Abstract
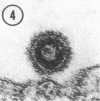
Small numbers of virus-like particles were observed by electron microscopy in each of two cloned lines of 3T3 cells transformed by murine sarcoma virus, even though these lines were free of detectable quantities of infectious leukemia and sarcoma virus. The morphology and occurrence of the particles were identical to those of the murine leukemia-sarcoma group. Moreover, the particles incorporated uridine and had a buoyant density of 1.16 g/ml in sucrose gradients. No evidence of sarcoma or leukemia virus infectivity was associated with the particles in cells of several susceptible species under various conditions, including both cosedimentation with leukemia virus and infection in the presence of inactivated Sendai virus. The particles may represent a form of murine sarcoma virus deficient in one or more of the viral components necessary for infectivity.
Keywords: density gradient centrifugation, electron microscopy, [3H]uridine
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Simons P. J., Chesterman F. C., Harvey J. J. Murine sarcoma virus (Harvey): characteristics of focus formation in mouse embryo cell cultures, and virus production by hamster tumor cells. Int J Cancer. 1968 Mar 15;3(2):265–272. doi: 10.1002/ijc.2910030212. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S. Virus particles associated with "nonproducer" Rous sarcoma cells. Virology. 1965 Nov;27(3):351–359. doi: 10.1016/0042-6822(65)90115-7. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H. Enhancing effect of diethylaminoethyl-dextran on the focus-forming titer of a murine sarcoma virus (Harvey strain). J Virol. 1968 Jun;2(6):643–644. doi: 10.1128/jvi.2.6.643-644.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- ERIKSON R. L., FENWICK M. L., FRANKLIN R. M. REPLICATION OF BACTERIOPHAGE RNA: STUDIES ON THE FATE OF PARENTAL RNA. J Mol Biol. 1964 Dec;10:519–529. doi: 10.1016/s0022-2836(64)80070-x. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., O'Conner T. E. Viral infection across species barriers: reversible alteration of murine sarcoma virus for growth in cat cells. Science. 1969 Aug 15;165(3894):714–716. doi: 10.1126/science.165.3894.714. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., O'Connor T. E. Productive infection and morphologic alteration of human cells by a modified sarcoma virus. J Natl Cancer Inst. 1970 Feb;44(2):429–438. [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Further studies on RSV production form transformed cells. Virology. 1968 Apr;34(4):630–636. doi: 10.1016/0042-6822(68)90084-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Virus production by Rous sarcoma cells. Curr Top Microbiol Immunol. 1970;51:114–123. doi: 10.1007/978-3-642-46213-9_6. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Parkman R., Levy J. A., Ting R. C. Murine sarcoma virus: the question of defectiveness. Science. 1970 Apr 17;168(3929):387–389. doi: 10.1126/science.168.3929.387. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. A virus released by "nonproducing" Rous sarcoma cells. Proc Natl Acad Sci U S A. 1967 Sep;58(3):801–808. doi: 10.1073/pnas.58.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Sarma P. S., Huebner R. J. Presence of avian tumor virus group-specific antigen in nonproducing Rous sarcoma cells of the chicken. Virology. 1965 Oct;27(2):233–236. doi: 10.1016/0042-6822(65)90168-6. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]