Abstract
Objectives
This study was conducted to determine if routine staging chest computed tomography (CT) or positron emission tomography (PET) scanning alters the clinical management of patients with newly diagnosed pancreatic adenocarcinoma.
Methods
All new pancreas cancers seen in medical oncology, radiation oncology and surgery from 1 June 2008 to 20 June 2010 were retrospectively reviewed. Patients with metastatic disease on chest CT or PET, that had been unsuspected on initial imaging, were identified.
Results
Pancreatic adenocarcinoma was present in 247 consecutive patients. Abdominal CT demonstrated metastases in 108 (44%) and localized disease in 139 (56%) patients. Chest CT and PET were not performed in 15 (11%) of these 139 patients. In the remaining 124 patients, CT imaging suggested resectable disease in 46, borderline resectable disease in 52 and locally advanced disease in 26 patients. Chest CT demonstrated an unsuspected lymphoma in one patient with borderline resectable disease and PET identified extrapancreatic disease in two patients with locally advanced disease. Chest CT and PET added no information in 121 (98%) of the 124 patients.
Conclusions
The addition of chest CT and PET to high-quality abdominal CT is of little clinical utility; additional sites of metastasis are rarely found. As the quality of abdominal imaging declines, the yield from other imaging modalities will increase. Dedicated pancreas-specific abdominal CT remains the cornerstone of initial staging in suspected or biopsy-proven pancreatic cancer.
Introduction
Current National Comprehensive Cancer Network (NCCN) guidelines for staging patients with pancreatic adenocarcinoma have been revised to include a dedicated chest computed tomography (CT) scan to evaluate for lung metastases.1,2 Pulmonary evaluation is important because the presence of pulmonary metastases will influence subsequent treatment recommendations, affect clinical trial eligibility, and have obvious implications for surgery directed at the primary tumour. For example, patients with resectable pancreatic adenocarcinoma (resectable primary tumour), in whom indeterminate lung lesions are found on imaging, are classified as borderline resectable according to the Katz classification (type B) in view of concern for distant metastases.3 In such patients, resection of the primary tumour is usually not performed as the first treatment modality. Type B patients are generally offered neoadjuvant chemotherapy, with or without chemoradiation.4–6 This strategy allows for the lung lesions to be further clarified over time, during which systemic therapy is provided to treat both local and potentially distant disease.
Accurate staging tools are needed to improve preoperative patient selection and offer stage-appropriate therapies to patients with newly diagnosed cancer of the pancreas. Computed tomography of the chest and positron emission tomography (PET) can potentially influence the management of patients with resectable pancreas cancer. However, there are very few data to support the routine use of chest CT or PET scanning, or the combination of both, in these patients. Chest CT scanning has been shown to be more sensitive and specific than chest X-ray in identifying lung cancer and in identifying lung metastases in patients with periampullary tumours.7,8 Lung metastases are often a later finding in patients who have already developed liver metastases, peritoneal carcinomatosis or malignant ascites. Many practices now incorporate CT and PET or combined CT/PET within the staging workup.9–11 However, there is no firm consensus on the precise roles of these and additional staging studies in patients with newly diagnosed pancreatic cancer.
The present group hypothesized that staging chest CT and PET fail to identify new sites of disease beyond those seen in abdominal CT and chest X-ray in patients with newly diagnosed pancreatic cancer. If lung metastases were found in patients with disease that was otherwise considered to be resectable according to conventional imaging methods, such patients would not be considered for surgery. The aim of this study was therefore to determine if routine staging chest CT or PET scanning alters the clinical management of patients presenting with newly diagnosed pancreatic adenocarcinoma.
Materials and methods
Subsequent to institutional review board approval, all new pancreatic cancer patients seen in the Departments of Medical Oncology, Radiation Oncology and Surgery at the Medical College of Wisconsin from 1 June 2008 to 30 June 2010 were retrospectively reviewed. Pancreas protocol CT (PPCT), chest X-ray, chest CT and PET were all re-reviewed by three of the present authors (SGP, APM, PPT). A pulmonary nodule scoring system designed to allow findings on these imaging studies to be objectively reviewed and compared was developed (Table 1). Although no specific criteria or definitions have been described for the evaluation of pulmonary metastasis in chest CT, the nodule scoring system was used to categorize the initial imaging studies of patients in the present series. In addition, PET scans were reviewed and qualitatively scored as positive or negative for additional suspicious findings in comparison with chest CT scans.
Table 1.
Pulmonary nodule scoring system
| Pulmonary nodule score | Lung findings |
|---|---|
| 0 No evidence of malignancy | 1 Normal lungs |
| 2 Stable pulmonary nodules for 2 years | |
| 3 Homogeneous calcified nodules | |
| 4 Opacities not related to malignancy (i.e. atelectasis, congestive heart failure, pulmonary scarring/fibrosis, emphysema) | |
| 1 Indeterminate | 1 Solitary pulmonary nodules <5 mm |
| 2 Pulmonary nodules <5 mm with short-interval stability (<2 years) | |
| 3 Pleural effusion, pleural plaques, and infectious/inflammatory ground glass and tree-in-bud opacities | |
| 2 Suspicious | 1 Solitary pulmonary nodule of >5 mm |
| 2 Multiple pulmonary nodules | |
| 3 Definite metastasis(es) | 1 Multiple, bilateral, peripheral, lower lobe pulmonary nodules of varying size (haematogenous spread) |
| 2 Diffuse nodular interstitial thickening (lymphangitic spread) | |
| 3 New and/or enlarged pulmonary nodule(s) over time | |
| 4 Biopsy-proven metastasis | |
| 5 Malignant lymphadenopathy | |
All patient examinations were reviewed with particular attention to the presence of suspicious pulmonary nodules. The majority of staging studies had been performed at the present institution; however, a subset of patients had already undergone imaging prior to evaluation at this institution. In this scenario, these studies were added to the present institution's imaging archive and were evaluated. Outside examinations were included only when deemed diagnostic by the interpreting radiologists and were excluded if they presented artefact or technical inadequacy. Examinations were reviewed retrospectively by two experienced radiologists. Examination findings were categorized accordingly by consensus. A third radiologist reviewed any cases in which disagreement occurred.
Patients with newly diagnosed pancreas cancer were clinically and radiographically staged based on CT imaging findings and clinical performance status.5,6 Resectable disease (stage I or II) was defined, based on CT images, as a normal tissue plane between the tumour and adjacent arterial structures and a patent superior mesenteric vein–portal vein (SMV–PV) confluence. Patients with borderline resectable disease represented three different subtypes as previously described (Katz types A, B and C).3 Type A patients had tumour abutment (≤180 °) of the superior mesenteric or coeliac arteries and an occluded SMV–PV confluence with an adequate segment of vein above and below the area of tumour involvement to allow for venous resection and reconstruction. Type B and C patients included those with questionable metastatic disease and marginal performance status, respectively. Locally advanced (stage III) tumours were considered as those that exhibited tumour encasement (>180 °) of the adjacent arteries or an occluded SMV–PV confluence with no technical option for venous reconstruction. Patients with metastatic disease (stage IV) at presentation had radiographic evidence of liver, lung, peritoneal or other sites of distant metastases.1
Patients with non-pancreatic periampullary tumours, and those with pancreatic tumours that were not adenocarcinomas (i.e. intraductal papillary mucinous neoplasms, neuroendocrine tumours) were excluded.
Results
Cytologic or histologic confirmation of pancreatic adenocarcinoma was present in 247 consecutive patients. Abdominal CT demonstrated metastatic disease in 108 (44%) patients and localized disease in 139 (56%) (Fig. 1). In 15 (11%) of these 139 patients, chest CT or PET were not performed. In the remaining 124 patients, CT imaging suggested resectable disease in 46, borderline resectable disease in 52 and locally advanced disease in 26 patients. Chest CT demonstrated an unsuspected mediastinal mass (unrelated to pancreas cancer) in one patient with borderline resectable disease and PET identified extrapancreatic disease in two patients with locally advanced disease. Chest CT and PET added no new information in 121 (98%) of the 124 patients.
Figure 1.
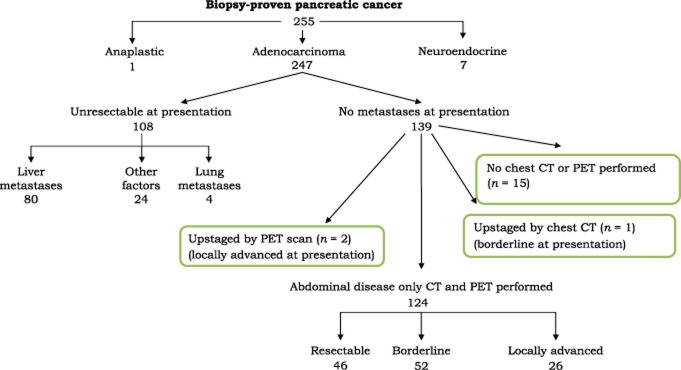
Findings in 247 consecutive patients with pancreas cancers at the Medical College of Wisconsin. CT, computed tomography; PET, positron emission tomography
In the 46 patients with resectable disease, chest CT and PET scans were performed and were available for comparison with pancreas CT and chest X-ray. In 43 (93%) of the 46 patients, chest CT and PET provided no additional information. New findings on chest CT and/or PET emerged for three patients. In two patients, the chest CT nodule score was suspicious for metastatic disease; the PET scan was read as negative in both patients. One patient underwent lung biopsy which demonstrated a hamartoma and the other patient submitted to a supraclavicular lymph node biopsy, which was also negative for malignancy. In one resectable patient, chest CT findings were indeterminate and PET was negative. This patient underwent a surgery-first approach and did not receive neoadjuvant therapy. The same patient progressed to develop liver and lung metastases within 3 months of surgery.
In the 52 patients with borderline resectable disease, chest CT and PET scans were performed and were available for comparison with pancreas CT and chest X-ray. In 46 (88%) of these 52 patients, the addition of chest CT and PET scanning to conventional staging studies added no further information. In four patients chest CT showed suspicious nodules. All patients had negative PET scans and all patients went on to submit to pancreatic cancer resection without significant interval change on repeat imaging prior to surgery. In one patient an obvious mass was detected on chest CT and PET. This patient had a concomitant primary lymphoma, was treated for this and subsequently underwent resection of the primary pancreatic cancer.
In the 26 patients with locally advanced disease, chest CT and PET scans were performed and were available for comparison with CT of the pancreas and chest X-ray. In 24 (92%) of these patients, chest CT and PET demonstrated no additional findings. In two patients, PET scanning after initial systemic therapy identified disease not seen on abdomen CT or chest X-ray; this involved metastatic disease to the adrenal gland in one patient and to the supraclavicular fossa in the other. Both patients had normal or indeterminate findings on chest CT. The majority of patients (108 of 247, 44%) had metastases at presentation and only four patients presented with lung-only metastases. In all four patients, the lung metastases were visible and suspicious on review of the lower lung fields of the pancreas CT. Eighty of the 108 (74%) patients with metastatic disease were found to be unresectable as a result of liver metastases. The remaining 24 were unresectable for other reasons (ascites, peritoneal carcinomatosis, etc.)
Discussion
Staging chest CT or PET scans rarely altered the clinical management strategy for patients presenting with newly diagnosed pancreatic adenocarcinoma. In fact, there were no instances in the resectable and borderline resectable categories in which staging chest CT or PET identified occult metastatic disease that precluded surgery. The one resectable patient in whom chest CT showed indeterminate findings underwent a surgery-first approach and suffered disease progression after surgery in the liver and lung. Prior imaging was not available for this patient, who was found to have multiple bilateral small (<5 mm) pulmonary nodules that would have warranted further imaging according to the nodule scoring system used in this study. The two resectable patients in whom chest CT findings were suspicious both had negative PET scans and neither progressed after receiving preoperative neoadjuvant therapy. These patients were felt to represent false positive chest CT results on retrospective review.
The precise role of additional imaging studies in patients with newly diagnosed cancer of the pancreas is not known. Although the use of PET scanning alone or in combination with CT scanning as a diagnostic procedure may seem reasonable, studies have failed to demonstrate that these techniques improve the accuracy of radiographic staging or alter recommendations for the sequencing of therapy.8 However, high-quality PPCT, as performed in this series, may not be available at all institutions. The roles of additional or complementary staging studies may vary based on the quality of CT or magnetic resonance imaging. Although staging chest CT and PET scans are included within the staging workup of pancreas cancer patients enrolled in clinical trials, the incremental benefit of this practice for off-protocol patients is not apparent based on the present review.
None of the patients with stage I or stage II pancreatic head carcinoma demonstrated suspicious findings on chest CT; the additional chest imaging did not change clinical management. Neither did chest CT or PET change clinical management in patients with stage IV disease. Patients with locally advanced stage III disease are not considered for surgery and are usually treated with systemic therapy prior to consideration for chemoradiation. In such patients, the finding of an additional site of possible metastatic disease in the lungs or bone may affect eligibility for clinical trials, but would not affect treatment sequencing if systemic therapy is delivered prior to consideration of locoregional chemoradiation. Most patients with localized (resectable or borderline resectable) pancreas cancer harbour radiographically occult metastatic disease even if this is not apparent on imaging studies and therein lies the rationale for the provision of combined modality therapy prior to consideration for surgery.4 This group therefore favours neoadjuvant therapy or chemoradiation, even in patients with resectable pancreatic adenocarcinoma.
For patients with borderline resectable pancreatic cancer, neoadjuvant therapy enjoys a more widespread consensus. It is notable that of the 52 borderline patients, four were found to have indeterminate or suspicious lung nodules on chest CT, all had negative PET scans, and all four failed to show progression in the lungs following neoadjuvant chemotherapy and surgery. Chest CT demonstrated an unsuspected mediastinal mass (unrelated to pancreas cancer) in one of the 52 patients with borderline resectable disease. These data would suggest that additional imaging studies prior to the initiation of systemic therapy in patients with borderline resectable disease will not identify clinically significant disease that alters the management of this subgroup of patients.
In patients with localized pancreatic cancer, lung metastases rarely occur in the absence of other contraindications for surgical resection (other sites of distant disease or locally advanced primary disease).8 Although the roles of chest CT and PET are well established in the staging of other solid tumours, their roles in the staging of periampullary cancer have not been fully evaluated.12–14 The only apparent benefit identified in the present study referred to stage III disease, in which PET scanning identified extrapancreatic disease in two (8%) of 26 patients. These findings are similar to data published previously by Nordback et al., who found a benefit of routine chest CT imaging prior to the initiation of anti-cancer therapy.8 In fact, only three (6%) of 53 patients had lung metastases. Nordback et al. found no patients in whom treatment sequencing was changed as all patients had imaging findings which precluded surgical resection of the pancreatic cancer.8 In the present report, only four (2%) of 247 patients had apparent isolated lung metastases, but, interestingly, these were readily identifiable on both the chest X-ray and the lower lung fields of the abdominal CT. The additional staging benefit to routine chest CT and PET scanning, in this study, appears to be negligible.
Patients with indeterminate pulmonary nodules, as defined in this report, may not need further evaluation prior to treatment when the initial treatment is systemic chemotherapy. It is not routine practice at the present study centre to biopsy such lung lesions found at the time of initial staging workup if neoadjuvant systemic therapy is planned. Although a positive lung biopsy (for adenocarcinoma of pancreatic origin) would change the stage of disease (to stage IV) in patients with localized pancreatic cancer, it would alter patient management only if a surgery-first treatment strategy was planned. Therefore, this centre typically follows such indeterminate or suspicious abnormal chest CT findings with serial imaging, especially in patients with borderline resectable disease (Katz types A, B or C). This treatment paradigm prevents any delay in the initiation of treatment and avoids the potential morbidities associated with lung biopsy and procedure-related complications. In fact, in two instances, the performance of lung biopsies delayed the initiation of systemic therapy and both biopsies were non-diagnostic.
Further study may be necessary to make a firm recommendation on the utility of screening chest CT and PET in all patients with pancreatic adenocarcinoma. It will also be important to study the clinical significance of isolated lung metastases as systemic therapies improve. Clinical observation suggests that lung metastases are often somewhat indolent and certainly much less aggressive than liver or peritoneal metastases. At present, this group supports the use of high-quality abdominal CT imaging as the cornerstone of initial staging and follow-up in patients with pancreatic cancer.
In conclusion, the addition of chest CT and PET to abdominal CT is of limited clinical utility; additional sites of metastasis are rarely found. However, these data were acquired at an institution which performs very high-quality abdominal imaging (to include the lower lung fields); as the quality of abdominal imaging declines, the yield from other imaging modalities will increase. Dedicated pancreas-specific abdominal CT remains the cornerstone of initial staging in patients with suspected or biopsy-proven pancreatic cancer.
Conflicts of interest
None declared.
References
- 1.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 2.Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, 3rd, Casper ES, et al. Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 5.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736–1744. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 6.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Spiro SG, Porter JC. Lung cancer – where are we today? Current advances in staging and non-surgical treatment. Am J Respir Crit Care Med. 2002;166:1166–1196. doi: 10.1164/rccm.200202-070SO. [DOI] [PubMed] [Google Scholar]
- 8.Nordback I, Saaristo R, Piironen A, Sand J. Chest computed tomography in the staging of pancreatic and periampullary carcinoma. Scand J Gastroenterol. 2004;39:81–86. doi: 10.1080/00365520310007323. [DOI] [PubMed] [Google Scholar]
- 9.Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 10.Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich S, Goerres GW, Schafer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235–243. doi: 10.1097/01.sla.0000172095.97787.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borg MF, Benjamin CS, Childs WJ. The value of routine computed tomography scanning of the chest in patients presenting with supradiaphragmatic Hodgkin's disease. Australas Radiol. 1993;37:244–248. doi: 10.1111/j.1440-1673.1993.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 13.White PM, Adamson DJ, Howard GC, Wright AR. Imaging of the thorax in the management of germ cell testicular tumours. Clin Radiol. 1999;54:207–211. doi: 10.1016/s0009-9260(99)91152-2. [DOI] [PubMed] [Google Scholar]
- 14.Fleming JB, Cantor SB, Varma DG, Holst D, Feig BW, Hunt KK, et al. Utility of chest computed tomography for staging in patients with T1 extremity soft tissue sarcomas. Cancer. 2001;92:863–868. doi: 10.1002/1097-0142(20010815)92:4<863::aid-cncr1394>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]


