Abstract
Background
The intraoperative placement of an enteral feeding tube (FT) during pancreaticoduodenectomy (PD) is based on the surgeon's perception of need for postoperative nutrition. Published preoperative risk factors predicting postoperative morbidity may be used to predict FT need and associated intraoperative placement.
Methods
A retrospective review of patients who underwent PD during 2005–2011 was performed by querying the National Surgical Quality Improvement Program (NSQIP) database with specific procedure codes. Patients were categorized based on how many of 10 possible preoperative risk factors they demonstrated. Groups of patients with scores of ≤1 (low) and ≥2 (high), respectively, were compared for FT need, length of stay (LoS) and organ space surgical site infections (SSIs).
Results
Of 138 PD patients, 82 did not have an FT placed intraoperatively, and, of those, 16 (19.5%) required delayed FT placement. High-risk patients were more likely to require a delayed FT (29.3%) compared with low-risk patients (9.8%) (P = 0.026). The 16 patients who required a delayed FT had a median LoS of 15.5 days, whereas the 66 patients who did not require an FT had a median LoS of 8 days (P < 0.001).
Conclusions
In this analysis, subjects considered as high-risk patients were more likely to require an FT than low-risk patients. Assessment of preoperative risk factors may improve decision making for selective intraoperative FT placement.
Introduction
Pancreaticoduodenectomy (PD), or the Whipple procedure, for the resection of malignancies of the pancreatic head, ampulla and distal bile duct is still associated with significant postoperative morbidity, rates of which range from 30% to 60%.1–4 Commonly reported morbidities include surgical site and organ space infections, delayed gastric emptying (DGE), respiratory complications, and pancreatic fistula. Many of these postoperative complications, including pancreatic fistula and DGE, may necessitate supplementary postoperative nutritional support. There is controversy in the surgical literature regarding the institution of early enteral nutritional support prior to the establishment of adequate oral feeding.5–9 Additionally, there are no standard recommendations in pancreatic surgery for the intraoperative placement of an enteral feeding tube (FT) to initiate early enteral support. Currently, the decision to place an enteral FT is largely based on the estimated concern for postoperative enteral feeding needs, as well as the availability of multidisciplinary FT placement and support should they be needed postoperatively.
Recent publications indicate that using the patient preoperative characteristics and comorbidities recorded in the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database may help to identify patients who will suffer postoperative complications and require an extended length of stay (LoS) following PD.10,11 The most contemporary of this literature, published by Greenblatt et al., identified 10 preoperative risk factors captured within the NSQIP database that were significantly associated with increased risk for postoperative morbidity.10 The aim of this study was to evaluate whether these same preoperative risk factors could more accurately guide the decision to pre-emptively place an enteral FT during the surgical procedure, which would be considered safer and possibly less expensive than delayed placement. The Huntsman Clinical Cancer Research PD patient database at the Huntsman Cancer Institute, University of Utah, and the NSQIP data were retrospectively reviewed to determine which patients might have benefitted from early enteral feeding by classifying them according to risk factors. Outcomes related to complications, hospitalization time and potential cost savings were analysed.
Materials and methods
Patients
Institutional review board approval granted in March 2011 (UU #46989) allowed the use of the NSQIP and Huntsman Clinical Cancer Research databases to identify all patients who underwent PD during 2005–2011 at the University of Utah and Huntsman Cancer Institute hospitals. Indications for PD were not restricted to a specific preoperative diagnosis. Charts were audited for preoperative demographics and comorbidities, perioperative outcomes, and nasojejunal FT management. Patients were categorized according to the presence or absence of the following 10 NSQIP preoperative risk factors: preoperative dependent functional status; presence of chronic obstructive pulmonary disease (COPD); age ≥80 years; male gender; elevated creatinine (mg/dl, based on laboratory normal ranges, gender-specific); leukocytosis (k/μl, based on laboratory normal ranges); steroid use; bleeding disorders; hypoalbuminaemia (≤3.5 g/dl), and an increased body mass index (BMI) of ≥25 kg/m2. The BMI cut-off of ≥25 kg/m2 is based on the published findings of Greenblatt et al.10 Patients in this analysis who demonstrated none or one of these risk factors (scores of ≤1) were considered to be low risk; those who demonstrated two or more (scores of ≥2) were considered to be high risk based on a decision by the authors. Variables examined included: standard demographics (age, gender); FT requirement; use of total parenteral nutrition (TPN); postoperative complications [organ space surgical site infection (SSI) rates as defined by the NSQIP]; 30-day readmission, and hospital mortality.
Surgical approach
Pancreaticoduodenectomies were performed by surgeons with extensive experience in pancreatic surgery. The operation was performed in either a classic or a pylorus-preserving fashion.
Nutrition
A nasojejunal FT was inserted at the time of surgery, at the surgeon's discretion, or during the postoperative period in patients who failed to achieve adequate nutrition via oral intake. If possible, oral feeding was instituted by postoperative day 5 in these patients regardless of intraoperative FT placement. If an FT was placed intraoperatively, feedings were initiated at a trophic rate starting on postoperative day 2 and advanced as required. Feedings were then discontinued if oral feeding was tolerated and provided sufficient support. Patients in whom an FT had been placed intraoperatively and who received enteral nutrition to and beyond postoperative day 9 were considered to have ‘required’ postoperative enteral nutritional support for the purposes of comparative analysis.
When the FT was placed intraoperatively, the anaesthesiologist inserted the FT via the nares and advanced the tube under the direct visualization of the attending surgeon to beyond the most distal anastomosis. Feeding tubes that were placed during the postoperative period were most often inserted in the radiology department under fluoroscopic guidance. A small number of patients required endoscopic placement by a gastroenterologist or bedside insertion under fluoroscopy while being treated in the intensive care unit.
Any patient who received TPN for at least a single day was considered to have been treated with TPN.
Outcomes
Patients were categorized according to whether the FT was inserted intraoperatively or postoperatively, and according to their need for enteral support. Patients were also grouped according to whether they demonstrated any of the 10 possible preoperative risk factors.
Data analysis consisted of comparisons between the low- and high-risk groups to verify relationships with complication rates and general hospital outcomes (LoS, 30-day readmission). Based on the determination of need for enteral support, risk groups were also used to determine possible predictors of need for enteral support and how these related to the insertion timeframe.
Three separate comparisons were made based on groupings of FT need and time of placement (Fig. 1).
Figure 1.
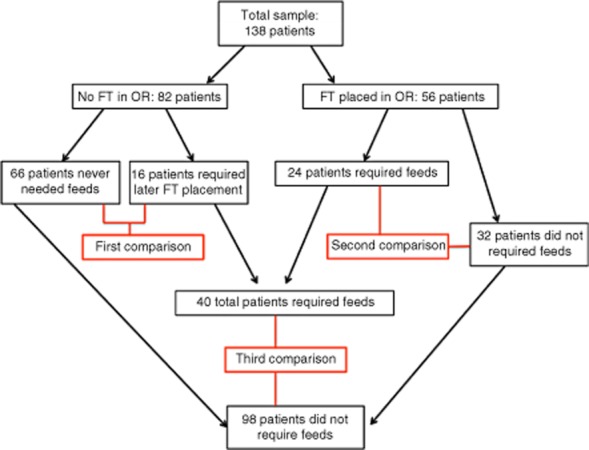
Patient flow though the study shows three sets of comparisons among patient groups categorized according to need for feeding tube and time of tube placement. FT, feeding tube; OR, operating room
Costs
Costs related to FT placement were examined. The average cost of an FT is US$175 (Dobbhoff Tube™; Covidien, Inc., Mansfield, MA, USA). When an FT is placed intraoperatively, the costs of doing so include only the cost of the product. When an FT is placed postoperatively, a fee for the associated procedure must be taken into account. This amounts to an average of US$325 per procedure for tubes placed under radiology, and at least US$750 per procedure for tubes placed endoscopically. For the purposes of this analysis, a procedure fee of US$500 was considered for tubes placed postoperatively.
Statistics
Data were compiled using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA) and analysed using pasw Statistics Version 18.0 (SPSS, Inc., Chicago, IL, USA). Data that were normally distributed are reported as the mean ± standard deviation (SD) and percentage. Data that were not normally distributed are reported as the median and interquartile range (IQR). Based on differences in the sizes of the analysis groups, univariate analysis with non-parametric testing using the Mann–Whitney U-test was performed with continuous variables; categorical variables were analysed using the chi-squared test or Fisher's exact test when any cell count was < 5. The Kruskal–Wallis rank test was used for continuous variables with more than two groups. A P-value of < 0.05 was considered to indicate statistical significance.
Results
A total of 138 patients underwent PD during 2005–2011. Of these, 67 (48.6%) patients underwent surgery for pancreatic adenocarcinoma, 29 (21.0%) for cystic lesions, 19 (13.8%) for an ampullary or bile duct cancer, 10 (7.2%) for neuroendocrine tumours and the remaining patients for other pancreas-related diagnoses. The mean ± SD age of the patient group was 63.3 ± 12.3 years (range: 25–88 years). Male patients comprised 53.6% (n = 74) of the population. Mean ± SD BMI was 26.6 ± 5.3 kg/m2 (range: 16.3–46.5 kg/m2). In 90 of the 138 patients (65.2%), a pylorus-preserving modification of the standard Whipple procedure was performed. Patients with an American Society of Anesthesiologists (ASA) score of 3 or 4 comprised 61.6% (n = 85) of the population. Mean ± SD preoperative albumin level (within 30 days of surgery) was 3.89 ± 0.5 g/dl. The incidence of hypoalbuminaemia was 21.1% (n = 28). Median hospital LoS was 9 days (IQR: 8–13 days). Three patients died during the index hospital admission, giving an overall mortality rate of 2.2%. Thirty of the 135 surviving patients required readmission within 30 days, giving a readmission rate of 22.2%.
Of the entire sample, 64 (46.4%) patients were categorized as low risk and 74 (53.6%) as high risk. Sixteen (11.6%) patients had none of the 10 possible risk factors, 48 (34.8%) had one risk factor and 47 (34.1%) had two. The highest number of risk factors seen was five in one patient only. Increased BMI (57.7%, n = 80) and male gender (53.6%, n = 74) were the most commonly seen risk factors. Table 1 describes the variables based on when the FT was inserted.
Table 1.
Comparison of preoperative and postoperative demographic variables according to whether feeding tube insertion was intraoperative, postoperative or never
| Never (n = 66) | Postoperative (n = 16) | Intraoperative (n = 56) | P-valuea | |
|---|---|---|---|---|
| Preoperative demographics | ||||
| Age, years, median (IQR) | 61.3 (53.3–68.2) | 75 (60.0–79.9) | 66.3 (55.6–74.0) | 0.030 |
| Male gender, n | 34 | 9 | 31 | 0.734, 0.672 |
| Body mass index, median (IQR) | 26.4 (23.1–28.5) | 25.5 (23.5–29.3) | 25.9 (21.5–30.5) | 0.937 |
| ASA class 3 or 4, n | 38 | 10 | 37 | 0.720, 0.337 |
| Albumin ≤3.5 g/dl, n | 7 | 5 | 16 | 0.057, 0.009 |
| Pylorus-preserving surgery, n | 47 | 8 | 35 | 0.105, 0.307 |
| High risk, n | 29 | 12 | 33 | 0.026, 0.099 |
| Postoperative outcomes | ||||
| Total parenteral nutrition, n | 4 | 8 | 15 | <0.001, 0.002 |
| Organ space SSI, n | 3 | 5 | 11 | 0.006, 0.009 |
| Length of stay, days, median (IQR) | 8 (7.0–9.0) | 15.5 (12.2–23.2) | 10 (8.0–15.0) | <0.001 |
| 30-day readmission, n | 12 | 6 | 12 | 0.091, 0.647 |
| Hospital mortality, n | 1 | 1 | 1 | 0.354, 1.000 |
Individual chi-squared test P-values are reported for ‘Never’ versus ‘Postoperative’, and ‘Never’ versus ‘Intraoperative’.
IQR, interquartile range; ASA, American Society of Anesthesiologists; SSI, surgical site infection.
The first set of comparisons (Fig. 1) analysed outcomes in the group of patients who did not receive an FT intraoperatively (n = 82). Patients who never required tube placement (n = 66) were compared with those who did require postoperative tube placement for enteral nutritional support (n = 16). Data are presented in Table 1.
The second set of comparisons (Fig. 1) involved the 56 patients in whom an FT was inserted intraoperatively and aimed to evaluate the need for postoperative enteral support. Of the 56 patients, 24 patients (42.9%) were categorized as having ‘required’ enteral support. Table 2 details comparative data for these 56 patients. The third set of comparisons (Fig. 1) were carried out in order to analyse outcomes in all patients who required an FT in comparison with outcomes in patients who did not. Data for the 16 patients in whom FTs were placed postoperatively were combined with data for 24 of the 56 patients in whom FTs were placed intraoperatively and who were deemed to have ‘required’ the FT, to give a total of 40 patients (Table 3). Outcomes in this group of 40 patients were then compared with those in the remaining 98 patients, of whom 66 never had an FT and 32 did not ‘require’ their intraoperatively placed FT. Table 3 details the variables for this comparison.
Table 2.
Comparison of preoperative and postoperative demographic variables in patients in whom feeding tubes (FTs) were placed intraoperatively determined according to the use of FTs to postoperative day 9
| Needed FT (n = 24) | Did not need FT (n = 32) | P-value | |
|---|---|---|---|
| Preoperative demographics | |||
| Age, years, median (IQR) | 69.2 (59.9–75.3) | 60.1 (54.9–72.3) | 0.065 |
| Male gender, n | 12 | 19 | 0.485 |
| Body mass index, median (IQR) | 25.9 (22.1–32.6) | 25.9 (21.1–30.4) | 0.541 |
| Albumin ≤3.5 g/dl, n | 6 | 10 | 0.697 |
| High risk, n | 14 | 19 | 0.938 |
| Postoperative outcomes | |||
| Total parenteral nutrition, n | 10 | 5 | 0.029 |
| Organ space SSI, n | 8 | 3 | 0.041 |
| Length of stay, days, median (IQR) | 14 (10.2–16.7) | 8 (8.0–10.0) | <0.001 |
| 30-day readmission, n | 5 | 7 | 0.990 |
| Hospital mortality, n | 1 | 0 | 0.429 |
SSI, surgical site infection; IQR, interquartile range.
Table 3.
Comparison of preoperative and postoperative demographic variables of feeding tube (FT) need; using overall combined grouping
| Needed FT (n = 40) | Did not need FT (n = 98) | P-value | |
|---|---|---|---|
| Preoperative demographics | |||
| Age, years, median (IQR) | 70.7 (60.0–78.5) | 60.9 (54.4–69.2) | 0.003 |
| Male gender, n | 21 | 53 | 0.866 |
| Body mass index, median (IQR) | 25.8 (22.8–30.2) | 26.2 (22.7–28.8) | 0.733 |
| Albumin ≤3.5 g/dl, n | 11 | 17 | 0.158 |
| High risk, n | 26 | 48 | 0.087 |
| Postoperative outcomes | |||
| Total parenteral nutrition, n | 18 | 9 | <0.001 |
| Organ space SSI, n | 13 | 6 | <0.001 |
| Length of stay, days, median (IQR) | 15 (11.2–21.0) | 8 (7.0–10.0) | <0.001 |
| 30-day readmission, n | 11 | 19 | 0.239 |
| Hospital mortality, n | 2 | 1 | 0.201 |
IQR, interquartile range; SSI, surgical site infection.
A comparison based on risk grouping was performed. It showed that 35.1% (n = 26) of high-risk patients required enteral nutritional support compared with 21.9% (n = 14) of low-risk patients (P = 0.087). Table 4 highlights further differences between the two groups.
Table 4.
Comparison of high- and low-risk patients
| Low-risk patients (n = 64) | High-risk patients (n = 74) | P-value | |
|---|---|---|---|
| Needed a feeding tube, % | 35.1 | 21.9 | 0.087 |
| Non-pylorus-sparing, % | 18.8 | 48.6 | <0.001 |
| Organ space SSI, % | 10.9 | 16.2 | 0.369 |
| ASA scores of 3 or 4, % | 51.6 | 70.3 | 0.024 |
| Total parenteral nutrition, % | 9.4 | 28.4 | 0.005 |
| 30-day readmission, % | 14.0 | 29.6 | 0.030 |
| Hospital mortality, % | 0.0 | 4.1 | 0.248 |
SSI, surgical site infection; ASA, American Society of Anesthesiologists.
Of the 10 NSQIP risk factors used to determine preoperative risk, the only factor to prove independently significant in terms of FT need was age ≥80 years (Table 5). The study sample included 10 patients aged ≥80 years, six of whom were among the 40 patients who needed an FT; of the 98 patients who did not require an FT, only four were aged ≥80 years (P = 0.035). This need for an FT would translate to a relative risk 2.3 times greater in those aged ≥80 years than in those aged < 80 years.
Table 5.
Distribution of risk factors used to determine risk groups based on overall combined grouping
| Needed FT (n = 40) | Did not need FT (n = 98) | |
|---|---|---|
| Dependent functional status, n | 0 | 0 |
| Bleeding disorder, n | 2 | 0 |
| Chronic obstructive pulmonary disease, n | 1 | 2 |
| Steroid medication, n | 3 | 2 |
| Leukocytosis, k/μla, n | 1 | 5 |
| Age ≥80 years, n | 6 | 4 |
| Elevated creatinine, mg/dlb, n | 9 | 13 |
| Hypoalbuminaemia ≤3.5 g/dl, n | 11 | 17 |
| Male gender, n | 21 | 53 |
| Body mass index ≥25 kg/m2, n | 23 | 56 |
Normal data based on laboratory site.
Normal data based on laboratory site and gender.
FT, feeding tube.
There were no complications related to FT placement, regardless of when the FT was placed. Of the 56 patients in whom FTs were placed intraoperatively, nine (16.1%) required additional placements by interventional radiology as a result of clogging (n = 2), inadvertent removal (n = 6), and need for a second tube after oral feeding had been deemed inadequate after the removal of the first tube (n = 1). Of the 16 patients in whom FTs were placed postoperatively, eight required more than a single attempt at placement in order to position the FT correctly, one required the replacement of a clogged FT, and two required endoscopic placement. Figure 2 outlines costs in a theoretical population of 100 patients.
Figure 2.
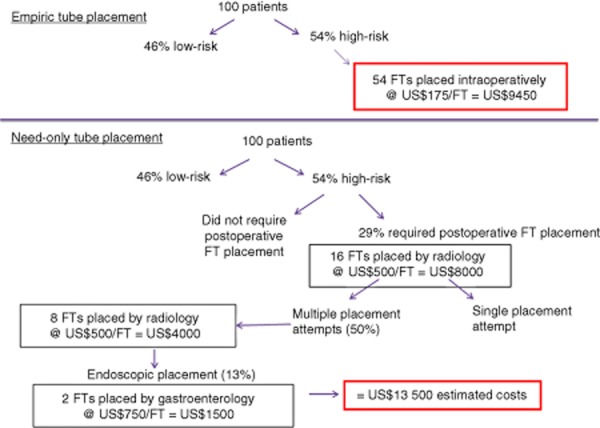
Estimated costs of feeding tube (FT) placement in a theoretical population of 100 patients
Discussion
Studies have demonstrated that early postoperative feeding following gastrointestinal surgery can decrease the overall rate of complications and LoS and aid in wound healing.12–15 Controversy exists regarding the optimal timing and method of administration of postoperative feeding following PD. Various articles examining the effects of postoperative nutrition on patients submitted to the Whipple procedure have come to varying conclusions. A systematic review published in 2006 by Goonetilleke and Siriwardena examined 10 studies investigating nutritional support following PD.6 Its overall conclusions favoured enteral feeding via a nasojejunal FT following PD.6 This article also showed that early mandatory, postoperative TPN was not associated with improved outcomes. Moreover, Gianotti et al. found the administration of TPN to be associated with increased morbidity.16 The review by Goonetilleke and Siriwardena also implied that administration of postoperative enteral nutrition helps to decrease infectious complications.6 A 2004 article by Baradi et al. evaluated the effect of early postoperative enteral feeding on outcomes following PD.9 This article also supported the provision of early postoperative nasoenteric nutrition because it was associated with lower readmission rates, complications and decreased TPN use.9 However, there are several case series which are not supportive of this approach in PD patients, implying an association between between enteral nutritional supplementation and DGE.8,17
There is no consensus regarding the need for or optimal strategy for enteral feeding support following PD. Given these findings, the present study aimed to identify patients who might benefit from the intraoperative placement of a nasojejunal FT in anticipation of the need for postoperative enteral feeding support. A 2011 article by Greenblatt et al. identified preoperative risk factors associated with increased morbidity following PD.10 These risk factors include dependent functional status, older age, male gender, being overweight or obese, COPD, steroid use, the presence of a bleeding disorder, preoperative leukocytosis, elevated serum creatinine and hypoalbuminaemia. These risk factors were used in an attempt to identify patients who might benefit from the intraoperative placement of an FT. Patients were categorized as being at low or high risk for requiring an FT according to whether they demonstrated the presence of none or one versus more than one risk factor.
In this small series, 35.1% of the high-risk patients and 21.9% of the low-risk patients required enteral nutritional support. These data showed a trend towards significance in support of the potential intraoperative placement of FTs in order to avoid difficult postoperative placements. The analysis of data for subsets of patients, such as those who needed an FT versus those who did not, showed that patients who required an FT were more likely to have a longer LoS, higher rates of organ space SSI and a need for TPN. It cannot be concluded from these data that high-risk status alone is an indicator for FT placement, but they may indicate a potential way to discriminate patients preoperatively for intraoperative placement. Operative insertion and stabilization could avoid a challenging postoperative FT placement, which, as the present data show, often requires multiple attempts.
There were complications associated with FT placement arose and the overall incidence of any complications related to the tubes was low. Only six of 56 intraoperatively placed FTs clogged (10.7%). This is relatively low as the literature cites rates of up to 34%.18 The finding that eight of 16 (50.0%) postoperatively placed FTs required multiple placement attempts was unexpected and is likely to reflect the complex post-PD anatomy and to highlight the advantage afforded by intraoperative placement in terms of ability to place the FT in the appropriate anatomic position. Of the 40 patients who required an FT, 18 (45.0%) eventually required TPN. The fact that a higher proportion of these patients (eight of 16, 50.0%) belonged to the group in which FTs were placed postoperatively may also reflect a failure to place the tube appropriately in this group. The rate of TPN use may reflect the number of patients with a pancreatic fistula, who were intolerant of any form of enteral nutrition, and thus indicates that an intraoperatively placed FT does not necessarily obviate the need for TPN.
A theoretical analysis was performed to examine costs related to FT placement. In the postoperative period, nasojejunal FT placement is generally performed under fluoroscopic guidance by an interventional radiologist or a trained technician, or by a gastroenterologist using endoscopic placement. These procedures can be timely, add a procedure cost, and can increase morbidity.18 Although no additional complications arose as a result of FT placement in the current study, half of the postoperatively placed tubes required multiple attempts at placement for appropriate positioning. Costs were hypothesized in a theoretical population of 100 patients and costs of empiric placement in high-risk patients were compared with costs in the theoretical group of high-risk patients who would require postoperative placement (Fig. 2). This exercise resulted in estimated potential cost savings of US$4050 for a population of 100 patients or US$75 per high-risk patient, with empiric placement.
This review is subject to the limitations of a small retrospective review. Rates of DGE were not evaluated because surgeons at the study center do not routinely place nasogastric tubes postoperatively, and the most recent definition of DGE proposed by the International Study Group of Pancreatic Surgery (ISGPS) is dependent on nasogastric tube output volume.19,20 Presumably, the majority of patients who required and benefitted from enteral support had some degree of DGE. The other disadvantage of this study refers to its inability to measure the possible benefits of early enteral nutrition as a result of the small size of the study population and low complication rate. Additionally this review of perioperative predictive factors was limited to those morbidities and patient demographics screened for in the NSQIP database. Other patient or intraoperative characteristics may also affect the need for an enteric FT.
In this retrospective review, patients with two or more preoperative risk factors were more likely to require FT placement and those aged ≥80 years had significantly higher risk for FT need. Patients who required an FT had a longer LoS and an increased complication rate. These data support the notion that selective intraoperative FT placement for at-risk patients may potentially benefit patients, as well as reduce costs. In conclusion, surgeons should consider selective intraoperative enteral FT placement in all patients aged ≥80 years and in any patients with two or more of the preoperative comorbidity risk factors.
Acknowledgments
The authors would like to acknowledge the Clinical Cancer Research Department at the Huntsman Cancer Institute, University of Utah, for use of its database.
The authors thank Dr Xiaoming Sheng PhD, Department of Paediatrics, University of Utah, for his assistance and advice with statistical methods.
Conflicts of interest
None declared.
References
- 1.Billinglsey KG, Hur K, Henderson WG, Daley J, Khuri SF, Bell RH. Outcome after pancreaticoduodenectomy for periampullary cancer: an analysis from the Veterans Affairs National Surgical Quality Improvement Program. J Gastrointest Surg. 2003;7:484–494. doi: 10.1016/S1091-255X(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 2.Pecorelli N, Balzano G, Capretti G, Zerbi A, DiCarlo V, Braga M. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg. 2012;16:518–523. doi: 10.1007/s11605-011-1777-2. [DOI] [PubMed] [Google Scholar]
- 3.Chalikondas S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;16:2397–2402. doi: 10.1007/s00464-012-2207-6. [DOI] [PubMed] [Google Scholar]
- 4.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Hilal M, Hemandas AK, McPhail M, Jain G, Panagiotopoulou I, Scibelli T, et al. A comparative analysis of safety and efficacy of different methods of tube placement for enteral feeding following major pancreatic resection. A non-randomized study. JOP. 2010;11:8–13. [PubMed] [Google Scholar]
- 6.Goonetilleke KS, Siriwardena AK. Systematic review of perioperative nutritional supplementation in patients undergoing pancreaticoduodenectomy. JOP. 2006;7:5–13. [PubMed] [Google Scholar]
- 7.Akizuki E, Kimura Y, Nobuoka T, Imamura M, Nagayam M, Sonoda T, et al. Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy. Ann Surg. 2007;249:986–994. doi: 10.1097/SLA.0b013e3181a63c4c. [DOI] [PubMed] [Google Scholar]
- 8.Martignoni ME, Friess H, Sell F, Ricken L, Shrikhande S, Kulli C, et al. Enteral nutrition prolongs delayed gastric emptying in patients after Whipple resection. Am J Surg. 2000;180:18–23. doi: 10.1016/s0002-9610(00)00418-9. [DOI] [PubMed] [Google Scholar]
- 9.Baradi H, Walsh RM, Henderson JM, Vogt D, Popovich M. Postoperative jejunal feeding and outcome of pancreaticoduodenectomy. J Gastrointest Surg. 2004;8:428–433. doi: 10.1016/j.gassur.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–2135. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 11.Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702–708. doi: 10.1097/SLA.0b013e31823598fb. [DOI] [PubMed] [Google Scholar]
- 12.Moss G, Greenstein A, Levy S, Bierenbaum A. Maintenance of GI function after bowel surgery and immediate enteral full nutrition. Doubling of canine colorectal anastomotic bursting pressure and intestinal wound mature collagen content. Clinical experience, with objective demonstration of intestinal absorption and motility. JPEN J Parenter Enteral Nutr. 1980;4:535–538. doi: 10.1177/0148607180004006535. [DOI] [PubMed] [Google Scholar]
- 13.Neumayer LA, Smout RJ, Horn HG, Horn SD. Early and sufficient feeding reduces length of stay and charges in surgical patients. J Surg Res. 2001;95:73–77. doi: 10.1006/jsre.2000.6047. [DOI] [PubMed] [Google Scholar]
- 14.McCarter MD, Gomez ME, Daly JM. Early postoperative enteral feeding following major upper gastrointestinal surgery. J Gastrointest Surg. 1997;1:278–285. doi: 10.1016/s1091-255x(97)80121-7. [DOI] [PubMed] [Google Scholar]
- 15.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianotti L, Braga M, Gentilini O, Balzano G, Zerbi A, Di Carlo V. Artificial nutrition after pancreaticoduodenectomy. Pancreas. 2000;21:344–351. doi: 10.1097/00006676-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rayar M, Sulpice L, Meunier B, Boudjema K. Enteral nutrition reduces delayed gastric emptying after standard pancreaticoduodenectomy with Child reconstruction. J Gastrointest Surg. 2012;16:1004–1011. doi: 10.1007/s11605-012-1821-x. [DOI] [PubMed] [Google Scholar]
- 18.Gerritsen A, Besselink MG, Cieslak KP, Vriens MR, Steenhagen E, Hillegersberg R, et al. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg. 2012;16:1144–1151. doi: 10.1007/s11605-012-1887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malleo G, Crippa S, Butturini G, Salvia R, Partelli S, Rossini R, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB. 2010;12:610–618. doi: 10.1111/j.1477-2574.2010.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]


