Abstract
Objectives
This study aimed to determine the impact of a standardized pathological protocol on resection margin status after pancreaticoduodenectomy (PD) for ductal adenocarcinoma.
Methods
A total of 150 patients operated during 2008–2010 were included in a prospective multicentre study using a ‘quality protocol’. Multicolour inking by the surgeon identified three resection margins: the portal vein–superior mesenteric vein margin (PV-SMVm) or mesenterico–portal vein groove; the superior mesenteric artery margin (SMAm), and the posterior margin. Resection margins were stratified by 0.5-mm increments (range: 0–2.0 mm). Pancreatic neck, bile duct and intestinal margins were also analysed. Correlations between histopathological factors and survival in the 0-mm resection margin group were analysed.
Results
Thirty-six patients (24%) had a PV-SMV resection (PV-SMVR). An analysis of resections categorized according to margin distances of 0 mm, <1.0 mm, <1.5 mm and <2.0 mm confirmed R1 resections in 35 (23%), 91 (61%), 94 (63%) and 107 (71%) patients, respectively. The most frequently invaded resection margin was the PV-SMVm (35% of all patients) and PV-SMVR was the only factor correlated with a higher risk for at least one 0-mm positive resection margin on multivariate analysis (P < 0.001). Two-year progression-free survival (PFS) and median PFS time in patients with R0 and R1 resections (at 0 mm), respectively, were 42.0% and 26.5%, and 19.5 months and 10.5 months, respectively (P = 0.02). A positive PV-SMVm and SMAm had significant impact on PFS, whereas a positive posterior margin had no impact.
Conclusions
Pancreaticoduodenectomy requiring PV-SMVR was associated with a higher risk for R1 resection. The standardization of histopathological analysis has a clinically relevant impact on PFS data.
Introduction
With more than 278 000 new diagnoses each year worldwide, pancreatic ductal adenocarcinoma (PDAC) accounts for 10% of all digestive system cancers.1 Some 266 000 of these newly diagnosed patients will die.1,2 The annual incidence rate approximates the mortality rate, which exceeds annual prevalence. Curative resection with negative resection margins (R0 resection) represents the only chance of cure, but the rate of such resections is remarkably low as a result of the lack of early specific biological markers, non-specific symptoms at presentation, delayed diagnosis and early metastasis.2–4 The prognosis remains extremely poor, with 5-year survival rates of <5% in the EU and USA.2–4 As a generally accepted oncological principle after the resection of a solid tumour, microscopic resection margin involvement (R1) has been reported as an independent predictor of poor longterm survival following pancreaticoduodenectomy (PD) for PDAC in several studies,5–21 but not in others.22,23 Underreporting of microscopic margin involvement may cause some discrepancy between margin status and clinical outcome, and hence the clinical significance of R1 resection remains unclear.24,25 There has recently been increased interest in resection margin involvement in PDAC, as well as in its prognostic and therapeutic implications. The standardization of pathological examination increased the rate of R1 resections after PD from 20% to 50%10,12 and to >70% when an intensified histopathological workup was applied.20,26–34
Adjuvant chemotherapy after PD for PDAC is presently the standard of care in the EU.6,35 Significant progress has been made in preoperative imaging, and major improvements have been achieved surgically in terms of postoperative morbidity and mortality.36 However, assessment of the quality of histopathological reporting on margin status, as well as the quality of the resection, should represent a crucial step towards patient stratification. The results of randomized multicentre trials evaluating adjuvant therapy for PDAC should be interpreted according to those settings.37 In the present study, a surgical quality protocol and a standardized pathological workup were applied prospectively to 150 consecutive pancreatic head resections for PDAC performed in French tertiary referral centres.
Materials and methods
Patient series
A prospective French multicentre study approved by the National Cancer Institute (INCA) commenced in August 2008 (ClinicalTrials.gov identifier: NCT00918853). Enrolment closed in May 2010. The collection of data for primary outcome measures was completed in May 2012. A total of 214 patients with periampullary tumours provided informed consent to their inclusion in the study before laparotomy, and 150 patients (70%) with true macroscopically margin-free PDAC underwent PD and entered the present study. Exclusion criteria applied after surgery included findings of macroscopic residual tumour in the operative field (R2 resection), non-adherence to the surgical protocol, non-ductal adenocarcinoma, and margin analysis performed without using the predefined criteria. Sixty-four (30%) patients with distal bile duct cancers, ampullary tumours, neuroendocrine tumours, non-invasive intraductal papillary mucinous neoplasm (IPMN) and periampullary tumours of various aetiologies were excluded after histopathological review. Any doubtful diagnosis led to the exclusion of that patient from the study.
Standardized PD by ‘quality protocol’
Circumferential dissection of the portal vein–superior mesenteric vein (PV-SMV) axis and dissection of the right hemi-circumference of the superior mesenteric artery (SMA) to the right of the coeliac trunk were required to obtain a good medial clearance (Fig. 1). The dissection of the SMA removed all soft tissue to the right of the adventitia, which corresponded to the SMA margin (SMAm) on the specimen.22,38–40 This technique obviated the need for intraoperative frozen-section analysis of the SMAm. A standard lymphadenectomy plus resection of lymph nodes to the right of the coeliac trunk, hepatic artery and hepatoduodenal ligament were carried out in all patients.41 The pancreatic neck transection margin and the common bile/hepatic duct transection margins were evaluated using frozen-section analysis and, if results were positive, additional tissue was resected to achieve negative margins at these two sites. En bloc resection of the SMV, PV or PV-SMV confluence was performed in 36 patients (PV-SMVR: 24%); PV-SMVR was planned in half of these patients and performed in the other half in response to intraoperative suspicion of venous wall invasion. Four patients were subjected to combined arterial resection as a result of the abutment or encasement of a short segment of the hepatic artery. Three patients underwent en bloc right colectomy for invasion of the mesocolon.
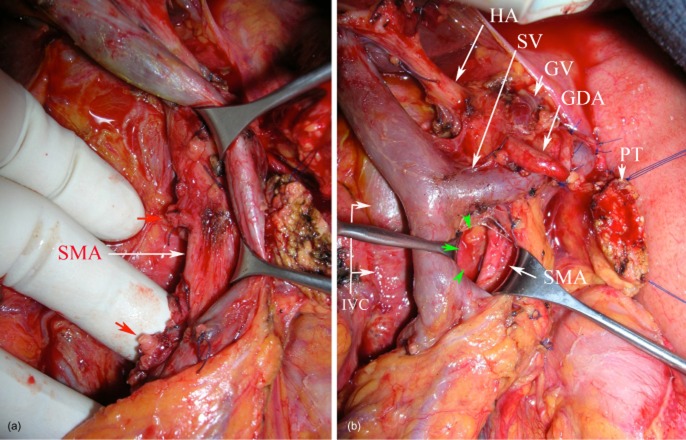
Figure 1.
Standardized pancreaticoduodenectomy. Operative view showing: (a) the dissection of the right hemi-circumference of the superior mesenteric artery (SMA) with elective division of the pancreaticoduodenal arteries (red arrows); (b) clearance of the medial margin (green arrows) with no residual soft tissue to the right of the SMA. Note the circumferential dissection of the mesenterico–portal venous axis. HA, hepatic artery; SV, splenic vein; GV, gastric vein; GDA, stump of the gastroduodenal artery; PT, pancreatic transection; IVC, inferior vena cava
The surgeon clearly identified the margins in the operative room with multicolour coded inking of: (i) the mesenterico–portal vein groove or PV-SMV margin (PV-SMVm); (ii) the SMA margin (SMAm), and (iii) the posterior margin (Fig. 2). In cases of PV-SMVR, the venous segment was clearly identified on the specimen (Fig. 3). The anterior surface was not considered as a transection margin and was not inked.
Figure 2.
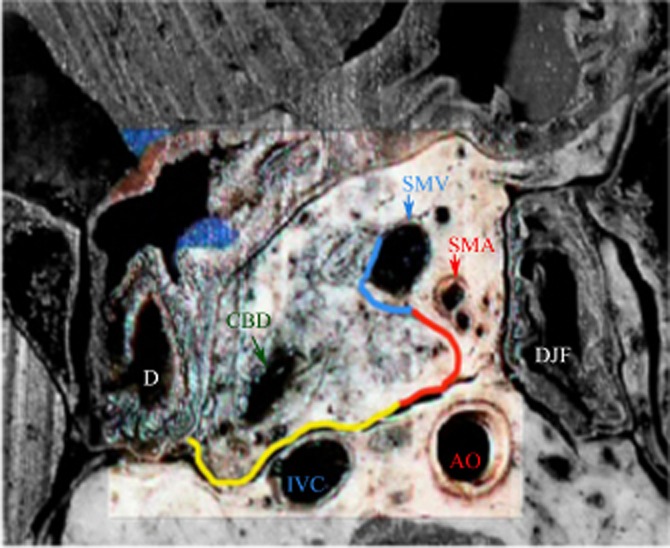
Cross-section of the pancreatic head showing the inked margins: the mesenterico–portal vein groove (medial circumferential resection margin) in blue, the superior mesenteric artery (SMA) margin (uncinate margin) in red and the posterior margin in yellow. D, duodenum; DJF, duodenojejunal flexure; CBD, common bile duct; AO, aorta; IVC, inferior vena cava; SMV, superior mesenteric vein
Figure 3.
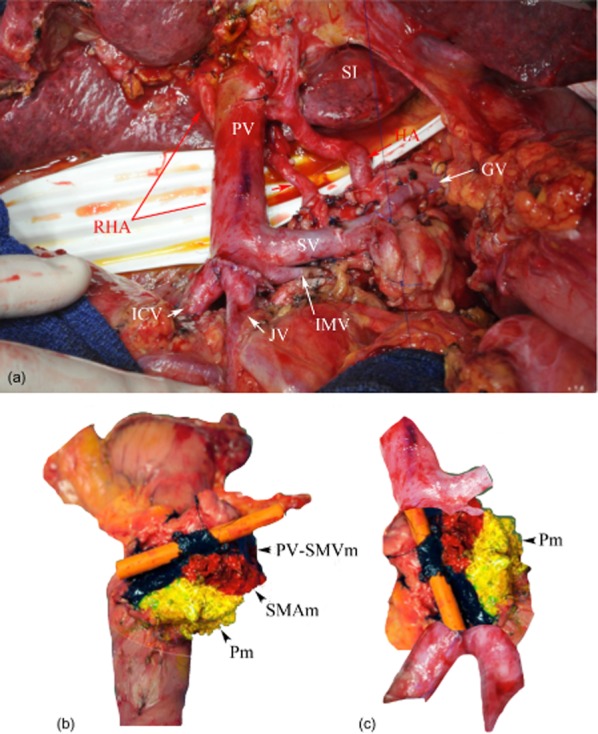
Pancreaticoduodenectomy with venous resection. (a) Operative view of venous confluence reconstruction. (b) A simple trick to identify the vein for the pathologist: a small drain is gently inserted into the venous lumen; the vein is inked to show the portal vein groove (PV-SMVm). (c) Photographic reconstruction of the venous axis with a medial and posterior view of the specimen. RHA, right hepatic artery; PV, portal vein; SI, liver segment I; GV, gastric vein; ICV, ileo-colic vein; JV, jejunal vein; SV, splenic vein; IMV, inferior mesenteric vein; SMAm, superior mesenteric artery margin; Pm, posterior margin
Standardization of the protocol for pathological examination
Serial slicing of the entire pancreatic head specimen was performed in a single axial plane (i.e. perpendicular to the longitudinal axis of the duodenum) according to the guidelines of the Royal College of Pathologists and the Leeds Pathology Protocol.26,27,29,40,42 Thus, large slices were obtained (median number: 12), allowing a precise study of each inked margin in increments of 0.5 mm from 0 mm to 2.0 mm. Margin involvement (R1) was defined for the 0-mm margin if tumour cells were present at the inked margin; R1 was also defined for each margin width if tumour cells were present within the margin, independently of the mode of tumour spread. The ‘vascular’ margin was defined as the PV-SMVm plus SMAm inked margins. Patients undergoing PV-SMVR for local invasion were considered to have a positive PV-SMVm if tumour was present at the resection margin, not simply intraluminally. The resection was considered as curative (R0) if no tumour cells were identified at any of the resection margins (including bile duct and pancreatic neck non-inked transection margins), again for each margin width (see Appendix). The pathological protocol also included the maximal transverse diameter of the tumour, the tumour–node–metastasis (TNM) classification, the grade of differentiation, the presence or absence of perineural, lymphatic and/or vascular spread, and the number of lymph nodes retrieved from the specimen, enabling the calculation of the lymph node ratio (LNR). The presence and grading of pre-neoplastic lesions were also recorded. In PV-SMVR, the length of the resection was recorded and the margins of the vessel segment were examined; if the vein was invaded, the depth of invasion was specified (invasion of adventitia, media or intima). Pathological data for PD specimens with PV-SMVR were compared with data for PD specimens without PV-SMVR.
Power and sample size considerations
The primary objective of this prospective multicentre study was to confirm the negative impact on longterm clinical outcome of resection margin involvement (R1 or R0) in patients with PDAC who underwent PD according to a standardized protocol. As defined in the protocol, the main evaluation criterion was overall survival (OS): a minimum of 87 deaths were required to reach 90% power using a two-sided 0.05-level log-rank test to detect a significant difference in hazard ratio (HR) between R1 and R0 status, assuming a true HR difference (HR = 2.0). In response to an unexpectedly low 2-year death rate, the initial sample size (n = 156) was increased. A total of 214 patients were recruited prior to laparotomy, of whom 150 with proven macroscopically margin-free PDAC entered this prospective 2-year follow-up study period. The first patient was accrued in August 2008 and the last in May 2010.
Follow-up
According to the protocol, patients were scheduled to be followed for a 2-year fixed period. Postoperative mortality was defined as in-hospital mortality or mortality within 30 days of discharge (five patients). Patients were followed every 4 months after discharge and evaluated using computed tomography (CT) and carbohydrate antigen 19-9 (CA 19-9) levels, or reviewed for any abnormal symptom. If an isolated elevation of CA 19-9 occurred [i.e. without documented CT or positron emission tomography (PET)-CT recurrence], the patient was followed every 2 months. Recurrences were defined as locoregional or metastatic (including peritoneal carcinomatosis) events or both.
As of May 2012, when data collection was completed, all demographic clinical and histological characteristics were monitored and centrally reviewed; however, longterm clinical outcome data collection remains ongoing or under resolution. According to these preliminary longterm data, 66 patients died and 71 (47%) demonstrated disease progression. To preserve the power of this prospective study, the time to progression or death was defined as the primary evaluation criterion in a revised statistical analysis plan issued in blind conditions.
Statistical analysis
Descriptive statistics were used to summarize the clinical and histological characteristics of the full cohort of patients who entered the prospective follow-up study period (n = 150). Two groups of patients were first considered: patients who underwent standard PD (n = 114), and patients who underwent PD with PV-SMVR (n = 36). Differences between the groups in patient characteristics were assessed using the chi-squared test for categorical variables and the Wilcoxon rank sum test for continuous variables. The main objective of this study was to evaluate microscopic involvement on at least one of the inked resection margins and to identify factors correlated with resection margin involvement. A selection of factors that may influence microscopic involvement were established prior to data analysis and then categorized using predefined cut-offs [age, sex, body mass index (BMI), Karnowsky index, conventional pathological factors, venous resection, preoperative treatments]. Factors correlated with positive margins at each increment were identified using the non-parametric Fisher's (exact) chi-squared test. All factors found to be significant at the 0.05 level or to be of borderline significance at the 0.15 level in univariate analysis were entered into a multivariate logistic regression model to identify independent factors correlated with microscopic involvement at each margin increment. Similar univariate and multivariate analyses were carried out for each category of margin involvement defined according to the presence of tumour cells at the inked margin and within resection margins in 0.5-mm increments. Preliminary longterm clinical outcome data were used to measure the prognostic impact of microscopic involvement on at least one inked margin. Median survival, OS and progression-free survival (PFS) were calculated for the 0-mm margin. Thus, the following results are preliminary data because the positive margin is defined by the presence of tumour cells at 0 mm.38,39 Patients alive without disease progression and patients alive at the end of follow-up were considered as censored on the right at the date of last contact. Time to progression or death (PFS) and time to death (OS) defined from the date of PD to the date of the event analysed were summarized using the Kaplan–Meier method and comparisons were made using the log-rank test. All statistical analyses were carried out using sas Version 9.3 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was accepted at the 5% level with no adjustment for multiple testing.
Results
Demographics are shown in Table 1. The study group included 95 men and 55 women, with a median age of 63 years (range: 41–84 years). Twenty-two (15%) patients had IPMN with invasive cancer. Twenty-nine (19%) patients received neoadjuvant treatment (FOLFIRINOX: n = 12; gemcitabine or 5-fluorouracil and platinum-based chemoradiation: n = 17), mostly for suspicion of involvement of more than half of the circumference of the venous axis on the portal phase of the preoperative CT. Nine of the 36 PV-SMVRs and all four arterial resections were performed after neoadjuvant treatment.
Table 1.
Comparison of clinicopathological data for the entire cohort and in the standard pancreaticoduodenectomy (PD) group and the PD with portal vein–superior mesenteric vein resection (PV-SMVR) group
| All patients (n = 150) | Standard PD (n = 114) | PD with PV-SMVR (n = 36) | P-value (χ2 or Wilcoxon test) | |
|---|---|---|---|---|
| Age, years, median (range) | 63 (41–84) | 64 (41–84) | 62 (41–78) | 0.135 |
| Male, n (%) | 95 (63%) | 72 (63%) | 23 (64%) | 0.909 |
| Female, n (%) | 55 (37%) | 42 (37%) | 13 (36%) | |
| pT3, n (%) | 111 (74%) | 80 (70%) | 31 (86%) | 0.057 |
| pN1, n (%) | 108 (72%) | 78 (68%) | 30 (83%) | 0.417 |
| Grade 3, n (%) | 24 (19%) | 14 (15%) | 10 (28%) | 0.037 |
| Tumour size, cm, median (range) | 3.5 (0.21–12) | 3 (0.21–9) | 4 (1.5–12) | <0.001 |
| LN retrieved, n, median (range) | 23 (3–80) | 19 (3–55) | 37 (7–80) | <0.001 |
| Disease-positive LN, n, median (range) | 3 (0–50) | 2 (0–16) | 3 (0–50) | 0.211 |
| LNR, median (range) | 0.11 (0–0.75) 42 (29%) | 0.12 (0–0.75) 34 (32%) | 0.08 (0–0.72) 8 (22%) | 0.552 0.785 |
| Neoadjuvant treatment, n (%) | 29 (19%) | 20 (18%) | 9 (25%) | 0.323 |
| Positive margin status for the 0-mm margin | ||||
| At least one positive margin, n (%) | 35 (23%) | 17 (15%) | 18 (50%) | <0.001 |
| Pm, n (%) | 11 (7%) | 4 (4%) | 7 (19%) | 0.009 |
| PV-SMVm, n (%) | 27 (18%) | 12 (11%) | 15 (42%) | <.001 |
| SMAm, n (%) | 15 (10%) | 6 (5%) | 9 (25%) | <.001 |
| Vascular margina, n (%) | 34 (23%) | 16 (14%) | 18 (50%) | <.001 |
| Positive margin status for the 1.0-mm margin | ||||
| At least one positive margin, n (%) | 91 (61%) | 63 (55%) | 28 (78%) | 0.016 |
| Pm, n (%) | 52 (37%) | 35 (31%) | 17 (47%) | 0.069 |
| PV-SMVm, n (%) | 61 (41%) | 39 (34%) | 22 (61%) | 0.004 |
| SMAm, n (%) | 37 (25%) | 24 (21%) | 13 (36%) | 0.067 |
| Vascular margina, n (%) | 73 (49%) | 48 (42%) | 25 (69%) | 0.004 |
| Positive margin status for the 1.5-mm margin | ||||
| At least one positive margin, n (%) | 94 (63%) | 66 (58%) | 28 (89%) | 0.031 |
| Pm, n (%) | 40 (27%) | 21 (18%) | 19 (53%) | 0.131 |
| PV-SMVm, n (%) | 62 (41%) | 40 (35%) | 22 (61%) | 0.006 |
| SMAm, n (%) | 40 (27%) | 26 (23%) | 14 (39%) | 0.057 |
| Vascular margina, n (%) | 74 (49%) | 49 (43%) | 25 (69%) | 0.006 |
| Positive margin status for the 2.0-mm margin | ||||
| At least one positive margin, n (%) | 107 (71%) | 76 (67%) | 31 (86%) | 0.025 |
| Pm, n (%) | 54 (36%) | 34 (30%) | 20 (56%) | 0.187 |
| PV-SMVm, n (% | 78 (52%) | 54 (47%) | 24 (67%) | 0.043 |
| SMAm, n (%) | 54 (36%) | 36 (32%) | 18 (50%) | 0.045 |
| Vascular margina, n (%) | 87 (58%) | 59 (52%) | 28 (78%) | 0.006 |
Vascular margin: SMAm + PV-SMVm.
LN, lymph node; LNR, lymph node ratio; Pm, posterior margin; PV-SMVm, portal vein–superior mesenteric vein margin; SMAm, superior mesenteric artery margin (uncinate margin).
Clinicopathological data for the entire cohort are reported in Table 1. A total of 78% of the tumours were classified as T3 tumours (T1: 8%; T2: 9%; T4: 5%). More than half of the tumours were moderately differentiated (grade 1: 30%; grade 2: 52%; grade 3: 19%). Significantly more poor prognostic factors were observed in the PD with PV-SMVR group (Table 1).
Margin status
The mean and median distances separating tumour cells from each inked margin are reported in Table 2. Microscopic involvement was present on at least one of the inked margins in 35 patients (23%) at the 0-mm margin and in 91 (61%), 94 (63%) and 107 (71%) patients at the 1.0-mm, 1.5-mm and 2.0-mm margins, respectively (Table 1). The number of patients in whom two or three margins were involved increased from 14 for the 0-mm margin (14/35, 40%) to 40 (40/91, 44%), 43 (43/94, 46%) and 66 (66/107, 62%) for the 1.0-mm, 1.5-mm and 2.0-mm margins, respectively (Table 3).
Table 2.
Mean and median clearances observed for each inked margin. In some patients the margin status was specified as positive or negative for each of the increments, but the precise distance between the margin and the tumor cells was not clearly specified
| Inked margins (number of patients with a specified distance in mm) | Mean clearance (mm ± SD) | Median clearance (mm; range) |
|---|---|---|
| Pm (129/150) | 5.6 ( 7) | 3.00 [0.10–35.00] |
| PVSMVm (115/150) | 6.8 ( 6.9) | 3.00 [0.10–20.00] |
| SMAm (123/150) | 9.0 ( 9.6) | 5.00 [0.20–30.00] |
| «Vascular» margin (PVSMVm + SMAm; 106/150) | 5.8 ( 6.6) | 2.25 [0.10–20.00] |
Table 3.
R1 resection rate for each margin increment (0–2.0 mm) and proportions of patients with at least one, two or three positive margins, respectively. R1 resection rates were comparable across the 1.0-mm and 1.5-mm margin widths
| Margin widths, mm | R1 resections, n/n (%) | One positive margin, n/n (%) | Two positive margins, n/n (%) | Three positive margins, n/n (%) |
|---|---|---|---|---|
| 0 | 35/150 (23%) | 21/35 (60%) | 10/35 (29%) | 4/35 (11%) |
| 1.0 | 91/150 (61%) | 51/91 (56%) | 21/91 (23%) | 19/91 (21%) |
| 1.5 | 94/150 (63%) | 51/94 (54%) | 23/94 (24%) | 20/94 (21%) |
| 2.0 | 107/150 (71%) | 41/107 (38%) | 38/107 (36%) | 28/107 (26%) |
The rates of positive inked margins within each increment are reported in Table 1. More invaded margins were observed in the PV-SMVR group (Table 1). The most frequently invaded margin was the PV-SMVm.
Invasion of the ‘vascular’ margin (PV-SMVm plus SMAm) was present in 23% of specimens at the 0-mm margin and in close to 50% at the 1.0-mm and 1.5-mm margins. Despite the intraoperative frozen-section analysis, 1% (n = 2) of bile duct transections and 5% (n = 8) of pancreatic neck transections were found to be invaded on final examination.
In summary, if R1 resection is defined by a positive margin of 0 mm,38,39,43,44 23% of the present patients achieved R1 resection. If R1 resection is defined by the presence of tumour cells within 1.0 mm40,42,45 or 1.5 mm,46 61% and 63%, of the present patients achieved R1 resection. Bile duct and pancreatic neck transection invasion resulted in an additional 7% of R1 resections for any definition of R1 on the inked margins. Thus, the rate of R1 resections was 30% when R1 was defined according to the 0-mm rule and 68% when R1 was defined according to the 1.00-mm rule (ratio: 2.3).
Univariate and multivariate analyses of factors associated with the risk for a positive inked margin at each increment are shown in Tables 4 and 5.
Table 4.
Univariate analysis of factors associated with at least one positive margin for each of the margins at each width from 0 mm to 2.0 mm. Three non-pathological factors were significantly associated with a positive margin: male sex; portal vein–superior mesenteric vein resection (PV-SMVR), and neoadjuvant treatment. Values in bold refer to factors that reached significance at a P-value of <0.05; values in italics refer to factors that did not reach significance at a P-value of <0.05, but tended towards significance at a P-value of <0.10
| Margin widths, mm | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| Margins | Variables | P-value Odds ratio (95% confidence interval) | ||||
| At least one positive inked margin | Tumour size | 0.018 3.5 (1.2–9.7) | – | 0.005 2.8 (1.4–6) | 0.005 3 (1.4–6.3) | 0.051 2.2 (1–5) |
| T stage | 0.029 9.7 (1.2–74) | – | 0.003 4 (1.6–10) | 0.018 2.94 (1.2–7) | 0.007 3.6 (1.4–9) | |
| N stage | 0.018 4.5 (1.3–16) | 0.009 3.2 (1.3–7.7) | 0.004 3.2 (1.4–7) | 0.005 3 (1.4–7) | – | |
| LNR>0.2 | – | 0.017 2.5 (1.2–5.2) | 0.040 2.4 (1–5.6) | – | – | |
| PV-SMVR | <0.001 5 (2.2–12) | 0.012 2.7 (1.2–6) | – | – | – | |
| Posterior margin | N-stage | – | – | 0.012 3.45 (1.3–9) | 0.043 2.49 (1–6) | 0.035 2.42 (1–5.5) |
| LNR >0.2 | 0.075 3.1 (0.9–11) | 0.029 2.5 (1.1–5.6) | 0.005 2.9 (1.4–6) | 0.015 2.5 (1.2–5.2) | 0.022 2.4 (1.1–5) | |
| PV-SMVR | 0.007 6.0 (1.7–22) | – | – | – | – | |
| Neoadjuvant treatment | – | – | 0.045 0.4 (0.1–1) | – | 0.077 0.464 (0.2–1.1) | |
| PV-SMVm | Tumour size | 0.053 3.1 (1.0–9.6) | – | 0.001 3.7 (1.6–8.4) | 0.001 3.9 (1.7–8.8) | 0.002 3.2 (1.5–6.7) |
| T-stage | – | 0.049 4.5 (1.0–20) | 0.007 4.8 (1.5–15) | 0.016 3.6 (1.3–10) | – | |
| N-stage | – | 0.042 3.2 (1.0–9.8) | 0.032 2.5 (1.1–5.9) | 0.026 2.6 (1.1–6) | – | |
| PV-SMVR | <0.001 5.6 (2.3–14) | 0.011 2.9 (1.3–6.5) | 0.012 2.7 (1.2–5.8) | 0.016 2.6 (1.2–5.6) | – | |
| SMAm | Tumour size | – | – | 0.023 3.0 (1.16–8.06) | 0.009 3.6 (1.4–9.3) | <0.001 5.4 (2.2–13) |
| N-stage | – | – | – | 0.038 2.99 (1.06–8.39) | – | |
| LNR >0.2 | 0.010 4.3 (1.4–13) | 0.014 3.6 (1.3–9.9) | 0.004 3.2 (1.4–7) | 0.013 2.7 (1.2–5.8) | – | |
| Grade 3 | 0.050 3.6 (0.8–16) | 0.002 4.39 (1–19) | 0.014 4.83 (1.5–16) | 0.008 5.7 (1.74–18.8) | 0.002 8.00 (2.44–26.2) | |
| PV-SMVR | 0.002 5.8 (1.9–18) | 0.011 3.8 (1.4–11) | – | – | – | |
| Male versus female | – | 0.024 0.2 (0.04–0.8) | 0.054 0.4 (0.2–1.0) | – | – | |
| Vascular margin PV-SMVm + SMAm | Tumour size | 0.009 4.4 (1.4–14) | 0.027 2.7 (1.1–6.4) | <0.001 4.47 (2.0–9.8) | <0.001 4.7 (2.1–10) | <0.001 4.2 (1.9–8.9) |
| T-stage | 0.033 9.2 (1.2–71) | 0.042 3.7 (1.0–13) | 0.002 5.3 (1.8–15) | 0.005 4.2 (1.5–11) | 0.009 3.4 (1.3–8.4) | |
| N-stage | 0.022 4.3 (1.2–15) | 0.018 3.4 (1.2–9.6) | 0.011 2.9 (1.3–6.6) | 0.001 3.0 (1.3–6.8) | 0.043 2.2 (1.0–4.9) | |
| LNR >0.2 | 0.016 2.56 (1.2–5.5) | 0.015 2.6 (1.2–5.6) | 0.020 2.5 (1.1–5.3) | 0.043 2.4 (1.0–5.4) | ||
| Grade 3 | 0.049 4.0 (1.3–12) | 0.067 3.6 (1.1–11) | ||||
| PV-SMVR | <0.001 5.5 (2.4–13) | 0.006 3.0 (1.4–6.6) | 0.018 2.6 (1.2–6) | 0.023 2.5 (1.1–5.7) | 0.028 2.7 (1.1–6.4) | |
| Male versus female | – | – | 0.022 0.45 (0.22–0.88) | 0.037 0.48 (0.24–0.95) | – | |
LNR, lymph node ratio; PV-SMVm, portal vein–superior mesenteric vein margin; SMAm, superior mesenteric artery margin (uncinate margin).
Table 5.
Multivariate analysis of factors associated with at least one positive margin for each of the margins at each width from 0 mm to 2.0 mm. Values in bold refer to factors that reached significance at a P-value of <0.05; values in italics refer to factors that did not reach significance at a P-value of <0.05, but tended towards significance at a P-value of <0.10
| Margin widths, mm | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 mm | ||
| Margins | Variables | P-value Odds ratio (95% confidence interval) | ||||
| At least one positive inked margin | Tumour stage | – | – | 0.073 2.5 (1.0–6.9) | – | 0.039 2.8 (1.0–7.3) |
| PV-SMVR | <0.001 4.7 (2.0–12) | 0.016 2.8 (1.2–6.4) | – | – | – | |
| Posterior margin | LNR >0.2 | 0.036 4.4 (1.10–17.7) | 0.053 2.2 (0.989–5.13) | 0.059 2.2 (0.968–5.12) | – | – |
| PV-SMVR | 0.005 7.5 (1.8–31) | – | – | – | – | |
| PV-SMVm | Tumour size | – | – | – | 0.066 2.2 (1.0–5.0) | 0.055 2.1 (1.0–4.4) |
| PV-SMVR | 0.001 4.5 (1.8–11) | 0.035 2.5 (1.0–5.7) | – | – | – | |
| SMAm | Tumour size | – | – | – | – | 0.020 3.0 (1.2–8.0) |
| Grade 3 | – | – | 0.049 4.05 (1.1–14) | 0.046 4.39 (1.2–16) | 0.032 5.5 (1.5–20) | |
| LNR >0.2 | 0.019 5.8 (1.3–25) | – | – | – | – | |
| PV-SMVR | 0.009 6.8 (1.6–28) | 0.047 3.9 (1.0–15) | – | – | – | |
| Vascular margin PV-SMVm + SMAm | Tumour size | – | – | 0.065 2.2 (0.97–5.1) | 0.034 2.5 (1.0–5.7) | 0.017 2.6 (1.2–5.6) |
| LNR >0.2 | – | 0.065 2.3 (0.95–5.5) | – | – | 0.099 2.17 (0.86–5.47) | |
| PV-SMVR | <0.001 5.2 (2.0–13) | 0.012 3.06 (1.3–7.4) | – | – | 0.080 2.31 (0.91–5.88) | |
aVascular margin: SMAm + PV-SMVm.
PV-SMVR, portal vein–superior mesenteric vein resection; LNR, lymph node ratio; PV-SMVm, portal vein–superior mesenteric vein margin; SMAm, superior mesenteric artery margin (uncinate margin).
Clinical impact of resection margin status
The median follow-up was 24.1 months (range: 23.7−24.8 months). Six (4%) patients were lost to follow-up (median follow-up: 7.5 months; range: 1.4−16.1 months). During the follow-up period, 66 (44%) patients died and disease progression was observed in 71 (47%) patients; five (3%) patients died from perioperative causes. A total of 54 (37%) patients developed one or more metastases, with (n = 15, 10%) or without (n = 39, 27%) local recurrence. In 16 (11%) patients, local recurrence was isolated. Eight patients with isolated local recurrence had R0 resections.
Median OS in the 150 patients was 20 months [95% confidence interval (CI) 16.4–22.6]. Patients with at least one positive inked margin had median survival of 17.7 months (11.7–36.4), whereas patients with R0 resections had a median survival of 32.9 months [P = 0.10; 95% CI 22.7 – not reached (NR)]. Two-year OS rates in patients with R0 and R1 resections were, respectively, 59.3% (95% CI 48–69) and 44.6% (95% CI 27–61) (P = 0.106; HR = 0.64, 95% CI 0.37–1.11) (Fig. 4). Median and 2-year rates of PFS by margin status are shown in Fig. 5 and Table 6. The relationship between margin status at each increment and survival is yet to be determined.
Figure 4.
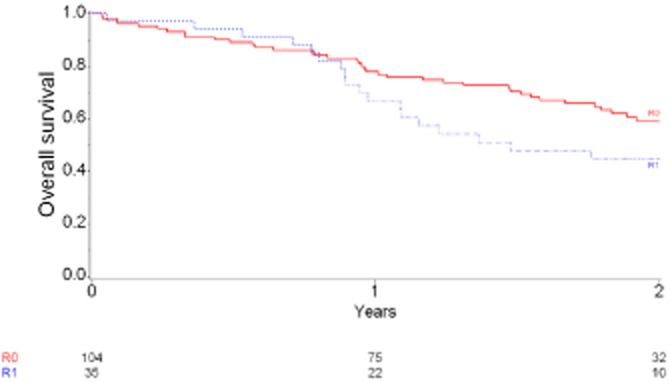
Overall survival in 139 patients for whom data were available at the time of the study. Hazard ratio: 0.64, 95% confidence interval 0.37–1.11; P = 0.106
Figure 5.
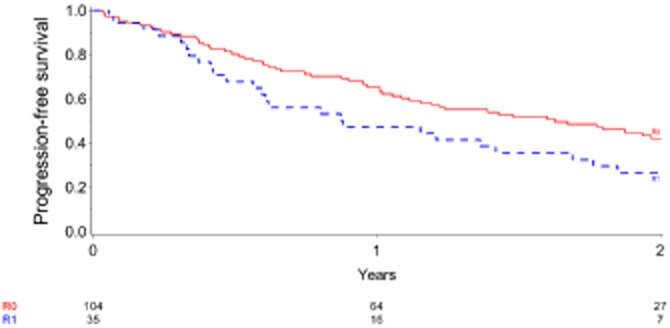
Progression-free survival in 139 patients for whom data were available at the time of the study. Cases of postoperative mortality were excluded (n = 5). Numbers of events were, respectively, 57 in the R0 group and 27 in the R1 group. Patients in whom an R0 resection was achieved had a significantly better outcome than those in whom an R1 resection was performed (at least one positive margin according to the 0-mm definition). Hazard ratio: 0.59, 95% confidence interval 0.37–0.94; P = 0.023
Table 6.
Median length and rate of 2-year progression free-survival for each 0-mm positive margin
| Margin status (n assessed) | Months, median (95% CI) | 2-year, % (95% CI) | P-value (log-rank test) | |
|---|---|---|---|---|
| At least one positive margin | R0 (n = 104) | 19.5 (13.0–NR) | 42% (32–52) | |
| R1 (n = 35) | 10.5 (5.6–20.3) | 27% (13–42) | 0.023 | |
| PV-SMVm | R0 (n = 114) | 17.0 (12.7–23.6) | 40% (30–49) | |
| R1 (n = 27) | 10.5 (5.0–21.0) | 27% (12–45) | 0.051 | |
| SMAm | R0 (n = 122) | 19.5 (13.8–24.0) | 42% (33–51) | |
| R1 (n = 15) | 9.6 (3.7–17.0) | 13% (2–35) | 0.017 | |
| PV-SMVm + SMAm (vascular margin) | R0 (n = 105) | 19.5 (13.4–24.5) | 41% (31–51) | |
| R1 (n = 34) | 10.5 (5.6–20.3) | 27% (14–43) | 0.026 | |
| Posterior margin | R0 (n = 128) | 16.7 (12.2–22.5) | 39% (30–47) | |
| R1 (n = 11) | 16.4 (0.9–21.2) | 20% (3–48) | 0.281 | |
95% CI, 95% confidence interval; NR, not reached; PV-SMVm, portal vein–superior mesenteric vein margin; SMAm, superior mesenteric artery margin (uncinate margin).
Discussion
This study is the first prospective multicentre study of resection margins in PDAC specimens to use a surgical quality protocol and a standardized pathological workup. Despite recommendations published since 1996,47–51 there is no consensus today on PD specimen handling and the assessment of resection margins. There is also a lack of consensus regarding the definition of microscopic margin involvement. However, this topic has recently attracted increasing interest.20,21,26–34,46
There is marked heterogeneity in published rates of R1 resection. Most series have reported rates well below 20–40%, by contrast with those that report rates of >70% in series using a standardized protocol for the pathological examination of PD specimens.24,28–34 These discrepancies are mainly caused by differences in pathological assessment rather than in surgical procedure and patient selection,26–28,30–34 although outcomes in low-volume centres negatively affect the rate of positive resection margins.52 The Leeds24 and Heidelberg28 groups were the first to demonstrate that the standardization of histopathological study resulted in a significant increase in R1 resection rates, without requiring any change in surgical technique (respectively, from 53% to 85%24 and 14% to 76%28). Thus, a high rate of R1 resection in PDAC is clearly a marker of high-quality pathology and depends firstly on the number of peri-pancreatic soft tissue resection margins examined, secondly on the number of blocks analysed,27,42 and thirdly on the minimum clearance in millimetres used to define microscopic margin involvement (R1).
In a study published recently by Campbell et al., tumour involvement within 1.0 mm of, but not directly reaching, one or more resection margins represented 45% of the 79% of resection margins identified as positive.30 In the most recent series, comparisons of R1 rates achieved using the Union for International Cancer Control (UICC) criteria (R1: 0 mm definition), which are commonly used in North America,22,38,39,43,44 and those achieved using the UK Royal College of Pathologists (RCPath) criteria (R1: 1.0 mm definition)42 show ratios ranging from 1.3 to 1.8.28,30–32,34 The ratio was 2.3 in the present study. Katz et al. reported a ratio of 5.5 (4–22%) in a study in which only the SMAm was assessed and in which 76% of patients had received preoperative radiochemotherapy; this study also showed that preoperative CT overestimated the SMAm in 73% of patients.53 Hartwig et al. reported a maximum ratio of 8.4 in a study comparing the 0 mm definition to the revised ‘R1 = 1.0 mm’ definition (4.8–40.5%).15
The confusing terminology used to define resection margins, which is sometimes ambiguous, makes it difficult to compare rates of microscopic invasion for each of the margins27 (see Appendix). The assessment of resection margins is often limited to those of the pancreatic neck and bile duct transections and the SMAm or medial margin.12,22,37,47–49 However, the medial circumferential resection margin (CRM),26 which faces the superior mesenteric vessels, and the posterior soft tissue margins were the most frequently involved.8,12,18,20–22,26–34 In the present study, assessments of the SMAm and SMVm were clearly distinct,8,40 and these medial resection margins, which represent true transection resection margins,34 were distinguished from the posterior margin. For the 0-mm and 1.0-mm margin definitions, the SMVm was the most commonly involved (18% and 41%, respectively), as in the study by Pingpank et al.,8 which contrasts with the particularly low rates of 3–10% reported by others.21,46 For the 0-mm and 1.0-mm margin definitions, the SMAm was invaded in 10% and 25% of specimens, respectively, and the vascular resection margin was invaded in 23% and 49% of specimens, respectively. In the present study, no significant difference in R1 rates emerged between the 1.0-mm and 1.5-mm margins. The median and mean clearances were lower for the PV-SMVm than for the SMAm. The assessment of resection margins along both vessels gives an accurate picture of resection margin status because these areas represent the most critical part of the CRM in terms of the occurrence of microscopic residual disease. Optimization of this crucial resection margin using the artery-first approach has been advocated,54 and the issue of a systematic en bloc PV-SMVR after a first SMA approach was addressed recently by Turrini et al.55
In the present study, the anterior surface of the specimen (anterior part of the whole CRM) was not inked. Assessment of the anterior resection margin as a part of the CRM was recommended several years ago in Japan,56 and subsequently in Europe27 and more recently in North America.40,43 Invasion of the anterior surface has been reported as marking a margin relevant to the definition of ‘radicality’ in the Japanese pancreatic cancer registry.57 However, the anterior/serosal margin has not been considered as a transection margin.28,34,46 Invasion has been reported in 7–15% of patients,26–28 but isolated infiltration of the anterior surface is unusual28 and has sometimes not been considered as indicating R1 resection.30 It was therefore proposed that assessment of this margin should be excluded from a standardized pathological protocol,28 or that the 0-mm clearance rule should be used.27,29 In the current study, despite the lack of inking of the anterior surface of the specimen, nearly 70% of patients were found to have at least one positive margin according to the 1.00-mm clearance rule, which is closest to the rates reported in recent series using a standardized pathological protocol.26,28,30–32,34
In the present study, the fact that 19% of patients had received neoadjuvant treatment, including irradiation in nearly 60%, may have biased the evaluation of resection margins. Low rates of R1 resection are usually observed after neoadjuvant radiochemotherapy, especially for the SMAm8,22,58,59 and SMVm.8 However, neoadjuvant therapy has been reported as a non-significant predictor of SMAm status.22,58 In the present study, neoadjuvant treatment was correlated with a reduced risk for a positive posterior margin at only the 1.0-mm definition in univariate analysis.
What constitutes an adequate margin in PDAC resection specimens was recently discussed by Verbeke et al.60 Indeed, the 1.0-mm margin rule42 has been extrapolated from the circumferential margin recognized as discriminant of recurrence in rectal cancer, but has never been validated for PDAC. Its relevance is questionable because of the infiltrative, dispersed and discontinuous growth pattern of PDAC.24,60 Chang et al. reported that a minimum clearance of 1.5 mm was an independent predictor of survival in multivariate analysis.46 A revision of the current 0-mm and 1.0-mm definitions of R1 resection should be considered for PDAC, as for rectal cancer.32,60,61
Curative resection (R0) is one of the key factors influencing survival after PD for PDAC.3,5–7,9,15 However, as in the present study, positive margins are often correlated with other strong pathologically prognostic factors that may be potential confounding variables in an assessment of the correlation of a positive resection margin with local recurrence and survival.
Numerous published multivariate analyses have emphasized the prognostic value of tumour size,7,10,22,34,46 tumour stage,34 grade 3,7,10,15,34,62,63 lymph node involvement,10,16,21,46 LNR15,64,65 and vascular invasion.46 Regardless of pre- or postoperative treatment, other clinical and biological variables have been reported as prognostic factors, including preoperative variables such as insulin-dependent diabetes and a CA 19-9 level of ≥400 U/ml,15 surgical variables such as blood loss7,22,53 and postoperative complications,11,63 and postoperative variables such as persistently elevated levels of CA 19-9.66 In the present study, patients with a PV-SMVR had more negative histological factors, including more positive margins, although they had more often received neoadjuvant treatment. An increased frequency of positive resection margins has been reported in patients who required PV-SMVR.21,58 As in the present study, a recent report by Gnerlich et al. showed that the invasion of at least one margin was significantly more frequent (34% versus 22%), and the PV-SMVm was more often invaded (52% versus 24%) in patients who required PV-SMVR.21 However, Tseng et al. reported that the significantly increased risk for a microscopically positive SMAm in patients who required PV-SMVR was associated with tumour size and not with vascular resection.58
Thus, the effect of positive resection margins per se remains controversial,25 and some authors consider the finding of a positive margin to represent a function of tumour size and anatomical location,22 rather than a surrogate marker of tumour biology.5,16,46
The suggestion that positive margins may be predictive of the risk for local recurrence makes sense.13,20,21,28,53 The rate of local recurrence after resection of PDAC based on autopsy findings67 seems to be at odds with the R1 rate reported in series using a standardized pathological protocol for the examination of PD specimens.27 However, the rate of isolated local recurrence is usually below 25%, and in clinical practice, the majority of patients had metastases at the time of diagnosis of local recurrence and died of metastases.13,21 In the present study, in which follow-up was relatively short, 16 of 31 instances of local recurrence were isolated and, as previously reported, resection margin status did not affect the pattern of first recurrence.58
Microscopic resection margin involvement (R1) has been reported as an independent predictor of poor longterm survival following PD for PDAC in several studies,5–21,63 but R1 status was not identified as a significant factor for survival in a meta-analysis of data collected from randomized controlled trials (RCTs) of adjuvant treatment.23 In some studies, R1 resection was found to correlate with poorer survival on univariate but not multivariate analysis.26,30 In other studies, as in the present study, median OS did not differ significantly, whereas survival time of patients with R0 resections varied by ≥6 months,22,28 which may be considered a substantial difference in PDAC. The ‘vascular’ margins (medial part of the CRM) seem to be the most important.8,21,26,28,31,34 As Jamieson et al. have reported, the involvement of ‘transection’ margins was an independent predictor of poor outcome even in patients with node-negative disease; by contrast, outcomes in patients with posterior margin and anterior surface involvement (‘mobilization’ margins) were similar to those in patients with R0 resections.34 In the present study, the median and 2-year PFS were significantly worse in patients with at least one 0-mm positive margin; positive vascular margins significantly decreased PFS, but a positive posterior margin had no impact. Pingpank et al. reported a significant decrease in disease-free survival in patients with SMVm and SMAm involvement, but not in those with posterior margin or pancreatic margin involvement.8 Furthermore, multiple margin involvement, which has been reported in 20–45% of PD specimens,21,26,28,30,34,68 significantly increased the risk for local recurrence21 and was correlated with a significantly worse outcome.34,68 In the current study, the impacts of R1 status (defined as a 0-mm invasion of at least one margin) on PFS and OS estimated by the Cox model are close (HR = 0.59 and 0.64, respectively): this suggests that the non-significance of the impact of R1 status on OS is related to the lack of power of the study (resulting from an insufficient number of observed deaths).
Adjuvant chemotherapy is currently the standard treatment for patients following a potentially curative PD for PDAC in Europe.5,6,35 It is noteworthy that the R1 rates in RCTs that have led to changes in clinical practice were very low (19% in ESPAC-15 and 17% in CONKO-0016). Two recent meta-analyses and a large, prospective monocentre database have suggested that in patients with R1 status, postoperative radiochemotherapy may be useful and probably better than chemotherapy alone.23,63,69 Thus, postoperative radiotherapy may be considered if radiotherapy has not been administered before the intervention. Neoadjuvant treatment is promising70 and may decrease the rate of microscopically positive resection margins and the rate of local recurrence.22,58,59 However, there is currently a lack of randomized trials showing that neoadjuvant treatment abrogates the adverse effect of R1 status and results in a significant increase in disease-free or overall survival.8,59 In any case, the use of a standardized pathological protocol for the stratification of patients in clinical trials of adjuvant treatment is relevant today.37
Conclusions
The present prospective study shows that recently reported results of positive margins are reproducible in a multicentre trial and that rates obviously depend on the definitions of microscopic invasion used. Achieving better understanding of the biology of pancreatic cancer will undoubtedly represent the most important step towards improving survival. Meanwhile, the standardization of histological examination is not only necessary to provide accurate prognostic information, but may represent a significant step forward in the design of future RCTs and the optimization of adjuvant treatment strategies.
Acknowledgments
The authors thank Professors Thierry Andre and Pascal Hammel for their contributions to the development of the study protocol, Dr Louise Barbier for the revision of the manuscript, and participating pathologists G. Averous, A. Bardier, P. Callard, A. Couvelard, G. Di Giuro, J. F. Flejou, A. Liprandi, S. Maiterie, M. J. Payan and J. Selves, and surgeons M. Adham, E. Buc, S. Houry, C. Laurent, J. Y. Mabrut, J. L. Peix, P. H. Rouannet and J. C. Vaillant.
This work was supported by a grant from the French National Cancer Institute (INCA).
Appendix
Correspondence between terms used to define resection margins in pancreatoduodenectomy specimens according to the Royal College of Pathologists (RCPath) (UK) (http://www.rcpath.org) and the College of American Pathologists (CAP) (http://www.cap.org/)a
| Terminology | RCPath,42 Verbeke et al.26 | AJCC, CAP38,39 | Jamieson et al.34 |
|---|---|---|---|
| CRM | Anterior, medial and posterior RMs | Transection versus mobilization margins | |
| SMAm | SMAm + Pm = posterior CRM | Mesenteric or uncinatec | Transection |
| SMVm | Medial part of the CRMb | Transection | |
| Vascular margind | SMAm + SMVm | Medial transection margin | |
| Pm | Part of the posterior CRM | Deep radial posterior margin | Posterior mobilization |
| Anterior surface | Anterior CRM | Anterior mobilization | |
In the present study, the superior mesenteric artery margin (SMAm) and the mesenterico–portal vein groove or superior mesenteric vein margin (SMVm) were assessed separately.
Surface of the pancreatic head that faces the SMV and separates the anterior from the posterior CRM.28
The soft tissue adjacent to the right lateral border of the proximal 3–4 cm of the SMA. The term ‘retroperitoneal margin’, commonly used in place of SMAm or uncinate margin, should be abandoned.
In the present study, the SMAm and SMVm were assessed separately; they both define the vascular margin (or medial transection margin34 of the mesopancreas31,34).
The section of the pancreatic neck, which is not included in this table, is a transection margin.
AJCC, American Joint Committee on Cancer; RM, resection margin; CRM, circumferential resection margin; SMAm, superior mesenteric artery margin; SMVm, superior mesenteric vein margin (or mesenterico–portal vein groove); Pm, posterior margin.
Conflicts of interest
None declared.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. 2010. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide. International Agency for Research on Cancer, Lyon, France. Available at http://globocan.iarc.fr/factsheet.asp (last accessed 12 June 2012)
- 2.Carpelan-Holmström M, Nordling S, Pukkala E, Sankila R, Lüttges J, Klöppel G, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385–387. doi: 10.1136/gut.2004.047191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a US population-based study. Am J Gastroenterol. 2007;102:1377–1382. doi: 10.1111/j.1572-0241.2007.01202.x. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control. 2008;15:308–313. doi: 10.1177/107327480801500405. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 8.Pingpank JF, Hoffman JP, Ross EA, Cooper HS, Meropol NJ, Freedman G, et al. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2001;5:121–130. doi: 10.1016/s1091-255x(01)80023-8. [DOI] [PubMed] [Google Scholar]
- 9.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–558. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to longterm survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–1345. doi: 10.1016/j.gassur.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer. 2008;14:5. doi: 10.1186/1471-2407-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Broeck A, Sergeant G, Ectors N, van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Fatima J, Schnelldorfer T, Barton J, Wood CM, Wiste HJ, Smyrk TC, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg. 2010;145:167–172. doi: 10.1001/archsurg.2009.282. [DOI] [PubMed] [Google Scholar]
- 15.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 16.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, et al. Pancreatic ductal adenocarcinoma: longterm survival does not equal cure. Surgery. 2012;152 (Suppl.):43–49. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510–519. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP) HPB. 2009;11:18–24. doi: 10.1111/j.1477-2574.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, et al. A prospective comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2318–2328. doi: 10.1245/s10434-011-1560-3. [DOI] [PubMed] [Google Scholar]
- 20.Rau BM, Moritz K, Schuschan S, Alsfasser G, Prall F, Klar E. R1 resection in pancreatic cancer has significant impact on longterm outcome in standardized pathology modified for routine use. Surgery. 2012;152 (Suppl.):103–111. doi: 10.1016/j.surg.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Gnerlich JL, Luka SR, Deshpande AD, Dubray BJ, Weir JS, Carpenter DH, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147:753–760. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 22.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, et al. Pancreatic Cancer Meta-Analysis Group. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg. 2008;143:75–83. doi: 10.1001/archsurg.2007.17. [DOI] [PubMed] [Google Scholar]
- 24.Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg. 2012;99:1036–1049. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- 25.Buchler MW, Werner J, Weitz J. R0 in pancreatic cancer surgery: surgery, pathology, biology, or definition matters? Ann Surg. 2010;251:1011–1012. doi: 10.1097/SLA.0b013e3181e07dad. [DOI] [PubMed] [Google Scholar]
- 26.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 27.Verbeke CS. Resection margins and R1 rates in pancreatic cancer – are we there yet? Histopathology. 2008;52:787–796. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 28.Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 29.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB. 2009;11:282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell F, Smith RA, Whelan P, Sutton R, Raraty M, Neoptolemos JP, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology. 2009;55:277–283. doi: 10.1111/j.1365-2559.2009.03376.x. [DOI] [PubMed] [Google Scholar]
- 31.Gaedcke J, Gunawan B, Grade M, Szöke R, Liersch T, Becker H, et al. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg. 2010;395:451–458. doi: 10.1007/s00423-009-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlitter AM, Esposito I. Definition of microscopic tumour clearance (R0) in pancreatic cancer resections. Cancers. 2010;2:2001–2010. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liszka Ł, Pajak J, Zielińska-Pajak E, Gołka D, Mrowiec S, Lampe P. Different approaches to assessment of lymph nodes and surgical margin status in patients with ductal adenocarcinoma of the pancreas treated with pancreaticoduodenectomy. Pathology. 2010;42:138–146. doi: 10.3109/00313020903494060. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2010;251:1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 35.Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs. observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 36.Warshaw AL, Lillemoe KD, Fernandez-Del Castillo C. Pancreatic surgery for adenocarcinoma. Curr Opin Gastroenterol. 2012;8:488–493. doi: 10.1097/MOG.0b013e3283567f2c. [DOI] [PubMed] [Google Scholar]
- 37.Katz MH, Merchant NB, Brower S, Branda M, Posner MC, William Traverso L. Standardization of surgical and pathologic variables is needed in multicentre trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann Surg Oncol. 2011;18:337–344. doi: 10.1245/s10434-010-1282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. [Google Scholar]
- 39.Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Fitgibbons P, et al. 2012. Protocol for the Examination of Specimens From Patients with Carcinoma of the Exocrine Pancreas. Avalaible at http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/PancreasExo_12protocol_3200.pdf (last accessed 30 June 2012)
- 40.National Comprehensive Cancer Network (NCCN) 2012. Clinical Practice Guidelines in Oncology, Pancreatic adenocarcinoma, Version 2. Available at http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (last accessed 19 August 2012)
- 41.Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265–273. doi: 10.1002/bjs.5716. [DOI] [PubMed] [Google Scholar]
- 42.Campbell F, Bennett M, Foulis AJ. Minimum Dataset for Histopathological Reporting of Pancreatic, Ampulla of Vater and Bile Duct Carcinoma. London: Royal College of Pathologists; 2002. Available at http://www.rcpath.org (last accessed 17 July 2012) [Google Scholar]
- 43.Hruban RH, Pitman MB, Klimstra D. Tumors of the Pancreas. Washington, DC: Armed Forces Institute of Pathology; 2007. [Google Scholar]
- 44.Sobin LH, Gaspodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th edn. New York, NY: Wiley & Sons; 2009. [Google Scholar]
- 45.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Fernandez-Del Castillo C, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for r1 resections versus locally advanced unresectable tumors? What is a ‘true’ r0 resection? Ann Surg. 2012 doi: 10.1097/SLA.0b013e318263da2f. September 10, in press. [DOI] [PubMed] [Google Scholar]
- 46.Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 47.Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–380. doi: 10.1097/00006676-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Luttges J, Vogel I, Menke M, Henne-Bruns D, Kremer B, Kloppel G. The retroperitoneal resection margin and vessel involvement are important factors determining survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Virchows Arch. 1998;433:237–242. doi: 10.1007/s004280050242. [DOI] [PubMed] [Google Scholar]
- 49.Chatelain D, Flejou JF. Pancreatectomy for adenocarcinoma: prognostic factors, recommendations for pathological report. Ann Pathol. 2002;22:422–431. [PubMed] [Google Scholar]
- 50.Compton CC, Henson DE. Protocol for the examination of specimens removed from patients with carcinoma of the exocrine pancreas: a basis for checklists. Cancer Committee, College of American Pathologists. Arch Pathol Lab Med. 1997;121:1129–1136. [PubMed] [Google Scholar]
- 51.Albores-Saavedra J, Heffess C, Hruban RH, Klimstra D, Longnecker D. Recommendations for the reporting of pancreatic specimens containing malignant tumours. The Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol. 1999;11:304–307. doi: 10.1093/ajcp/111.3.304. [DOI] [PubMed] [Google Scholar]
- 52.Torre ML, Nigri G, Ferrari L, Cosenza G, Ravaioli M, Ramacciato G. Hospital volume, margin status, and longterm survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2012;78:225–229. [PubMed] [Google Scholar]
- 53.Katz MH, Wang H, Balachandran A, Bhosale P, Crane CH, Wang X, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg. 2012;16:68–79. doi: 10.1007/s11605-011-1748-7. [DOI] [PubMed] [Google Scholar]
- 54.Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA. ‘Artery-first’ approaches to pancreatoduodenectomy. Br J Surg. 2012;99:1027–1035. doi: 10.1002/bjs.8763. [DOI] [PubMed] [Google Scholar]
- 55.Turrini O, Ewald J, Barbier L, Mokart D, Blache JL, Delpero JR. Should portal vein be routinely resected during pancreaticoduodenectomy for adenocarcinoma? Ann Surg. 2012 doi: 10.1097/SLA.0b013e318269d23c. September 10. doi: 10.1097/SLA.0b013e318269d23c. [DOI] [PubMed] [Google Scholar]
- 56.Japan Pancreas Society. Classification of Pancreatic Cancer. 2nd edn. Tokyo: Kanehara; 2003. [Google Scholar]
- 57.Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, et al. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219–230. doi: 10.1097/00006676-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 59.Barbier L, Turrini O, Grégoire E, Viret F, Le Treut YP, Delpero JR. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB. 2011;13:64–69. doi: 10.1111/j.1477-2574.2010.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verbeke CS, Knapp J, Gladhaug IP. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: implications for resection margin assessment. Histopathology. 2011;59:1111–1121. doi: 10.1111/j.1365-2559.2011.04056.x. [DOI] [PubMed] [Google Scholar]
- 61.Wittekind C, Compton C, Quirke P, Nagtegaal I, Merkel S, Hermanek P, et al. A uniform residual tumour (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115:3483–3488. doi: 10.1002/cncr.24320. [DOI] [PubMed] [Google Scholar]
- 62.Crippa S, Partelli S, Zamboni G, Barugola G, Capelli P, Inama M, et al. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery. 2012;152 (Suppl.):112–119. doi: 10.1016/j.surg.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 65.Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez JM, Cowgill SM, Al-Saadi S, Collins A, Ross SB, Cooper J, et al. CA 19-9 velocity predicts disease-free survival and overall survival after pancreatectomy of curative intent. J Gastrointest Surg. 2009;13:349–353. doi: 10.1007/s11605-008-0696-3. [DOI] [PubMed] [Google Scholar]
- 67.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 68.Sasson AR, Hoffman JP, Ross EA, Kagan SA, Pingpank JF, Eisenberg BL. En bloc resection for locally advanced cancer of the pancreas: is it worthwhile? J Gastrointest Surg. 2002;6:147–115. doi: 10.1016/s1091-255x(01)00063-4. [DOI] [PubMed] [Google Scholar]
- 69.Stocken DD, Buchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH, et al. Meta-analysis of randomized adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372–1381. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]