 26-antibody recombinant polyclonal against smallpox (Vaccinia). After characterization of the antibody repertoire in ten immunized individuals, an antibody combination was identified which mirrors the diversity and specificity of the human response with affinities ranging from 10 to 0.1 nm [41••]. While enriched immunoglobulin required 30 mg to protect mice, a recombinant mixture recognizing mature and enveloped virions protected at 100 μg and a mixture only recognizing enveloped virions protected at 30 μg, a 1000-fold decrease. [42••]. Importantly, the antibodies were not characterized beyond their ability to bind virus. Symphogen has an additional Phase I trial on-going with Sym013, a mixture of six antibodies recognizing all three EGFR variants (EGFR, HER1, HER2; Table 1).
Excelimmune employed a set of five human antibodies recognizing S. aureus, including methicillin-resistance S. aureus (MRSA) strains, in in vivo studies. These showed protection exceeding that conferred by any monoclonal antibody, fully protecting mice against a lethal dose of bacteria at a 1 mg/kg dose [43, 44]. Of these five antibodies, only one binds an identified antigen, while the others bind either cell surface or soluble proteins. Many S. aureus toxins are encoded by phages, resulting in considerable diversity between strains. When a case of acute sepsis presents, the optimal clinical outcome may be obtained by treatment with a single therapeutic with broad strain coverage, rather than first identifying the specific strain before treating with a more specialized drug.
Finally, an excellent test case for recombinant polyclonal antibody therapy may be disease caused by Bordetellae, primarily B. pertussis. Bordetellae express over 20 known virulence factors with, critically, no clear serological correlate of protection. Currently available acellular vaccines include between one and five antigens known to induce strong humoral immune responses. In spite of broad vaccine coverage, disease persists and incidence has increased dramatically in recent years, with high infant morbidity and mortality rates. However, treatment with high-titer anti-pertussis toxin IVIG has been shown to reverse disease symptoms up to seven days after infection in mice [45] and in Phase II trials with infants, shown to reduce the number of whoops [46, 47, 48]. There are also several examples in the literature of synergistic antibody combinations with potencies surpassing that of polyclonal antisera in mice [49]. Since the key antigens are well-characterized with, in most cases, neutralizing antibodies and protective epitopes already identified [49, 50, 51], antibodies could be rationally combined to identify a potently protective cocktail. The antibodies could be individually engineered for traits likely to correlate with protection, including high affinity, recognition of all circulating clinical strains with characterized mechanisms of protection [50, 52].
Mining the natural polyclonal repertoire
A key question during development of recombinant cocktails or polyclonal antibody therapeutics is which antibodies should be combined? What minimal collection will be synergistic and potently protective yet robust against antigenic variation? The answer may lie in defining antibody repertoires responding to the different epitopes present on an antigen; from this comprehensive collection, protective antibodies binding different epitopes can be selected for further characterization and engineering. Older work used phage libraries generated from donor PBMCs, but this approach suffers from loss of native light-heavy chain pairing and heterologous expression constraints [53]. Exciting newer technologies have been developed to isolate and sequence the variable regions present in antigen-specific plasmablasts [54, 55, 56, 57], which correlate with serum antibodies observed proteomically [58••, 59••].
The overall serum response recognizing a single antigen appears to be oligoclonal with a discrete number of clades resulting from an initial VDJ recombination event followed by somatic hypermutation. In the circulating repertoire, multiple clade members — not just highest affinity or most highly mutated — persist, perhaps to provide the plasticity to respond to antigenic variants [60••]. Antibodies present in the circulating repertoire exhibit affinities between 10 and 0.1 nm [61••, 62], consistent with expectations based on the limits of B cell receptor signaling [63] and competition for limited antigen [64]. While the naïve repertoire can encode antibodies with high affinity for large, antigenically complex antigens, within a clade 10–100-fold increases in affinity are observed as the genes are progressively altered via somatic hypermutation [60••]. An analysis of plasma cells after tetanus toxin immunization observed 32 and 41 unique Vh and Vl rearrangements, respectively, while one donor immunized with the 2003 influenza vaccine yielded 17 unique Vh rearrangements. Overall the affinities ranged from 250 to 0.3 nm [65].
A single antigen can present multiple antigenic sites, which include neutralizing and non-neutralizing, immunodominant and weakly immunogenic epitopes. The native polyclonal response will include antibodies recognizing all of these, including ‘decoy’ epitopes that are highly immunogenic and dominate the polyclonal response but are only weakly or non-protective (Figure 1). For instance, RhD appears to possess one major epitope, fortuitously recognized by high affinity germline antibodies [37], while the head domain of H1N1 influenza HA has five unique sites. The key advantage of recombinant polyclonal therapeutics is that they can be comprised exclusively of high affinity antibodies binding potently neutralizing epitopes with characterized protective mechanisms to not just recapitulate but exceed the potency of the natural response.
Manufacture of oligoclonal antibodies
The manufacture of individual monoclonal antibodies is well-developed, primarily using stably transfected CHO cells in fed-batch culture, followed by downstream purification and product characterization according to standards set by the Center for Drug Evaluation and Research (CDER) at the FDA [66]. These steps typically involve protein A affinity and size exclusion chromatography followed by analyses of structural integrity (SDS-PAGE, peptide mapping, free sulfhydryl and monosaccharide and oligosaccharide analyses). A simple approach to generation of antibody cocktails, especially those which have already received approval as a monoclonal therapeutic, is to process the molecules in parallel, combining them after purification or at the point of administration (Figure 2 ) [32]. This may be favored from a regulatory perspective, considering the FDA combination drug rule discussed below.
Figure 2.
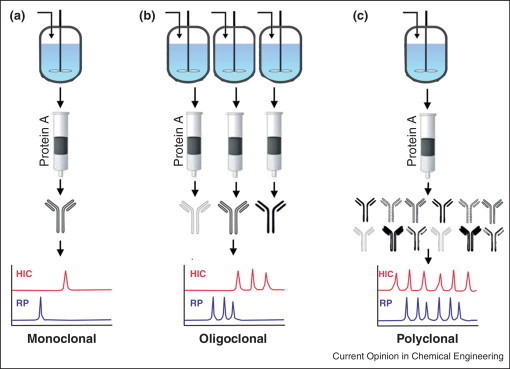
Manufacturing approaches for polyclonal antibody therapeutics. (a) Monoclonal or bi-specific antibodies are derived from a single cell line, which is expanded and grown in a bioreactor, followed by protein A and size exclusion chromatographic steps. (b) Oligoclonal cocktails are comprised of multiple antibodies (typically two to three) that are expressed and purified in parallel before combining to yield the final product. (c) Recombinant polyclonal antibodies are comprised of two to 26 unique molecular entities. These can be produced from a mixture of cell lines, each expressing a single heavy and light chain, in a single bioreactor and purified en masse. Alternatively, these can be produced from a single master cell line stably expressing a single light chain and up to 5 heavy chains to produce a mixture of bi-specific and mono-specific antibodies. Chromatographic and binding assays ensure the presence and activity of each individual antibody in the final preparation.
Biclonal and triclonal therapeutics comprised of novel antibodies are currently generated in a similar manner, with master cell banks prepared for each antibody. These are then expanded, expressed and purified separately before combining in defined ratios [32, 67•]. Theoretically, bi-and triclonal mixtures are not complicated, thus manufacturing them as monoclonals avoids overhauling established manufacturing platforms with the main concern being undesirable interactions between the component antibodies upon combination. For instance, the XOMA 3AB anti-BoNT triclonal cocktail is produced in this way, with the presence and integrity of each component antibody monitored by HPLC chromatography (hydrophobic interaction and reverse phase) and ELISA, using toxin domains specifically engineered to bind a single antibody in the mixture [67•]. This cocktail can be stored at 2–8°C for two years and is robust to lyophilization, retaining full-antigen-reactivity after five months at 50°C [68]. However, as the number of unique molecules increases above two or three, the manufacturing costs rise precipitously, since each molecule sequentially monopolizes facilities and requires individual characterization. Moreover, as each batch is subject to failure, producing multiple small batches eliminates the savings achieved by economies of scale in larger recombinant protein production facilities [69] (Figure 3 ).
Figure 3.
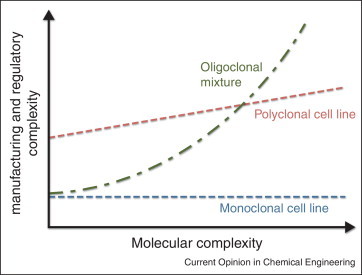
Comparison of costs associated with manufacturing and development of antibody combinations. Costs are fixed for production of a single monoclonal or bispecific antibody, since just one master cell line, manufacturing process and clinical trial is required. In contrast, for oligoclonal cocktails, manufacturing costs increase linearly with the number of unique antibodies processed in parallel, while regulatory costs increase exponentially since all pairwise combinations must be assessed for safety. For a recombinant polyclonal therapeutic, the initial manufacturing and regulatory costs are high to establish the process, but increase incrementally with further increases in molecular complexity [80••].
Manufacture of recombinant polyclonal antibodies
To constrain costs, strategies have been developed to produce recombinant polyclonal antibodies in a single pot, several of which have subsequently entered clinical trials. This process-as-product strategy bypasses the manufacturing hurdles associated with individual antibody production but introduces new challenges to demonstrate reproducible cell growth, genomic stability, constant antibody ratios and integrity of the component antibodies. Merus solved this by generating Oligoclonics™, a single PER.C6® stable cell line expressing one light common chain and three heavy chains, producing a consistent antibody mixture with three unique binding sites at 15–20 pg/cell/day for up to 67 cell divisions [70••]. The process works since in many cases, light chains are promiscuous, forming functional binding sites with a variety of heavy chains [18]. In this case, ligand-specific heavy chains were selected from a human Fab phage library containing a single rearranged light chain, although Merus has now developed a transgenic mouse with a single human light chain and diverse human heavy chains to further develop the technology [71].
In contrast, three approaches have been explored to combine stable cell lines to create a polyclonal master cell bank. Here, the challenge of consistent growth and antibody production rates is magnified, since small rate differences can lead to large differences in the final antibody mixture. One approach, Sympress I, relies on site-specific flp recombinase to introduce an antibody expression cassette into a single, transcriptionally permissive site in the genome, favoring consistent growth and expression [72]. Equal numbers of 25 unique cell lines were combined to yield the Sym001 working cell bank, with the resulting polyclonal antibody mixture showing similar composition as measured by the ion exchange profile and activity in four 400 L bioreactor lots. This consistency was achieved in spite of up to 10-fold different expression levels. In addition to the standard analyses used to characterize monoclonal antibodies, a robust product-specific liquid chromatography–mass spectrometry method was developed to unambiguously quantify antibody ratios based on the light chain [73•, 74••]. This consistency allowed Symphogen to carry Sym001 through the clinical trials approval process as a single therapeutic entity [40••].
A second approach, Sympress II, randomly integrates antibody genes into the DHFR−/− CHO DG44 cell line. After gene amplification with methotrexate, selected high expressing clones were expanded and combined to form a polyclonal master cell bank. This process has been evaluated with two groups of six antibodies each, recognizing vaccinia and respiratory syncytial virus. The polyclonal preparation produced ∼13 pg/cell/day with stable antibody compositions as assessed by ion exchange chromatography over ∼27 cell generations [75]. In a third approach, the adeno-associated virus rep protein mediates stable integration of antibody genes into HEK cells. While early, this appears to be rapid yet provides control over the final antibody ratios. Mixtures of five lines have shown consistent expression levels and stable antibody ratios for up to 100 days [76].
Regulatory approval routes for oligoclonal and recombinant polyclonal antibodies
Polyclonal immunoglobulin therapy has been safely used to treat human disease for decades, with clinical trials on-going for new indications (Table 1). Their regulation under CBER as a blood-derived product has been extensively described, with activity standards established for antigen-enriched IVIG [5]. Since they are derived from human volunteers or equine sources, they are subject to limitations in terms of scalability and supply shortfalls. As individual antibodies are never isolated, the preparation is characterized for bulk activity, necessarily including many antibodies which are inert and do not bind antigens relevant to the disease (Figure 1).
Monoclonal antibody cocktails may address IVIG supply issues as well as provide novel drugs for other diseases with negligible risk for transmissible diseases. Given that these are recombinant biologics, they are likely to fall under the regulations made by CDER, including the combination drug rule, Title 21 in the Code of Federal Regulations (21 CFR 3.2), which indicates that each component be assessed for safety and efficacy individually as well as in combination [66]. Thus, for a mixture of two antibodies, three clinical trials may be required (antibody 1, antibody 2 and the binary combination). This quickly becomes cost-prohibitive as the number of antibodies increases, unless only previously approved antibodies are considered, such as an anti-HER2 and anti-EGFR combination. Additionally, consistent production and the lack of lot-to-lot variability must be ensured. For antibody cocktails that are combined postpurification, each antibody in the mixture must adhere to the same product quality and consistency requirements as for a single monoclonal. Given that most monoclonals are produced in CHO cells, with glycosylation variation and other micro-heterogeneities affecting efficacy and long in vivo half-lives, the regulatory requirements are strict [77]. For instance, HepeX-B, a combination of two monoclonal antibodies against hepatitis B, or HBV, reported positive results of a Phase IIb study but was terminated, reportedly due to operational and economic challenges surrounding design of a Phase III trial [78].
Which rules apply to recombinant polyclonal antibodies produced in a single bioreactor? It depends on perspective: are recombinant polyclonals merely antibody cocktails produced in a single bioreactor? If so, then each component would be expected to be well-characterized and contribute to efficacy. If it is possible to produce each component individually, they can be considered a monoclonal antibody derivative regulated by CDER. Alternatively, are recombinant polyclonals a highly potent, less complex variant of IVIG? In this case, not all individual antibodies would be expected to contribute to the pharmacological effect, as long as the product is efficacious, safe and reproducibly manufactured. For instance, Symphogen's lead anti-RhD product Sym001 includes 25 unique molecular entities. Phase II trials demonstrated similar activity for Sym001 at much lower doses than anti-RhesusD IVIG [40••]. The key question is, are all 25 antibodies are essential [79••]? Whether 25, 24, or only three antibodies provide the pharmacological effect is relevant primarily for its effects on manufacturing complexity and dose. For infectious diseases, the more complex the polyclonal, the less important any single antibody becomes and the smaller the chance that a point mutation or combination of point mutations will render an organism completely resistant.
Conclusions
Monoclonal antibodies have established themselves as a versatile platform to treat many diseases, but a single antibody binding a single epitope is inherently limited, even when engineered for high ligand binding affinity, optimal pharmacokinetics and effector functions. An exciting new approach for the next generation of antibody therapeutics is mixtures of individual antibodies, selected to capitalize on functional synergy and inhibit generation of escape variants. Advances in antibody repertoire characterization are facilitating deconvolution of the polyclonal immune response to a minimal set of antibodies recognizing complementary epitopes with characterized mechanisms of protection and will prove key to selecting antibodies for inclusion in these cocktails. Similarly, transformative new technologies are emerging in terms of highly polyclonal recombinant antibody therapeutics, led by the pioneering anti-RhD product, Sym001. Once the first truly polyclonal recombinant antibody therapeutic enters the market, establishing a clear regulatory path and validated manufacturing processes, we expect to see many more follow.
Note added in proof
Two recent publications [85, 86] demonstrate dramatic reduction in viremia after administration of anti-HIV-1 antibody cocktails in rhesus macaques chronically infected with SHIV, highlighting the potential for clinical use of antibody combinations to treat infectious diseases.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by grants from the National Institutes of Health (#GM095638), the McMakin family and the Welch Foundation (F-1767) to JAM.
References
- 1.Reichert J.M. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–415. doi: 10.4161/mabs.19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wlaschin K.F., Hu W.S. Fedbatch culture and dynamic nutrient feeding. Adv Biochem Eng Biotechnol. 2006;101:43–74. doi: 10.1007/10_015. [DOI] [PubMed] [Google Scholar]
- 3.Maynard J., Georgiou G. Antibody engineering. Annu Rev Biomed Eng. 2000;2:339–376. doi: 10.1146/annurev.bioeng.2.1.339. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez P., Trenholme A., Abarca K., Griffin M.P., Hultquist M., Harris B., Losonsky G.A., Motavizumab Study Group A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatr. 2010;10:38. doi: 10.1186/1471-2431-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiehm E.R., Keller M.A., Vyas G.N. Preparation and use of therapeutic antibodies primarily of human origin. Biologicals. 2008;36:363–374. doi: 10.1016/j.biologicals.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Bakker A.B., Marissen W.E., Kramer R.A., Rice A.B., Weldon W.C., Niezgoda M., Hanlon C.A., Thijsse S., Backus H.H., de Kruif J. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J Virol. 2005;79:9062–9068. doi: 10.1128/JVI.79.14.9062-9068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demarest S.J., Hariharan K., Dong J. Emerging antibody combinations in oncology. MAbs. 2011;3:338–351. doi: 10.4161/mabs.3.4.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Kasus T., Schechter B., Lavi S., Yarden Y., Sela M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: relevance of receptor endocytosis. Proc Natl Acad Sci U S A. 2009;106:3294–3299. doi: 10.1073/pnas.0812059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dechant M., Weisner W., Berger S., Peipp M., Beyer T., Schneider-Merck T., Lammerts van Bueren J.J., Bleeker W.K., Parren P.W., van de Winkel J.G. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226. [DOI] [PubMed] [Google Scholar]
- 10.Spiridon C.I., Ghetie M.A., Uhr J., Marches R., Li J.L., Shen G.L., Vitetta E.S. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8:1720–1730. [PubMed] [Google Scholar]
- 11.Nahta R., Hung M.C., Esteva F.J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 12.Bostrom J., Yu S.F., Kan D., Appleton B.A., Lee C.V., Billeci K., Man W., Peale F., Ross S., Wiesmann C. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323:1610–1614. doi: 10.1126/science.1165480. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen M.W., Jacobsen H.J., Koefoed K., Hey A., Pyke C., Haurum J.S., Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70:588–597. doi: 10.1158/0008-5472.CAN-09-1417. [DOI] [PubMed] [Google Scholar]
- 14••.Koefoed K., Steinaa L., Soderberg J.N., Kjaer I., Jacobsen H.J., Meijer P.J., Haurum J.S., Jensen A., Kragh M., Andersen P.S. Rational identification of an optimal antibody mixture for targeting the epidermal growth factor receptor. MAbs. 2011;3:584–595. doi: 10.4161/mabs.3.6.17955. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a roadmap to select synergistic antibody combinations from the native repertoire.
- 15.Spangler J.B., Neil J.R., Abramovitch S., Yarden Y., White F.M., Lauffenburger D.A., Wittrup K.D. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci U S A. 2010;107:13252–13257. doi: 10.1073/pnas.0913476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skartved N.J., Jacobsen H.J., Pedersen M.W., Jensen P.F., Sen J.W., Jorgensen T.K., Hey A., Kragh M. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res. 2011;17:5962–5972. doi: 10.1158/1078-0432.CCR-11-1209. [DOI] [PubMed] [Google Scholar]
- 17.Volk W.A., Bizzini B., Snyder R.M., Bernhard E., Wagner R.R. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect Immun. 1984;45:604–609. doi: 10.1128/iai.45.3.604-609.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kruif J., Kramer A., Visser T., Clements C., Nijhuis R., Cox F., van der Zande V., Smit R., Pinto D., Throsby M. Human immunoglobulin repertoires against tetanus toxoid contain a large and diverse fraction of high-affinity promiscuous V(H) genes. J Mol Biol. 2009;387:548–558. doi: 10.1016/j.jmb.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Lang A.B., Cryz S.J., Jr., Schurch U., Ganss M.T., Bruderer U. Immunotherapy with human monoclonal antibodies. Fragment A specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J Immunol. 1993;151:466–472. [PubMed] [Google Scholar]
- 20•.Nowakowski A., Wang C., Powers D.B., Amersdorfer P., Smith T.J., Montgomery V.A., Sheridan R., Blake R., Smith L.A., Marks J.D. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A. 2002;99:11346–11350. doi: 10.1073/pnas.172229899. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seminal paper showing the profound increase in protection by biclonal and triclonal mixtures of neutralizing antibodies.
- 21••.Cheng L.W., Stanker L.H., Henderson T.D., 2nd, Lou J., Marks J.D. Antibody protection against botulinum neurotoxin intoxication in mice. Infect Immun. 2009;77:4305–4313. doi: 10.1128/IAI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; Performs careful PK analysis of toxin in the presence and absence of neutralizing antibodies to identify therapeutic windows after intoxication when antibody therapy is likely to be effective.
- 22.Tomic M., Garcia C., Lou J., Geren I., Meng Q., Conrad F., Wen W., Smith T., Brown J., Simpson L. Recombinant monoclonal antibody based anti-toxin for treatment of type A, B, and E botulism. Public Health Emergency Medical Countermeasures Enterprise; Washington, DC; 2011. [Google Scholar]
- 23•.Garcia-Rodriguez C., Levy R., Arndt J.W., Forsyth C.M., Razai A., Lou J., Geren I., Stevens R.C., Marks J.D. Molecular evolution of antibody cross-reactivity for two subtypes of type A botulinum neurotoxin. Nat Biotechnol. 2007;25:107–116. doi: 10.1038/nbt1269. [DOI] [PubMed] [Google Scholar]
- 24••.Garcia-Rodriguez C., Geren I.N., Lou J., Conrad F., Forsyth C., Wen W., Chakraborti S., Zao H., Manzanarez G., Smith T.J. Neutralizing human monoclonal antibodies binding multiple serotypes of botulinum neurotoxin. Protein Eng Des Sel. 2011;24:321–331. doi: 10.1093/protein/gzq111. [DOI] [PMC free article] [PubMed] [Google Scholar]; With Ref. [23], engineering of a single antibody to broadly neutralize multiple strains, presenting a possible alternative to oligoclonal antibody mixtures or a way to simplify highly complex polyclonal mixtures.
- 25.Kramer R.A., Marissen W.E., Goudsmit J., Visser T.J., Clijsters-Van der Horst M., Bakker A.Q., de Jong M., Jongeneelen M., Thijsse S., Backus H.H. The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur J Immunol. 2005;35:2131–2145. doi: 10.1002/eji.200526134. [DOI] [PubMed] [Google Scholar]
- 26.Goudsmit J., Marissen W.E., Weldon W.C., Niezgoda M., Hanlon C.A., Rice A.B., Kruif J., Dietzschold B., Bakker A.B., Rupprecht C.E. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. J Infect Dis. 2006;193:796–801. doi: 10.1086/500470. [DOI] [PubMed] [Google Scholar]
- 27•.Bakker A.B., Python C., Kissling C.J., Pandya P., Marissen W.E., Brink M.F., Lagerwerf F., Worst S., van Corven E., Kostense S. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine. 2008;26:5922–5927. doi: 10.1016/j.vaccine.2008.08.050. [DOI] [PubMed] [Google Scholar]; This paper provides strong clinical data supporting a bi-clonal anti-rabies therapeutic which they produce from a single stable cell line.
- 28.Lowy I., Molrine D.C., Leav B.A., Blair B.M., Baxter R., Gerding D.N., Nichol G., Thomas W.D., Jr., Leney M., Sloan S. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 29.ter Meulen J., van den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galun E., Eren R., Safadi R., Ashour Y., Terrault N., Keeffe E.B., Matot E., Mizrachi S., Terkieltaub D., Zohar M. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology. 2002;35:673–679. doi: 10.1053/jhep.2002.31867. [DOI] [PubMed] [Google Scholar]
- 31.Eren R., Landstein D., Terkieltaub D., Nussbaum O., Zauberman A., Ben-Porath J., Gopher J., Buchnick R., Kovjazin R., Rosenthal-Galili Z. Preclinical evaluation of two neutralizing human monoclonal antibodies against hepatitis C virus (HCV): a potential treatment to prevent HCV reinfection in liver transplant patients. J Virol. 2006;80:2654–2664. doi: 10.1128/JVI.80.6.2654-2664.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armbruster C., Stiegler G.M., Vcelar B.A., Jager W., Koller U., Jilch R., Ammann C.G., Pruenster M., Stoiber H., Katinger H.W. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother. 2004;54:915–920. doi: 10.1093/jac/dkh428. [DOI] [PubMed] [Google Scholar]
- 33.Trkola A., Kuster H., Rusert P., von Wyl V., Leemann C., Weber R., Stiegler G., Katinger H., Joos B., Gunthard H.F. In vivo efficacy of human immunodeficiency virus neutralizing antibodies: estimates for protective titers. J Virol. 2008;82:1591–1599. doi: 10.1128/JVI.01792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doria-Rose N.A., Louder M.K., Yang Z., O’Dell S., Nason M., Schmidt S.D., McKee K., Seaman M.S., Bailer R.T., Mascola J.R. HIV-1 neutralization coverage is improved by combining monoclonal antibodies that target independent epitopes. J Virol. 2012;86:3393–3397. doi: 10.1128/JVI.06745-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klitgaard J.L., Coljee V.W., Andersen P.S., Rasmussen L.K., Nielsen L.S., Haurum J.S., Bregenholt S. Reduced susceptibility of recombinant polyclonal antibodies to inhibitory anti-variable domain antibody responses. J Immunol. 2006;177:3782–3790. doi: 10.4049/jimmunol.177.6.3782. [DOI] [PubMed] [Google Scholar]
- 36•.Kumpel B.M. Efficacy of RhD monoclonal antibodies in clinical trials as replacement therapy for prophylactic anti-D immunoglobulin: more questions than answers. Vox Sang. 2007;93:99–111. doi: 10.1111/j.1423-0410.2007.00945.x. [DOI] [PubMed] [Google Scholar]; Her work and this paper show how much we still have to learn even with a relatively simple system such as a single protein.
- 37.Andersen P.S., Haahr-Hansen M., Coljee V.W., Hinnerfeldt F.R., Varming K., Bregenholt S., Haurum J.S. Extensive restrictions in the VH sequence usage of the human antibody response against the Rhesus D antigen. Mol Immunol. 2007;44:412–422. doi: 10.1016/j.molimm.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Godeau B., Oksenhendler E., Brossard Y., Bartholeyns J., Leaute J.B., Duedari N., Schaeffer A., Bierling P. Treatment of chronic autoimmune thrombocytopenic purpura with monoclonal anti-D. Transfusion. 1996;36:328–330. doi: 10.1046/j.1537-2995.1996.36496226146.x. [DOI] [PubMed] [Google Scholar]
- 39.Kjaersgaard M., Aslam R., Kim M., Speck E.R., Freedman J., Stewart D.I., Wiersma E.J., Semple J.W. Epitope specificity and isotype of monoclonal anti-D antibodies dictate their ability to inhibit phagocytosis of opsonized platelets. Blood. 2007;110:1359–1361. doi: 10.1182/blood-2007-03-079848. [DOI] [PubMed] [Google Scholar]
- 40••.Robak T., Windyga J., Trelinski J., von Depka Prondzinski M., Giagounidis A., Doyen C., Janssens A., Alvarez-Roman M.T., Jarque I., Loscertales J. Rozrolimupab, a mixture of 25 recombinant human monoclonal RhD antibodies, in the treatment of primary immune thrombocytopenia. Blood. 2012;120:3670–3676. doi: 10.1182/blood-2012-06-438804. [DOI] [PubMed] [Google Scholar]; First in class Phase I/II data demonstrating efficacy of a truly recombinant polyclonal antibody.
- 41••.Lantto J., Haahr Hansen M., Rasmussen S.K., Steinaa L., Poulsen T.R., Duggan J., Dennis M., Naylor I., Easterbrook L., Bregenholt S. Capturing the natural diversity of the human antibody response against vaccinia virus. J Virol. 2011;85:1820–1833. doi: 10.1128/JVI.02127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Zaitseva M., Kapnick S.M., Meseda C.A., Shotwell E., King L.R., Manischewitz J., Scott J., Kodihalli S., Merchlinsky M., Nielsen H. Passive immunotherapies protect WRvFire and IHD-J-Luc vaccinia virus-infected mice from lethality by reducing viral loads in the upper respiratory tract and internal organs. J Virol. 2011;85:9147–9158. doi: 10.1128/JVI.00121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]; With Ref. [41] presents a compelling proof-of-concept that a 26-antibody mixture can potently protect against smallpox in a mouse model.
- 43.V.W. Coljee, Staphylococcus aureus specific human recombinant polyclonal antibodies and uses thereof, US Patent 2010.
- 44.Zondervan Q., Carey K., Croal L., Dubey M., Porter J., Puro R., Reczek E., Russell H., Schneider R., Towle T. Human recombinant antibody (Ab) cocktails protect against Methicillin Resistant Staphylococcus aureus (MRSA) infection in mice. Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA; 2010. [Google Scholar]
- 45.Bruss J.B., Siber G.R. Protective effects of pertussis immunoglobulin (P-IGIV) in the aerosol challenge model. Clin Diagn Lab Immunol. 1999;6:464–470. doi: 10.1128/cdli.6.4.464-470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruss J.B., Malley R., Halperin S., Dobson S., Dhalla M., McIver J., Siber G.R. Treatment of severe pertussis: a study of the safety and pharmacology of intravenous pertussis immunoglobulin. Pediatr Infect Dis J. 1999;18:505–511. doi: 10.1097/00006454-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Granstrom M., Olinder-Nielsen A.M., Holmblad P., Mark A., Hanngren K. Specific immunoglobulin for treatment of whooping cough. Lancet. 1991;338:1230–1233. doi: 10.1016/0140-6736(91)92101-7. [DOI] [PubMed] [Google Scholar]
- 48.Halperin S.A., Vaudry W., Boucher F.D., Mackintosh K., Waggener T.B., Smith B. Is pertussis immune globulin efficacious for the treatment of hospitalized infants with pertussis? No answer yet. Pediatr Infect Dis J. 2007;26:79–81. doi: 10.1097/01.inf.0000247103.01075.cc. [DOI] [PubMed] [Google Scholar]
- 49.Sato H., Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990;58:3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutherland J.N., Maynard J.A. Characterization of a key neutralizing epitope on pertussis toxin recognized by the monoclonal antibody 1B7. Biochemistry. 2009;48:11982–21199. doi: 10.1021/bi901532z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.J., Gray M.C., Guo L., Sebo P., Hewlett E.L. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1999;67:2090–2100. doi: 10.1128/iai.67.5.2090-2095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maynard J.A., Maassen C.B.M., Leppla S.H., Brasky K., Patterson J.L., Iverson B.L., Georgiou G. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat Biotechnol. 2002;20:597–601. doi: 10.1038/nbt0602-597. [DOI] [PubMed] [Google Scholar]
- 53.Binley J.M., Ditzel H.J., Barbas C.F., 3rd, Sullivan N., Sodroski J., Parren P.W., Burton D.R. Human antibody responses to HIV type 1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res Hum Retroviruses. 1996;12:911–924. doi: 10.1089/aid.1996.12.911. [DOI] [PubMed] [Google Scholar]
- 54.Yu X., Tsibane T., McGraw P.A., House F.S., Keefer C.J., Hicar M.D., Tumpey T.M., Pappas C., Perrone L.A., Martinez O. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith K., Garman L., Wrammert J., Zheng N.Y., Capra J.D., Ahmed R., Wilson P.C. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang N., He J., Weinstein J.A., Penland L., Sasaki S., He X.S., Dekker C.L., Zheng N.Y., Huang M., Sullivan M. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5:ra119. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Cheung W.C., Beausoleil S.A., Zhang X., Sato S., Schieferl S.M., Wieler J.S., Beaudet J.G., Ramenani R.K., Popova L., Comb M.J. A proteomics approach for the identification and cloning of monoclonal antibodies from serum. Nat Biotechnol. 2012;30:447–452. doi: 10.1038/nbt.2167. [DOI] [PubMed] [Google Scholar]
- 59••.Wine Y., Boutz D.R., Lavinder J.J., Miklos A.E., Hughes R.A., Hoi K.H., Jung S.T., Horton A.P., Murrin E.M., Ellington A.D. Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. Proc Natl Acad Sci U S A. 2013;110:2993–2998. doi: 10.1073/pnas.1213737110. [DOI] [PMC free article] [PubMed] [Google Scholar]; With Ref. [58] demonstrates that the frequency of antibody sequences detected by high throughput sequencing of plasma B cells correlates with the frequency of specific antibody proteins present in the serum.
- 60••.Krause J.C., Tsibane T., Tumpey T.M., Huffman C.J., Briney B.S., Smith S.A., Basler C.F., Crowe J.E., Jr. Epitope-specific human influenza antibody repertoires diversify by B cell intraclonal sequence divergence and interclonal convergence. J Immunol. 2011;187:3704–3711. doi: 10.4049/jimmunol.1101823. [DOI] [PMC free article] [PubMed] [Google Scholar]; Suggests that a wide variety of clones recognizing a given antigen are maintained in circulation in to allow for faster responses to antigenic variation.
- 61••.Poulsen T.R., Jensen A., Haurum J.S., Andersen P.S. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol. 2011;187:4229–4235. doi: 10.4049/jimmunol.1000928. [DOI] [PubMed] [Google Scholar]; Carefully documents the ranges of on and off-rates for antibodies present in the natural polyclonal repertoire of vaccinated adults.
- 62.Poulsen T.R., Meijer P.J., Jensen A., Nielsen L.S., Andersen P.S. Kinetic, affinity, and diversity limits of human polyclonal antibody responses against tetanus toxoid. J Immunol. 2007;179:3841–3850. doi: 10.4049/jimmunol.179.6.3841. [DOI] [PubMed] [Google Scholar]
- 63.Foote J., Eisen H.N. Kinetic and affinity limits on antibodies produced during immune responses. Proc Natl Acad Sci U S A. 1995;92:1254–1256. doi: 10.1073/pnas.92.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batista F.D., Neuberger M.S. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 65.Meijer P.J., Andersen P.S., Haahr Hansen M., Steinaa L., Jensen A., Lantto J., Oleksiewicz M.B., Tengbjerg K., Poulsen T.R., Coljee V.W. Isolation of human antibody repertoires with preservation of the natural heavy and light chain pairing. J Mol Biol. 2006;358:764–772. doi: 10.1016/j.jmb.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 66.Lauritsen K.J., Nguyen T. Combination products regulation at the FDA. Clin Pharmacol Ther. 2009;85:468–470. doi: 10.1038/clpt.2009.28. [DOI] [PubMed] [Google Scholar]
- 67•.Meng Q., Li M., Silberg M.A., Conrad F., Bettencourt J., To R., Huang C., Ma J., Meyer K., Shimizu R. Domain-based assays of individual antibody concentrations in an oligoclonal combination targeting a single protein. Anal Biochem. 2012;421:351–361. doi: 10.1016/j.ab.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; Presents a novel ELISA format to detect the specific activity of antibodies present in a mixture using modified antigen to present only a single epitope.
- 68.Freeberg J., Li M., To R., Tomic R. Development of a lyophilized formulation for anti-BoNT A drug product XOMA 3AB. Interagency Botulism Research Coordinating Committee Meeting; Atlanta, GA; 2010. [Google Scholar]
- 69.Rasmussen S.K., Nielsen L.S., Muller C., Bouquin T., Naested H., Monster N.T., Nygaard F., Weilguny D., Frandsen T.P., Tolstrup A.B. Recombinant antibody mixtures; optimization of cell line generation and single-batch manufacturing processes. BMC Proc. 2011;5(Suppl 8):O2. doi: 10.1186/1753-6561-5-S8-O2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70••.de Kruif J., Kramer A., Nijhuis R., van der Zande V., den Blanken R., Clements C., Visser T., Keehnen R., den Hartog M., Throsby M. Generation of stable cell clones expressing mixtures of human antibodies. Biotechnol Bioeng. 2010;106:741–750. doi: 10.1002/bit.22763. [DOI] [PubMed] [Google Scholar]; Describes the Merus Oligoclonics™ process for generating a single cell line expressing mutiple mono-specific and bi-specific antibodies.
- 71.Moran N. Mouse platforms jostle for slice of humanized antibody market. Nat Biotechnol. 2013;31:267–268. doi: 10.1038/nbt0413-267. [DOI] [PubMed] [Google Scholar]
- 72.Wiberg F.C., Rasmussen S.K., Frandsen T.P., Rasmussen L.K., Tengbjerg K., Coljee V.W., Sharon J., Yang C.Y., Bregenholt S., Nielsen L.S. Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol Bioeng. 2006;94:396–405. doi: 10.1002/bit.20865. [DOI] [PubMed] [Google Scholar]
- 73•.Persson P., Engstrom A., Rasmussen L.K., Holmberg E., Frandsen T.P. Development of mass spectrometry based techniques for the identification and determination of compositional variability in recombinant polyclonal antibody products. Anal Chem. 2010;82:7274–7282. doi: 10.1021/ac101175w. [DOI] [PubMed] [Google Scholar]; This paper presents robust methods to detect and characterize each component antibody present in a complex recombinant mixture.
- 74••.Frandsen T.P., Naested H., Rasmussen S.K., Hauptig P., Wiberg F.C., Rasmussen L.K., Jensen A.M., Persson P., Wiken M., Engstrom A. Consistent manufacturing and quality control of a highly complex recombinant polyclonal antibody product for human therapeutic use. Biotechnol Bioeng. 2011;108:2171–2181. doi: 10.1002/bit.23166. [DOI] [PubMed] [Google Scholar]; This paper presents methods to make highly polyclonal recombinant antibody mixtures.
- 75.Nielsen L.S., Baer A., Muller C., Gregersen K., Monster N.T., Rasmussen S.K., Weilguny D., Tolstrup A.B. Single-batch production of recombinant human polyclonal antibodies. Mol Biotechnol. 2010;45:257–266. doi: 10.1007/s12033-010-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hicks S.W., Wilmes G.M., Lutz S.R., Stevenson J.A., Capacian M.D., Carey K.L., Colantonio A.D., Russell H.H., Reczek E.E., Coljee V.W. Non-viral adeno-associated virus-based platform for stable expression of polyclonal antibody mixtures and/or recombinant proteins. Immunology. 2012;137:658. [Google Scholar]
- 77.Read E.K., Park J.T., Brorson K.A. Industry and regulatory experience of the glycosylation of monoclonal antibodies. Biotechnol Appl Biochem. 2011;58:213–219. doi: 10.1002/bab.35. [DOI] [PubMed] [Google Scholar]
- 78.XTLbio: Annual Report; Valley Cottage, NY; 2006. [Google Scholar]
- 79••.Cuker A. Are 25 antibodies better than 1? Blood. 2012;120:3627–3628. doi: 10.1182/blood-2012-09-452771. [DOI] [PubMed] [Google Scholar]; This paper provides commentary on Robak et al. [40••], questioning whether the contributions of each of the 25 antibodies present in Sym001 needs to be elucidated for regulatory purposes.
- 80••.Rasmussen S.K., Naested H., Muller C., Tolstrup A.B., Frandsen T.P. Recombinant antibody mixtures: production strategies and cost considerations. Arch Biochem Biophys. 2012;526:139–145. doi: 10.1016/j.abb.2012.07.001. [DOI] [PubMed] [Google Scholar]; This paper performs a careful economic comparison of manufacturing costs for oligoclonal versus recombinant antibodies.
- 81.Micallef I.N., Maurer M.J., Wiseman G.A., Nikcevich D.A., Kurtin P.J., Cannon M.W., Perez D.G., Soori G.S., Link B.K., Habermann T.M. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118:4053–4061. doi: 10.1182/blood-2011-02-336990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prabakaran M., Prabhu N., He F., Hongliang Q., Ho H.T., Qiang J., Meng T., Goutama M., Kwang J. Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants. PLoS ONE. 2009;4:e 5672. doi: 10.1371/journal.pone.0005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Z., Moayeri M., Crown D., Emerson S., Gorshkova I., Schuck P., Leppla S.H., Purcell R.H. Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody. Infect Immun. 2009;77:3902–3908. doi: 10.1128/IAI.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johan Lantto M.W.P., Jacobsen H.J., Poulsen T.T., Kjær I., Koefoed K., Sen J.W., Weilguny D., Bjerregaard B., Andersen C.R., Horak I.D., Kragh M. Simultaneous inhibition of EGFR, HER2 and HER3 by an antibody mixture provides broad and potent tumor inhibition. Annual Meeting of the American Association for Cancer Research; Washington, DC; 2013. [Google Scholar]
- 85.Barouch D.H., Whitney J.B., Moldt B., Klein F., Oliveira T.Y., Liu J., Stephenson K.E., Chang H.W., Shekhar K., Gupta S. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013 doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shingai M., Nishimura Y., Klein F., Mouquet H., Donau O.K., Plishka R., Buckler-White A., Seaman M., Piatak M., Lifson J.D. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013 doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]


