Abstract
Background
By attenuating the systemic inflammatory response to major surgery, the pre-operative administration of steroids may reduce the incidence of complications.
Methods
A systematic review was conducted to identify randomized controlled trials (RCT) comparing pre-operative steroid administration with placebo during a liver resection. Meta-analyses were performed.
Results
Five RCTs were identified including a total of 379 patients. Pre-operative steroids were associated with statistically significant reductions in the levels of serum bilirubin and interleukin 6 (IL-6) on post-operative day one. There was a trend towards a lower incidence of post-operative complications and prothrombin time (PT), but this did not reach statistical significance.
Conclusion
Pre-operative steroids may be associated with a clinically significant benefit in liver resection.
Introduction
Major surgery is associated with an acute systemic inflammatory response mediated by endogenously generated cytokines and free radicals.1,2 When excessive or uncontrolled this may lead to the systemic inflammatory response syndrome (SIRS).3 The degree of SIRS after surgery may correlate with post-operative morbidity, mortality and a delayed return to function.4 There has been interest in how this response may be modified in other major surgical procedures.5,6 A liver resection is a procedure which may be associated with a marked cytokine response, that may be potentiated by the ischaemia–reperfusion injury caused by portal triad clamping and other methods of vascular control used in liver surgery.7–9 Steroids are known to have significant analgesic10 and antiemetic11–13 properties, but their immunological and anti-inflammatory effects may improve the outcomes of a liver resection. It has been postulated that the peri-operative use of steroids may decrease the cytokine response and thus improve surgical outcomes.14 The potential for the benefit of the use of steroids in liver surgery is supported by experimental studies.15–17 Nevertheless, the utility of pre-operative steroid use in clinical liver resection remains controversial and their use is not a widespread practice. The aim of this systematic review and meta-analysis was to critically appraise the available evidence, with particular reference to randomized controlled trials (RCT).
Methods
A systematic literature search was independently conducted by two authors (A.J.R. and V.L.). The following electronic databases were searched: Medline (1950–2012), Embase (1974–2012), Cochrane Controlled trials Register and the science citation index. Combinations of medical subject headings (MeSH) as well as key words were used including the following: glucocorticoids, prednisone, methylprednisone, dexamethasone, steroids, predniso$, methylprednis$, liver surgery, hepatic resection, liver resection, hemihepatectomy and hepatectomy.
The literature search was not restricted by language or year of publication but was restricted to human trials. The last search was done on the 26 March 2012. A manual search was done of all the relevant articles and independent experts were contacted to retrieve other relevant articles.
Study selection and primary endpoints
Only RCTs comparing peri-operative steroid administration with placebo were included in the review. Studies describing paediatric liver resections, cadaveric liver transplantation or laparoscopic liver resection were excluded, as were animal studies. The primary end points analysed were in-hospital mortality and complications. The secondary end points analysed were prothrombin time (PT), level of serum bilirubin and level of serum interleukin 6 (IL-6) on the first post-operative day. Those studies with insufficient data relating to the defined primary and secondary outcomes were excluded. The total number of complications was recorded as reported in the original papers and comprised myocardial infarction, chest infection, bile leak, intra-abdominal collections or pulmonary embolus. The recording was in accordance with the PRISMA criteria.18 Two reviewers independently performed article selection and these were reviewed by the third author (J.L.). The methodological quality of studies was assessed using the Cochrane Collaboration's tool for assessing the risk of bias19 using the following criteria: adequate sequence generation, allocation concealment, blinding, addressing of incomplete data and whether the article appeared to be free of selective reporting and other biases.
Statistical analysis
Meta-analyses were performed using Revman 5.1 (Review manager version 5.1; Cochrane collaboration 2011). Primary outcomes were expressed as an odds ratio (OR) with 95% confidence intervals (CIs) derived by the mean difference. A random effects model was used for the analysis. The Mantel–Haenzsel method was used for dichotomous outcomes and the inverse variance method was used for continuous outcomes. Heterogeneity was assessed using Cochran's Q statistic and an I2 statistic, where values of 25% or less were considered to indicate low heterogeneity and values of 75% or more were taken to indicate high heterogeneity.20 Forrest plots were constructed with P-values of less than 0.2 considered to be statistically significant.
Results
Description of studies
Five studies met the predefined inclusion criteria, were included in the meta-analysis and are summarized in Table 1.21–25 The search strategy results are shown in Fig. 1. One group published three papers covering the same group of patients. Two of these studies were rejected26,27 and only one study was included.23 Another study that focused mainly on renal function after cadaveric liver transplantation was excluded.28 Of the five included studies, two came from Japan, two from Italy and one from Germany. There were a total of 379 patients, with 190 patients in the pre-operative steroid group and 189 in the placebo group. More than half of the patients came from one study. There was no mortality reported in any of the analysed papers. Only one study used a classification of complication severity,25 albeit not standardized. Standardized definitions of complications in liver surgery29–31 or of complication severity32 were not used in any study. The characteristics of the procedures (extent of resection, method of transaction, use, type and duration of vascular control) and patients are summarized in Table 2. The indications for liver resection are set out in Table 3.
Table 1.
Summary of randomized controlled trials analysed
| First author | Institution | Year | Steroids administration regime | Steroids group number of patients | Placebo group number of patients | Total number of patients |
|---|---|---|---|---|---|---|
| Yamashita | Fukuoka, Japan | 2001 | MP 500 mg 2 h prior to surgery | 17 | 16 | 33 |
| Muratore | Torino, Italy | 2003 | MP 30 mg/kg 30 min prior to surgery | 25 | 28 | 53 |
| Aldrighetti | Milan, Italy | 2006 | MP 500 mg prior to induction of anaesthesia | 36 | 37 | 73 |
| Schmidt | Berlin, Germany | 2007 | MP 30 mg/kg 90 min prior to surgery | 10 | 10 | 20 |
| Hayashi | Tokyo, Japan | 2011 | MP 500 mg prior to hepatic pedicle clamping | 102 | 98 | 200 |
| 300 mg on post-operative day 1 | ||||||
| 200 mg on post-operative day 2 | ||||||
| 100 mg on post-operative day 3 | ||||||
| 190 | 189 | 379 | ||||
MP, Methylprednisolone.
Figure 1.
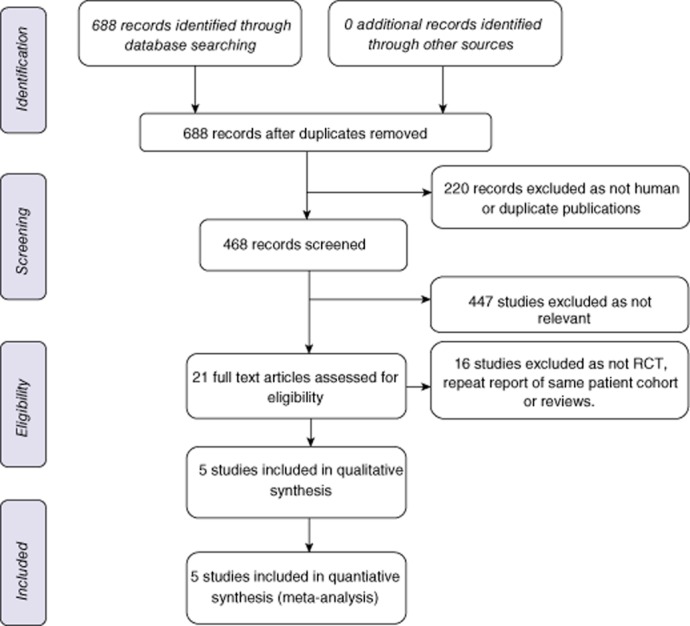
Flow chart showing the search strategy used to identify studies. RCT, randomized controlled trial
Table 2.
Summary of characteristics of procedures and patients included in the studies
| First author | Group | Major resectiona | Resection techniqueb | Vascular controlb | Ischaemic time (mins) | Cirrhosis |
|---|---|---|---|---|---|---|
| Yamashita | Steroids | 5/17 | NS | PTC or HVE with TVE | NS | NS |
| Placebo | 6/16 | NS | NS | |||
| Muratore | Steroids | 13/25 | KC or UD | PTC (continuous) | 41.4 (15.9)c | 7/25 |
| Placebo | 15/28 | 37.3 (17.8)c | 12/28 | |||
| Aldrighetti | Steroids | 26/36 | UD or US | PTC (intermittent) | 52.4 (20–89)d | 14/36 |
| Placebo | 27/37 | 48 (20–78)d | 12/36 | |||
| Schmidt | Steroids | 6/10 | UD | None used | NA | NS |
| Placebo | 5/10 | NA | NS | |||
| Hayashi | Steroids | 11/102 | NS | PTC (intermittent) | 72 (0–247)d | NS |
| Placebo | 15/98 | 60 (0–203)d | NS | |||
Resection of three or more adjacent segments.
The same method of vascular control used in both groups.
Mean (SD).
Median (range).
PTC, portal triad clamping; HVE, hemi-hepatic vascular occlusion; TVE, total vascular exclusion; NA, not applicable; NS, not stated; UD, ultrasonic dissector; KC, Kelly-clysis; US, ultrasonic shears.
Table 3.
Indications for liver resection
| Steroid group | Placebo group | |
|---|---|---|
| Hepatocellular carcinoma | 92 | 87 |
| Metastatic colorectal cancer | 47 | 43 |
| Cholangiocarcinoma | 10 | 12 |
| Living-related donor | 4 | 4 |
| Other | 12 | 15 |
Study quality
There was statistically significant heterogeneity observed in the analysis of length of stay (I2 = 77%), level of bilirubin on post-operative day 1 (I2 = 85%), PT on post-operative day 1 (I2 = 76%) and IL-6 on post-operative day 1 (I2 = 93%) but not with respect to complications (I2 = 0%). Given the small number of studies, funnel plot analysis could not be reliably interpreted and was not performed. A risk of bias diagram is shown in Fig. 2. Only one study reported on all the parameters analysed.23 No study was deficient in reporting on more than two parameters.
Figure 2.
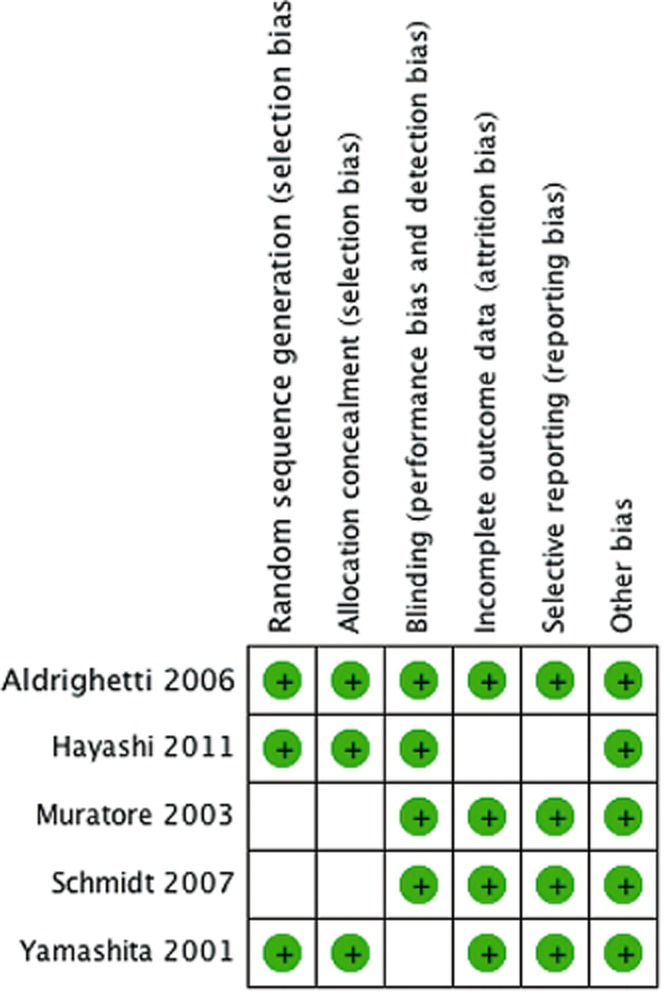
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Primary study endpoints
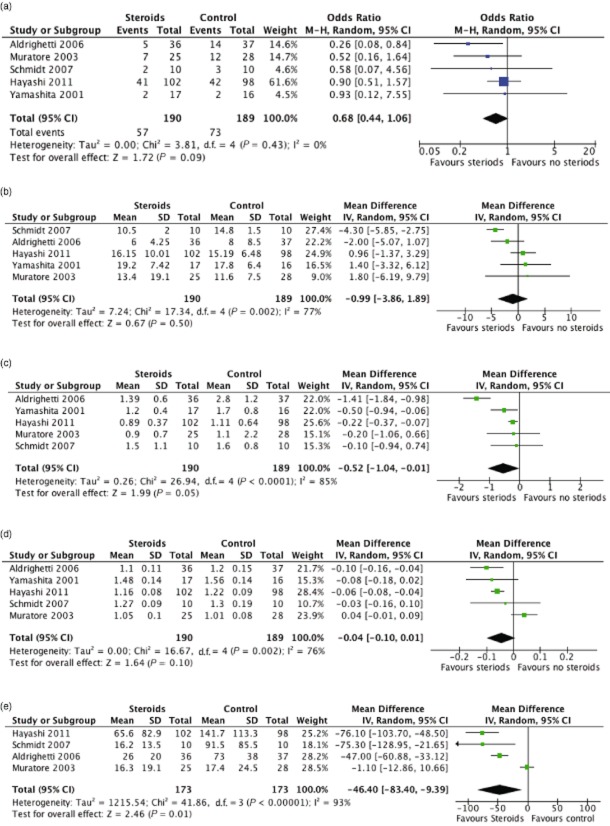
Data were available for all studies. There was no mortality in either group. There was a trend towards a reduction in the incidence of post-operative complications with steroid administration (Fig. 3a), but this did not reach statistical significance (P = 0.09, OR = 0.68 95% CI 0.44 to 1.06).
Figure 3.
Forest plots illustrating meta-analysis of outcomes in patients undergoing a liver resection with pre-operative steroid administration (steroids) or placebo (control). The outcomes analysed were post-operative complications (a), length of stay (b), serum bilirubin (c), prothrombin time (d) and IL-6 on postoperative day 1 (e). M-H, Mantel-Haenszel; 95% CI, 95% confidence
Secondary study endpoints
Data were available from all five studies with regard to length of stay (Fig. 3b), post-operative serum bilirubin (Fig. 3c) and serum PT (Fig. 3d). No effect was observed with respect to the length of stay (P = 0.5, OR = −0.99 95% CI −3.86 to 1.89). There was a statistically significant reduction in post-operative serum bilirubin associated with the use of steroids (P = 0.05, OR −0.43 95% CI −1.04 to −0.015). Steroid treatment was associated with a trend towards, although not statistically significant, reduced post-operative PT (P = 0.1, OR −0.04 95% CI −0.1 to 0.01). Data were available in four of the studies pertaining to post-operative serum IL-6 (Fig. 3e). There was a significant reduction in serum IL-6 in association with steroid administration (P = 0.01, OR −46.4 95% CI −83.4 to −9.39).
Discussion
This is the first systematic review of the use of pre-operative steroids focused exclusively on studies of liver resection. There were five RCT identified. Meta-analysis demonstrated reductions in the serum bilirubin and IL-6 on post-operative day one. There was a trend towards a reduction in the incidence of post-operative complications and PT, although this was not statistically significant. No effect was observed with respect to the length of stay.
One previous systematic review addressed different major abdominal procedures and found a decrease in length of stay and complications in those patients who were treated with pre-operative steroids.33 Similarly, there has been one systematic review of the use of pre-operative steroids in oesophageal resection which suggested a similar benefit, but the authors did point out that the quality of the included trials was poor and this tempered the firmness with which conclusions could be drawn.34 Additionally, there has been one systematic review of pharmacological interventions to reduce ischaemia–reperfusion injury in elective liver resection with vascular occlusion.35 This review suggested that pre-operative steroids may attenuate such an injury.
Inflammation is necessary for tissue healing after surgery, but it is thought that an excessive response may contribute to post-operative morbidity, mortality and may delay post-operative recovery.2,36–38 Additionally, an excessive inflammatory response may lead to SIRS and resultant multi-organ dysfunction syndrome.39,40 The hepatic acute-phase response41,42 is a physiological process believed to be focused on the restoration of homeostasis. This response is mainly modulated by inflammatory cytokines of which the most important appear to be IL-6, IL-8 and tumour necrosis factor alpha (TNF-a).4,27,43,44 This systematic review included four studies in which IL-6 was measured. IL-6 was significantly decreased on post-operative day 1 after treatment with steroids.
There may be a relationship between the use of occlusive vascular control (such as portal triad clamping), extent of resection and the acute phase response to liver resection. Although the first animal studies showing a protective effect of steroids on liver ischaemia appeared more than 30 years ago,45 the mechanism is still unclear. However, it may be related to suppression of inflammatory cytokines, increased tissue blood flow, stabilization of cell membranes and decreased lysosomal protease release.21,46–50 The level of serum bilirubin on post operative day 1 does have a prognostic value in terms of clinical outcome and in particular the occurrence of liver failure.51,52 Morbidity is related to the extent of resection as well as the ischaemic period. It may be that only a subset of high-risk patients (having a major resection or with prolonged vascular occlusion) are deriving benefit from pre-operative steroid administration. However, given the relatively small size of each of the studies in this review, there is insufficient data to resolve this question.
Although pre-operative steroids have been recommended for liver resection by some authors,53 there have been concerns about the effect this may have on post-operative liver regeneration. Liver regeneration involves a number of steps, but IL-6 and TNF-a are important initiators of the process.54–56 As we have concluded steroids decrease the levels of IL-6 and this could theoretically impair liver regeneration. However, it has been shown that overproduction of IL-6 may also inhibit liver regeneration and as such, steroid administration may have a positive effect.57 Glanemann et al.47 showed in an animal model that steroids had no effect on liver regeneration. It is also important to note that although we have demonstrated that IL-6 is suppressed by steroid administration, it is not completely abolished.
Another concern with steroid administration is the potential increased risk of infectious complications. This concern is not substantiated by the data from this meta-analysis. Steroid administration may in fact reduce the incidence of infectious complications. The mechanisms for this apparently paradoxical effect are not clear. However, there are a number of candidate mediators including immunosuppressive acidic protein (IAP) and candida antigen. IAP is a glycoprotein,58 levels of which are significantly increased by complex surgery59 and may correlate with post-operative infections.60 Similarly, candida antigen has been shown to be associated with an increase in infectious complications after a hepatectomy.61 Yamashita et al.21 showed a decrease in the levels of these two markers with steroid administration. Human leukocyte antigen (HLA)-DR expression on peripheral monocytes has been shown to correlate with an increased risk of infection after major surgery.62 Schmidt et al.24 found no evidence of an increase in HLA-DR expression, or of a decrease in cellular immunity in the group of patients treated with steroids.
In all five of the studies included in this analysis, the steroid administration regime was slightly different. However the regimes did have common features of pre-operative administration and the use of methylprednisolone. Corticosteroids are known to be less effective or indeed ineffectual if given after induction of the inflammatory response.63,64 Given that there is a delay to the onset of the anti-inflammatory effects of steroids for 1 to 2 h after administration,14 all studies gave steroids pre-operatively. Methylprednisolone was used in all of the studies as its anti-inflammatory actions are five times as strong as cortisol with less effect on electrolyte metabolism.65 The half life of methylprednisolone in the blood is 2.8 h and its biological action is prolonged for up to 36 h after administration.65 Therefore it seems reasonable that a single dose of 500 mg would be sufficient to suppress both early and late inflammatory effects.23,66 This variation may account for some of the heterogeneity observed in the meta-analysis.
There are some weaknesses in this meta-analysis. The analysis includes only RCT; however, they are of differing quality, providing variable information about potential sources of bias. Because the number of trials in the analysis is small, publication bias cannot be reliably assessed. Meta-analysis is primarily a means for addressing the issue of inadequate statistical power in studies with a small sample size.67 Given that there was a non-statistically significant trend in many outcomes between the steroid and placebo groups, the question naturally arises whether this is a result of a lack of power or could be a false-negative result owing to other causes. There was highly significant heterogeneity in the length of stay, bilirubin and PT on post-operative day one. This heterogeneity may be attributable to differences in the patients (such as underlying liver disease), intervention (dose of steroids), operations (extent of liver resection and use of vascular occlusion), outcome assessment and quality of reporting. This heterogeneity may obscure an important treatment effect.
More than half of the patients came from one study where the steroid-treated group was given larger doses and administration was continued over a number of days. From a western perspective, the patient cohort was different in that nearly half of the patients were having a resection for hepatocellular carcinoma. In most western series, the majority of patients will be having a resection for colorectal liver metastases and there will also be confounding issues related to chemotherapy-induced liver damage. In spite of these weaknesses, there does seem to be a clear benefit or trend towards a benefit in every parameter examined (except length of stay). Although the evidence is so far insufficient to justify recommending a change in routine clinical practice, there is a need for a larger multi-centre trial to further explore this strategy.
Acknowledgments
The authors wish to acknowledge the kind assistance, in providing additional data, of Dr Hayashi and Dr Takayama, Department of Digestive Surgery, Nihon University School of Medicine, Itabashi-ku, Tokyo, Japan.
Conflicts of interest
None declared.
References
- 1.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–459. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 2.Hill AG. Initiators and propagators of the metabolic response to injury. World J Surg. 2000;24:624–629. doi: 10.1007/s002689910103. [DOI] [PubMed] [Google Scholar]
- 3.Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8:1382–1389. doi: 10.1016/j.micinf.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–664. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 6.Sato N, Koeda K, Ikeda K, Kimura Y, Aoki K, Iwaya T, et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002;236:184–190. doi: 10.1097/00000658-200208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerin A, Pozar-Lukanovic N, Sojar V, Stanisavljevic D, Paver-Erzen V, Osredkar J. Balance of pro- and anti-inflammatory cytokines in liver surgery. Clin Chem Lab Med. 2003;41:899–903. doi: 10.1515/CCLM.2003.136. [DOI] [PubMed] [Google Scholar]
- 8.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 9.Badia JM, Ayton LC, Evans TJ, Carpenter AJ, Nawfal G, Kinderman H, et al. Systemic cytokine response to hepatic resections under total vascular exclusion. Eur J Surg. 1998;164:185–190. doi: 10.1080/110241598750004625. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H. Glucocorticoids for peri-operative analgesia: how far are we from general recommendations? Acta Anaesthesiol Scand. 2007;51:1133–1135. doi: 10.1111/j.1399-6576.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukami Y, Terasaki M, Okamoto Y, Sakaguchi K, Murata T, Ohkubo M, et al. Efficacy of preoperative dexamethasone in patients with laparoscopic cholecystectomy: a prospective randomized double-blind study. J Hepatobiliary Pancreat Surg. 2009;16:367–371. doi: 10.1007/s00534-009-0079-5. [DOI] [PubMed] [Google Scholar]
- 12.Feo CV, Sortini D, Ragazzi R, De Palma M, Liboni A. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006;93:295–299. doi: 10.1002/bjs.5252. [DOI] [PubMed] [Google Scholar]
- 13.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–1628. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 14.Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. 2002;195:694–712. doi: 10.1016/s1072-7515(02)01491-6. [DOI] [PubMed] [Google Scholar]
- 15.Saidi RF, Chang J, Verb S, Brooks S, Nalbantoglu I, Adsay V, et al. The effect of methylprednisolone on warm ischemia-reperfusion injury in the liver. Am J Surg. 2007;193:345–347. doi: 10.1016/j.amjsurg.2006.09.017. discussion 347–348. [DOI] [PubMed] [Google Scholar]
- 16.Santiago Delpin EA, Figueroa I, Lopez R, Vazquez J. Protective effect of steroids on liver ischemia. Am Surg. 1975;41:683–685. [PubMed] [Google Scholar]
- 17.Alegre ML, Vandenabeele P, Depierreux M, Florquin S, Deschodt-Lanckman M, Flamand V, et al. Cytokine release syndrome induced by the 145-2C11 anti-CD3 monoclonal antibody in mice: prevention by high doses of methylprednisolone. J Immunol. 1991;146:1184–1191. [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, et al. Effects of preoperative steroid administration on surgical stress in hepatic resection: prospective randomized trial. Arch Surg. 2001;136:328–333. doi: 10.1001/archsurg.136.3.328. [DOI] [PubMed] [Google Scholar]
- 22.Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg. 2003;90:17–22. doi: 10.1002/bjs.4055. [DOI] [PubMed] [Google Scholar]
- 23.Aldrighetti L, Pulitano C, Arru M, Finazzi R, Catena M, Soldini L, et al. Impact of preoperative steroids administration on ischemia-reperfusion injury and systemic responses in liver surgery: a prospective randomized study. Liver Transpl. 2006;12:941–949. doi: 10.1002/lt.20745. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt SC, Hamann S, Langrehr JM, Hoflich C, Mittler J, Jacob D, et al. Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepatobiliary Pancreat Surg. 2007;14:484–492. doi: 10.1007/s00534-006-1200-7. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama H, et al. Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg. 2011;253:50–55. doi: 10.1097/SLA.0b013e318204b6bb. [DOI] [PubMed] [Google Scholar]
- 26.Pulitano C, Aldrighetti L, Arru M, Finazzi R, Soldini L, Catena M, et al. Prospective randomized study of the benefits of preoperative corticosteroid administration on hepatic ischemia-reperfusion injury and cytokine response in patients undergoing hepatic resection. HPB. 2007;9:183–189. doi: 10.1080/13651820701216984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulitano C, Aldrighetti L, Arru M, Finazzi R, Catena M, Guzzetti E, et al. Preoperative methylprednisolone administration maintains coagulation homeostasis in patients undergoing liver resection: importance of inflammatory cytokine modulation. Shock. 2007;28:401–405. doi: 10.1097/shk.0b013e318063ed11. [DOI] [PubMed] [Google Scholar]
- 28.Turner S, Dhamarajah S, Bosomworth M, Bellamy MC. Effect of perioperative steroids on renal function after liver transplantation. Anaesthesia. 2006;61:253–259. doi: 10.1111/j.1365-2044.2006.04532.x. [DOI] [PubMed] [Google Scholar]
- 29.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB. 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasa S, Kahokehr AA, Yu TC, Hill AG. Preoperative glucocorticoid use in major abdominal surgery: systematic review and meta-analysis of randomized trials. Ann Surg. 2011;254:183–191. doi: 10.1097/SLA.0b013e3182261118. [DOI] [PubMed] [Google Scholar]
- 34.Engelman E, Maeyens C. Effect of preoperative single-dose corticosteroid administration on postoperative morbidity following esophagectomy. J Gastrointest Surg. 2010;14:788–804. doi: 10.1007/s11605-010-1168-0. [DOI] [PubMed] [Google Scholar]
- 35.Abu-Amara M, Gurusamy K, Hori S, Glantzounis G, Fuller B, Davidson BR. Systematic review of randomized controlled trials of pharmacological interventions to reduce ischaemia-reperfusion injury in elective liver resection with vascular occlusion. HPB. 2010;12:4–14. doi: 10.1111/j.1477-2574.2009.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilmore DW. Metabolic response to severe surgical illness: overview. World J Surg. 2000;24:705–711. doi: 10.1007/s002689910113. [DOI] [PubMed] [Google Scholar]
- 37.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 38.Zargar-Shoshtari K, Hill AG. Postoperative fatigue: a review. World J Surg. 2009;33:738–745. doi: 10.1007/s00268-008-9906-0. [DOI] [PubMed] [Google Scholar]
- 39.Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 40.Nystrom PO. The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother. 1998;41(Suppl. A):1–7. doi: 10.1093/jac/41.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 41.Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19:141–155. doi: 10.1055/s-2007-1007106. [DOI] [PubMed] [Google Scholar]
- 42.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 43.Biffl WL, Moore EE, Moore FA, Barnett CC, Jr, Carl VS, Peterson VN. Interleukin-6 delays neutrophil apoptosis. Arch Surg. 1996;131:24–29. doi: 10.1001/archsurg.1996.01430130026005. discussion 29–30. [DOI] [PubMed] [Google Scholar]
- 44.van der Poll T, Buller HR, ten Cate H, Wortel CH, Bauer KA, van Deventer SJ, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322:1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 45.Figueroa I, Santiago-Delpin EA. Steroid protection of the liver during experimental eschemia. Surg Gynecol Obstet. 1975;140:368–370. [PubMed] [Google Scholar]
- 46.Fujioka T, Murakami M, Niiya T, Aoki T, Murai N, Enami Y, et al. Effect of methylprednisolone on the kinetics of cytokines and liver function of regenerating liver in rats. Hepatol Res. 2001;19:60–73. doi: 10.1016/s1386-6346(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 47.Glanemann M, Strenziok R, Kuntze R, Munchow S, Dikopoulos N, Lippek F, et al. Ischemic preconditioning and methylprednisolone both equally reduce hepatic ischemia/reperfusion injury. Surgery. 2004;135:203–214. doi: 10.1016/j.surg.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Sakon M, Umeshita K, Okuyama M, Shiozaki K, Nagano H, et al. Prednisolone suppresses ischemia-reperfusion injury of the rat liver by reducing cytokine production and calpain mu activation. J Hepatol. 2001;34:278–283. doi: 10.1016/s0168-8278(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 49.Chiappa AC, Makuuchi M, Zbar AP, Biella F, Vezzoni A, Torzilli G, et al. Protective effect of methylprednisolone and of intermittent hepatic pedicle clamping during liver vascular inflow occlusion in the rat. Hepatogastroenterology. 2004;51:1439–1444. [PubMed] [Google Scholar]
- 50.Nagy P, Kiss A, Schnur J, Thorgeirsson SS. Dexamethasone inhibits the proliferation of hepatocytes and oval cells but not bile duct cells in rat liver. Hepatology. 1998;28:423–429. doi: 10.1002/hep.510280220. [DOI] [PubMed] [Google Scholar]
- 51.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 862–864. [DOI] [PubMed] [Google Scholar]
- 53.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 54.Fausto N. Liver regeneration. J Hepatol. 2000;32(1 Suppl):19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 55.Mangnall D, Bird NC, Majeed AW. The molecular physiology of liver regeneration following partial hepatectomy. Liver Int. 2003;23:124–138. doi: 10.1034/j.1600-0676.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 56.Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–312. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wustefeld T, Rakemann T, Kubicka S, Manns MP, Trautwein C. Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology. 2000;32:514–522. doi: 10.1053/jhep.2000.16604. [DOI] [PubMed] [Google Scholar]
- 58.Shibata Y, Tamura K, Ishida N. In vivo analysis of the suppressive effects of immunosuppressive acidic protein, a type of alpha 1-acid glycoprotein, in connection with its high level in tumor-bearing mice. Cancer Res. 1983;43:2889–2896. [PubMed] [Google Scholar]
- 59.Takenoshita S, Hashizume T, Asao T, Nakamura J, Tsukada K, Katoh R, et al. Influence of surgical insults for colorectal cancers on neuroendocrine and immune parameters. Oncol Rep. 1994;1:1029–1033. doi: 10.3892/or.1.5.1029. [DOI] [PubMed] [Google Scholar]
- 60.Takenoshita S, Tsukada K, Nakamura J, Shitara Y, Asao T, Kato R, et al. Immunosuppressive acidic protein (IAP) level in serum and peritoneal washings, and its implication in determining multidisciplinary treatments. Anticancer Res. 1996;16(4B):2269–2272. [PubMed] [Google Scholar]
- 61.Shirabe K, Takenaka K, Yamatomto K, Kawahara N, Itasaka H, Nishizaki T, et al. Impaired systemic immunity and frequent infection in patients with Candida antigen after hepatectomy. Hepatogastroenterology. 1997;44:199–204. [PubMed] [Google Scholar]
- 62.Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18:1022–1028. doi: 10.1007/s00464-003-9169-7. [DOI] [PubMed] [Google Scholar]
- 63.Guirao X, Lowry SF. Biologic control of injury and inflammation: much more than too little or too late. World J Surg. 1996;20:437–446. doi: 10.1007/s002689900069. [DOI] [PubMed] [Google Scholar]
- 64.Pearce D, Yamamoto KR. Mineralocorticoid and glucocorticoid receptor activities distinguished by nonreceptor factors at a composite response element. Science. 1993;259:1161–1165. doi: 10.1126/science.8382376. [DOI] [PubMed] [Google Scholar]
- 65.Stubbs SS. Corticosteroids and bioavailability. Transplant Proc. 1975;7:11–19. [PubMed] [Google Scholar]
- 66.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 67.O'Rourke K. An historical perspective on meta-analysis: dealing quantitatively with varying study results. J R Soc Med. 2007;100:579–582. doi: 10.1258/jrsm.100.12.579. [DOI] [PMC free article] [PubMed] [Google Scholar]