Abstract
Background
As a consequence of continuous technical developments in liver surgery, laparoscopic liver resection (LLR) is increasingly performed worldwide.
Methods
Between January 2004 and December 2011, 265 LLR were performed in 242 patients for various diseases. The experience of LLR is reported focusing on risk factors of conversion and their management.
Results
The overall conversion rate was 17/265 (6.4%), equally distributed over the period of the study. Statistically significant factors for conversion were found to be LLR of the postero-superior (P-S) segments (SI, SIVa; SVII; SVIII) (12.7% converted versus 2.5% non-converted groups, P = 0.01) and a major compared with a minor hepatectomy (15.2% vs. 4.6%, P = 0.02 respectively). A R0 resection was achieved in 93.2% of cases. According to Dindo's classification, complications were recorded as grade I (n = 20); grade II (6); grade III (11) and grade IV(1) events (total morbidity rate of 14%). Univariate analysis identified a major hepatectomy and resection involving P-S segments as prognostic factors for conversion whereas multivariate analysis identified the latter as an independent risk factor [P = 0.003, odds ratio (OR) = 5.9, 95% confidence interval (CI) = 1.8–18.8].
Conclusions
LLR can be safely performed with low overall morbidity. According to this experience and irrespective of the learning curve, resections of P-S segments were identified as an independent risk factor for conversion in LLR.
Introduction
Since the first laparoscopic liver resection (LLR) was performed in 1992, there has been an exponential growth of reports, with more than 4000 worldwide-published procedures to date.1–8 Meticulous knowledge of surgical anatomy of the liver, improvements in peri-operative care, development of newer instruments, enhanced diagnostic imaging and advancement in laparoscopic skills are considered the major advances that have been achieved in this field. The indications for a laparoscopic hepatic resection are usually the same as the standard approach, as stated by the Louisville Statement Consensus Conference.9 Although LLR was initially indicated for benign lesions, port-site cancer seeding has not been observed and the recurrence pattern has been shown to be similar to those described for an open resection of colorectal liver metastases (CRLM) and/or hepatocellular carcinoma (HCC), with outcomes at least as good as those reported for open surgery.10–15 In addition, a laparoscopic live donor hepatectomy has been shown to be feasible especially in left lateral sectionectomy procurement where it looks to be more appropriate compared with laparoscopic-assisted techniques in right lobe living liver donation.5,16–18 Laparoscopic liver surgery is still limited by both the hepatic and laparoscopic experience of the surgeon. In fact, there is a general agreement that LLR should be initiated in centres with expertise in both techniques.9,19 Although no intra-operative mortality has been recorded, the greatest limitation to the expansion of LLR remains the learning curve and, probably, the fear of major intra-operative complications (i.e. massive bleeding) that can put the patient in a life-threatening situation.2,9 The conversion rate is estimated at approximately 4%; however, if needed, this event overrides some of the benefits of the procedure.2
The purpose of this study was to identify predictive factors of conversion to an open approach on 265 laparoscopic liver resections performed over a 8-year period, focusing on causes of conversion, technical issues and outcomes. Assessment of morbidity, peri-operative parameters and outcome analysis are fully provided.
Methods
After approval of the local Independent Research Board, data were collected from the institutional database of patients undergoing liver surgery. Between January 2004 and December 2011, 265 (27.4%) out of 891 liver resections were performed by laparoscopy at the Ghent University Hospital Medical School. According to centre policy, hepatic resections requiring bile duct or vascular reconstruction or focal lesions not amenable to a parenchymal-sparing technique are not considered for a laparoscopic approach. For benign lesions, indications for LLR were liver cell adenomas, symptomatic focal nodular hyperplasia and haemangiomas. Variables considered for the analysis were: gender, age, indication, tumour site and size, type of resection, histology, R0 margins (minimum of 1 mm uninvolved tissue) versus R1 (0 mm margin), operation time, blood loss, overall morbidity and length of post-operative stay. Demographics and indications for LLR are depicted in Table 1. An indication for surgical treatment was made during a multidisciplinary conference which included hepato-biliary surgeons, oncologists, gastroenterologists, radiologists, radiotherapists and pathologists. Few patients were referred with a resectable HCC and cirrhosis, with the majority having multifocal disease and treated by loco-regional therapy or liver transplantation. Complications were scored according to Dindo's classification as follows: Grade I, complication without any deviation from the normal post-operative course or the need for pharmacological treatment or surgical, endoscopic and radiological interventions; Grade II, complications requiring pharmacological treatment with drugs other than such allowed for grade I complications; this includes blood transfusions; Grade III, complications requiring surgical, radiological or endoscopic treatment; Grade IV, life-threatening complications requiring intensive care unit management; and Grade V, death of a patient.20
Table 1.
Patient demographics and indications for a laparoscopic liver resection
| Demographics | N = 265 |
|---|---|
| Age | 55.7 ± 16 |
| Gender, male | 98 (47.5%) |
| Indication for resection | Colorectal metastases (49%) |
| Liver cell adenoma (17.5%) | |
| Focal nodular hyperplasia (12.5%) | |
| HCC (7%) | |
| Other non-CRLM (4%) | |
| Haemangioma (3.5%) | |
| Living liver donation (1.5%) | |
| Cystadenoma (1.5%) | |
| Cholangiocarcinoma (1.1%) | |
| Hydatid cyst (0.8%) | |
| Others (1.6%) | |
| Comorbidity | |
| Cardiac | 61 (23%) |
| Pulmonary | 14 (5.3%) |
| Renal | 3 (1.1%) |
| Diabetes | 17 (6.4%) |
| Previous abdominal surgery | 167 (63%) |
| Previous liver resection | 23 (8.7%) |
| Two-stage hepatectomy | 4 (1.5%) |
Surgical technique
LLR was performed with the patient in the supine and 30° anti-Trendelenburg position and with the surgeon standing between the patient's legs. An intermittent pneumatic compression device was applied to the lower extremities to minimize the risk of a deep venous thrombosis. For lesions situated in Segments V and VI, and for a right hepatectomy, a wedge-shaped cushion was positioned under the patient's right flank with the table turned on its left side. For resection of lesions located in postero-superior (P-S) segments (segments I, IVa, VII, VIII), the patient was turned 2/3 on his left side with the right arm alongside the body if possible; alternatively, with the right arm fixed above the head with special care to avoid nerve lesions. Basically, 4 to 6 port sites (5 mm; 10 mm and two-four 12-mm ports) were inserted in the upper abdominal quadrant: the 12-mm ports were placed to allow insertion of a 30° optical device and the linear stapler; the 10-mm port for the surgical aspirator or harmonic scissors, the 5-mm port was used mainly to allow irrigation and aspiration during surgery, and to hang the liver when necessary. Carbon dioxide pressure for pneumoperitoneum was kept as low as possible, aiming for 10–12 mmHg during hepatic parenchymal transection. The mean central venous pressure (CVP) was maintained at 4–6 mmHg, whereas urinary output was maintained above 0.5 ml/kg/h21. Assessment of the liver surface and surgical margins was undertaken by intra-operative ultrasonography (Aloka SSD 4000; Aloka, Tokyo, Japan). Parenchymal division was almost exclusively performed using a surgical aspirator (CUSA, Excel Valleylab until 2009 and SonoSurg-Olympus to present date) and the harmonic scalpel (Ultracision; Ethicon Endosurgery, Cleveland, OH, USA) for the glissonian approach. The Pringle manoeuver was seldom applied. Bipolar coagulation was used for minor bleeding or oozing. Larger vascular and biliary structures were controlled with endoclips (Hem-o-lock clips; TFX Medical Ltd, Durham, NC, USA) or vascular staplers (EndoGIA; Ethicon). Superficially located lesions were resected using the corkscrew technique.22 The technique for a left lateral sectionectomy has been described elsewhere.23 Left or right hepatectomies were performed with upfront unilateral inflow occlusion by opening of the hilar plate and by division of the hepatic veins (HV) before starting parenchymal division. Finally, the specimen was extracted using a plastic bag through the Pfannestiel incision, with additional port site enlargement if required or by partial opening of a previous abdominal scar.
Statistical analysis
Continuous data are reported as mean ± SD (range) and were compared using the two-side Student's t-test for normally distributed parameters. Continuous data non-normally distributed are reported as median with an interquartile range and were compared using the Wilcoxon–Mann–Whitney test. Comparisons between groups for categorical variables were performed using the χ2 test with Yates' correction or Fisher's exact test when appropriate. To identify variables that were independent predictors of conversion, a logistic regression analysis with backward stepwise selection was constructed, employing those variables with a significant level of P < 0.2. Statistical significance was set at P < 0.05. Statistical analysis was performed by IBM©-SPSS© Statistics 19.0 (SPSS Inc., Chicago, IL, USA).
Results
The volume of laparoscopic procedures progressively increased over the 8-year period from 18 (16.8%) in 2004 to 78 (52.3%) in 2011 (Fig. 1). During the same period, liver resection with open access progressively decreased, and in 2011 the number of LLRs exceeded the open procedures. The number of LLRs for malignant diseases progressively increased from 1 in 2004 to 55 in 2011 whereas the number of LLRs for benign lesions remained stable (Fig. 2). The type of resections is shown in Table 2. The main indications for LLR in patients with benign tumours were represented by 47 liver cell adenomas, 33 focal nodular hyperplasia and 9 haemangiomas. In four cases, the laparoscopic approach was utilized in a living liver donation: two right hepatectomies using the hybrid technique, one left hepatectomy and one left lateral sectionectomy (both fully harvested laparoscopically).17,23 Uncommon indications recorded were Caroli's disease mimicking a peripheral cholangiocarcinoma and post-traumatic liver ischaemia (segments 2 and 3). Colorectal liver metastases (CRLM) were the main indication for malignancy (n = 130), followed by HCC with cirrhosis (n = 18) and breast metastases (n = 6). All cirrhotic patients were Child–Pugh A score. Patients with malignancy showed a higher rate of cardiac co-morbidity with respect to the benign group (30.1% versus 13%, P = 0.004, respectively). Previous abdominal surgery was recorded in 84% of patients with malignancy compared with 37% in patients with benign lesions (P < 0.001). LLR was performed as a repeat hepatectomy in 15 (11.6%) patients with CRLM and in five (5.4%) with benign disease. A two-stage LLR was performed in four patients with CRLM unfit for one-time resection. Each patient underwent ligation and alcoholization of the right portal vein branch with concomitant wedge resection of up to three lesions among segments 2, 3 and 4. The rate of major hepatectomy was 17.4%: a right and left hepatectomy were the more frequent major procedures carried out. No chemotoxicity-related aborted resection was recorded in patients receiving neoadjuvant chemotherapy. Perioperative data are shown in Table 3.
Figure 1.
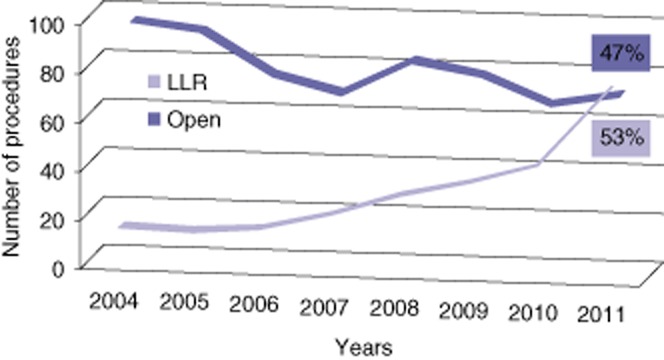
Evolution of a laparoscopic liver resection (LLR) during the time (2004–2011)
Figure 2.
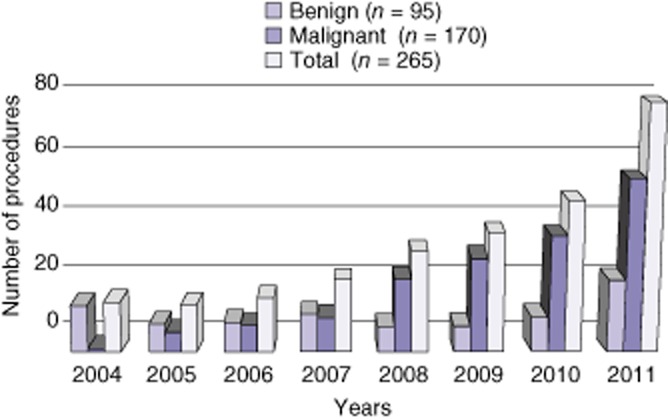
Evolution of a laparoscopic liver resection (LLR) according to the indication
Table 2.
Type of hepatectomy
| Type of resection | (N = 265) |
|---|---|
| Major hepatectomy | 46 (17.4%) |
| Left Hepatectomy | 21 |
| Right Hepatectomy | 18 |
| Right extended hepatectomy | 2 |
| Mixed segments (>2)a | 5 |
| Minor hepatectomy | 219 (82.6%) |
| Monosegmentectomy | 62 |
| Wedge resection | 55 |
| Left lateral segmentectomy | 44 |
| Bisegmentectomy | 35 |
| Mixed segments (≤2)a | 23 |
Mixed segments include resection of non-adjacent segments.
Table 3.
Peri-operative data
| Parameter | N = 265 |
|---|---|
| Mean anaesthesia time (SD) (range) (min) | 345 ± 119 (95–720) |
| Mean surgical time (min)(range) | 254 ± 111 (50–680) |
| Pringle manoeuver (n) | 17 (6.4%) |
| Mean blood loss (SD) (range)(ml) | 172 ± 150 (10–1250) |
| Overall number of conversions (rate) | 17 (6.4%) |
| Specimen extraction | |
| Pfannestiel incision | 118 (44.5%) |
| Trocar incision | 131 (49.5%) |
| Previous incision | 14 (5%) |
| New incision | 2 (1%) |
| Mean post-operative stay (SD) (range) (days) | 5.5 ± 3.6 (1–38) |
Histology assessment
Multiple nodules were present in 82 (30.9%) patients, whereas the overall mean size was 48.9 ± 35.8 mm (range 4–200). Benign nodules were significantly larger with respect to malignant nodules (58.7 ± 38 mm versus 43.3 ± 33 mm, P < 0.001). The maximum number of resected nodules was not related to the extent of hepatectomy (7 in major and minor hepatectomies). The overall median-free margin was 3 mm (1–8): a R0 resection was achieved in 93.5% of cases with malignancies (Table 4).
Table 4.
Pathology report. Living liver grafts, Caroli and post-traumatic liver ischaemia excluded
| Parameter | Benign (N = 89) | Malignant (N = 170) | P |
|---|---|---|---|
| Mean size (mm) | 58.7 ± 38 (8–200) | 43.3 ± 33 (4–196) | <0.001 |
| Multiple lesions | 24 (27%) | 48 (28%) | 0.44 |
| Mean lesion number | 1.34 ± 0.7 (1–5) | 1.64 ± 1.2 (1–7) | 0.029 |
| Bilobar | 8 (9%) | 27 (16%) | 0.12 |
| Free resection margins | NA | 159 (93.5%) | NA |
| Mean free margins (mm) | NA | 3 (1–8) | NA |
NA, not applicable.
Morbidity
Thirty-eight complications were recorded in 30 patients (11.3%). No 3-month mortality was seen. Morbidity was very low and equally distributed between the minor and major resections, and between the anterior and lateral (A-L) and P-S segments. Complications recorded included grade I (n = 20); grade II (6); grade III (11); and grade IVa (1). Specific post-operative complications were biliary fistulae (4; 1.5%), whereas a pneumothorax was recorded in LLR of the P-S segments after intercostal positioning of port sites (3; 1.1%, Table 5). In patients with CRLM, 1- to 3-year disease-free survival rates were 81% and 41%, respectively (median follow-up of 24 months, 44% of total recurrence rate).
Table 5.
Overall morbidity according to Dindo's classification and specific events
| Morbidity | Total | Minor/major resection | A-L/P-S segments |
|---|---|---|---|
| Minor (grade 1) | 20 | 14/6 | 9/11 |
| Major (grade 2-4) | 18 | 14/4 | 9/9 |
| Pneumonia | 5 (1.9%) | 4/1 | 2/3 |
| Ileus | 4 (1.5%) | 2/2 | 1/3 |
| Biliary leak | 4 (1.5%) | 3/1 | 2/2 |
| Ascites | 4 (1.5%) | 3/1 | 2/2 |
| Urinary tract infection | 3 (1.1%) | 1/2 | 1/2 |
| Pneumothorax | 3 (1.1%) | 3/0 | 0/3 |
| Bleeding | 3 (1.1%) | 2/1 | 2/1 |
| Pleural effusion | 3 (1.1%) | 1/2 | 2/1 |
| Fluid collections | 2 (0.7%) | 2/0 | 1/1 |
| Renal failure | 2 (0.7%) | 2/0 | 2/0 |
| Lung embolism | 1 (0.4%) | 0/1 | 0/1 |
| Wound haematoma | 1 (0.4%) | 1/0 | 1/0 |
| Pancreatitis | 1 (0.4%) | 0/1 | 1/0 |
| Cholangitis | 1 (0.4%) | 1/0 | 0/1 |
| ARDS | 1 (0.4%) | 0/1 | 0/1 |
ARDS, ac ute respiratory distress syndrome.
Conversion to a standard approach
Conversion was required in 17/265 (6.4%) patients (Table 6). A hand-assisted procedure was performed in only one case. Bleeding episodes were the main cause of conversion (3%). There were no significant differences in the conversion rate when comparing age, gender, learning curve (first 60 cases), previous abdominal surgery, number of lesions, tumour size, diagnosis, repeat hepatectomy, liver cirrhosis, neoadjuvant chemotherapy and a body mass index of more than 28. Patients experiencing a failure of the LLR approach had a significantly longer hospital stay, which was not related to an increased complication rate (mean of 11.1 ± 9.6 days versus 5.2 ± 2.5 days; P < 0.001). Predictive factors for conversion were found to be LLR of the P-S segments in the converted compared with the non-converted group (12.7% versus 2.5%, P = 0.01, respectively) and major hepatectomy compared with minor hepatectomy (15.2% versus 4.6%, P = 0.02). Multivariate analysis revealed resections involving P-S segments as an independent variable of conversion [P = 0.003, odds ratio (OR) = 5.9, 95% confidence interval (CI) = 1.8–18.8] (Table 7).
Table 6.
Reasons for conversion to an open approach
| No. LLR | Reason | Type of hepatectomy | Tumour position | Diagnosis |
|---|---|---|---|---|
| #13 | Bleeding | Monosegmentectomy | S5 | FNH |
| #30 | Bleeding | Left Hepatectomy | S3/S4 | CRLM |
| #31 | Bleeding | Bisegmentectomy | S5-S6 | FNH |
| #45 | Oncological | Segmentectomy | S7 | CRLM |
| #60 | Gas embolism | Bisegmentectomy | S6-7 | CRLM |
| #65 | Bleeding | Wedge | S4-8 | CRLM |
| #101 | Lack of progression | Right Hepatectomy | S5-6-8 | CRLM |
| #103 | Bleeding | Segmentectomy | S7 | CRLM |
| #109 | Anatomical | Right Hepatectomy | S5-7-8 | CRLM |
| #133 | Bowel perforation | Wedge | S6-S7 | CRLM |
| #135 | Stapler failure/bleeding | Left Hepatectomy | S2-3-4 | HEMANGIOMA |
| #142 | Oncological | Wedge | S2-3 | HCC |
| #167 | Bleeding | Right Hepatectomy | S5-6-7 | CRLM |
| #161 | Oncological | Bisegmentectomy | S2-5-6 | CRLM |
| #171 | Bleeding | Right Hepatectomy | S5-8 | ADENOMA |
| #185 | Bleeding | Segmentectomy | S8 | CRLM |
| #212 | Oncological | Segmentectomy | S1 | CRLM |
LLR, lap aroscopic liver resection.
Table 7.
Logistic regression analysis of risk factors for conversion
| Parameter | Fully LLR (n = 248) | Conversions (n = 17) | HR | 95% CI | P |
|---|---|---|---|---|---|
| Age ≥60 years | 125 (50.4%) | 10 (58.8%) | 1.48 | 0.36–6.0 | 0.58 |
| Gender (male) | 116 (46.8%) | 10 (58.8%) | 1.94 | 0.53–7.2 | 0.32 |
| Previous operation | 152 (61.3%) | 15 (88.2%) | 4.9 | 0.87–27 | 0.07 |
| Major hepatectomy | 39 (15.7%) | 7 (41.2%) | 3.34 | 0.94–11.9 | 0.02 |
| Lesion number (>1) | 75 (31.6%) | 7 (41.2%) | 1.26 | 0.40–3.9 | 0.69 |
| Tumour size ≥50 mm | 91 (38.2%) | 5 (29.4%) | 0.58 | 0.16–2.0 | 0.39 |
| Diagnosis (malignant) | 152 (61.3%) | 11 (64.7%) | 0.24 | 0.05–1.2 | 0.09 |
| Neo-adjuvant chemotherapy | 96 (38.7%) | 8 (47.1%) | 0.50 | 0.14–2.7 | 0.52 |
| BMI (>28) | 120 (48.4%) | 9 (52.9%) | 1.15 | 0.35–3.7 | 0.78 |
| Repeat hepatectomy | 21 (8.5%) | 2 (11.8%) | 1.25 | 0.23–6.7 | 0.79 |
| Posterior–superior segments | 89 (35.9%) | 13 (76.5%) | 5.87 | 1.45–19.8 | 0.01 |
| Resection close to major HV | 113 (45.7%) | 7 (41.2%) | 0.72 | 0.18–2.9 | 0.65 |
| Learning curve (≤60 LLR) | 55 (22.2%) | 4 (23.5%) | 2.48 | 0.57–10.8 | 0.22 |
| Multivariate analysis | |||||
| Posterior–superior segments | – | – | 5.9 | 1.8–18.8 | 0.003 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; HV, hepatic veins; LLR, laparoscopic liver resection.
Discussion
Although the development of LLR has been limited over time, LLR is a well-established worldwide procedure actually reported more for malignancies rather than for benign disease.2–7,9–13,16–19,22–24 The present series showed that LLR is safe and feasible for different liver diseases involving minimal blood loss, with very few complications and characterized by a short hospital stay. Although conversion is not considered as a failure of the laparoscopic approach, this event entails that some benefits of the laparoscopic approach are lost.9 Indeed, a delay in conversion in difficult cases or bleeding is related specifically to more complications and a longer hospital stay.25 Overall reported conversion rates are about 4% and bleeding is reported as the most common cause for this (range 2%–5%), accounting for as many as 34% of patients that required conversion.1,12,26 However, according to this review, some of the 23 instances of revision were not documented, leaving uncertainty about their cause. Undoubtedly, one of the main disadvantages of LLR involves a significant learning curve, which can account for initially higher conversion rates. According to a statistical study, a minimum number of 60 cases of LLR is needed to significantly reduce the risk of conversion.27
Multivariate analysis identified the P-S segments as a predictive factor of conversion irrespective of the learning curve: 76.5% compared with 35.9%, respectively, for converted compared with non-converted patients (P = 0.01). According to our initial experience in the first 60 cases, very few lesions (22%) were located in P-S segments, indicating the precautionary need for initial patient selection. Moreover, the second risk factor for conversion that we found resulted in laparoscopic major hepatectomy that always includes a P-S segment (P = 0.02). These segments are probably more difficult to access because the angle of the instruments is limited by the costal margin, making bleeding control and tumour margins more challenging.1 A second reason could be represented by our policy of performing a parenchymal saving procedure in any case. According to Cho et al., as much as 71% of lesions in P-S segments required a major hepatectomy.28 In our experience, by attempting a parenchymal-sparing approach, only one-third of the P-S lesions were resected by a major hepatectomy, accounting for a total rate of 17.4%, less than in other reported major experiences.1,5,7,13,28 However, limited resections in P-S segments could turn out to be more complex than a straightforward right or left hepatectomy.1,28,29A robotic-assisted liver resection could improve the approach to P-S segments eventually limiting the conversion rate; however, no experiences are available to date.
Bleeding episodes were the main cause of conversion in our series. To laparoscopically manage acute bleeding, the intermittent Pringle manoeuver, compression with gauze, the use of clips or staplers and, if necessary, the hand-assisted technique have been proposed.26,30 In addition, close cooperation with the anaesthetist is mandatory. The Trendelenburg position may reduce the risk of gas embolism by increasing the CVP when a tear in the major intrahepatic vessels occur.31 Maintenance of low CVP conditions is easily achieved by positioning of the patient in anti-Trendelenburg during surgery. Blood volume is simply pooled in the lower limbs, readily achieving low CVP conditions. When necessary, the blood volume can immediately be transferred by changing to the Trendelenburg position. Others argue that having some intravascular filling pressure is a safe condition to counteract a life-threatening situation such as acute bleeding from the hepatic veins.32,33 Although the clinical consequences of a gas embolism is not an issue, reduction of the intra-abdominal pressure could be recommended as a strategy to prevent this.32,34,35 In the case of acute bleeding, it is advisable to laparoscopically manage the critical moment by grasping the vein, and applying sutures. This approach makes it possible to reduce bleeding while converting. Once the decision to convert has been made, filling the abdominal cavity with saline solution while opening the cavity may be helpful. One of the techniques that could be used in acute bleeding is hand-assisted laparoscopic surgery, which has been reported in up to 18% of overall conversions.2 Although the hand-assisted technique represents a useful approach in elective surgery, increasing the tactile feedback and facilitating compression of the bleeding segment was used only once.36 It is possible that this procedure may help when in a hurry with the aim to control the bleeding while deciding on how to go on with the resection (conversion or not). High intra-abdominal pressure has been suggested to increase the haemostatic effect of CO2 especially to control oozing but has recently been discouraged owing to its increased risk of gas embolism, with deleterious impairment of kidney perfusion and also of liver regeneration as demonstrated in two different experimental animal models.37,38 However, when acute bleeding is difficult to manage by laparoscopy, conversion is the only procedure that ensures the best results avoiding life-threatening situations.
The decision to convert case 13 even after trying a hand-assisted procedure was followed by a massive bleed leading to acute kidney insufficiency. The liberal use of the harmonic scalpel instead of a more accurate and meticulous dissection with the CUSA (cases 30, 31 and 103) predisposed to a lesion of the major hepatic veins difficult to manage laparoscopically. Stapler misfire is an event which may occur and has already been described elsewhere.5,39 Massive bleeding originated by stapler failure makes laparoscopic management very difficult, especially if this occurs at the level of major hepatic veins. Hilar bleeding could be treated with sutures or by positioning additional clips.
Adequate intra-operative tumour assessment was provided by laparoscopy, resulting, also in our experience, in a high percentage of free margins confirmed by the pathology report and no port-site metastases at follow-up. Indeed, our results are in line with similar published experiences. Most recent data have shown that oncological outcomes of laparoscopic surgery in selected patients produce equivalent results to open surgery.10–14,40,41 However, four patients (23.5%) required a conversion for oncological reasons: three of them because of uncertainty about the free-margins; the last because of a ‘missed’ lesion during the US evaluation. One of the main problems in LLR is the intra-operative ultrasonography evaluation in assessing safe tumoural margins while dividing the parenchymal and by the bad definition of a lesion previously treated by chemotherapy (missing lesions). According to the concept of a parenchymal-sparing resection in a patient with CRLM, we preferred to do a conversion and thus avoid a larger resection. To solve the problems related to a better definition of tumoural lesions, intra-operative contrast-enhanced ultrasound could be useful in assessing not only tumour margins but also in visualizing missing lesions.42 Although such software has shown that it is possible to make it easier to define intra-operatively the position of the tumour through the laparoscopic view, the absence of a real-time 3-D identification forces us to use the major portal pedicles or hepatic veins as the only landmarks. The use of optical and electromagnetic sensors could allow a continuous tracking of the liver with compensation of breathing artifacts.43 Laparoscopic surface scanning is able to predict the location of intraparenchymal targets with a more precise resection line distant from the major veins leading to a more parenchymal-sparing resection and potentially reducing the conversion rate for bleeding and oncological reasons.44,45 Post-operative outcomes were very favourable with half of the complications classified as grade 1 and only four biliary leaks (1.5%). One early concern was whether LLR would result in an increased incidence in bile leaks, particularly with the liberal use of staplers to transect the parenchymal. We think that this rather low incidence of biliary leaks recorded is probably as a result of the accuracy of the liver dissection technique using the CUSA associated with a limited use of linear staplers. When approaching P-S segments, we believe that the higher positioning of some trocars (i.e. between the last ribs) could be helpful when dividing the liver parenchymal with the CUSA or the harmonic scalpel, because this increases the viewing and working angle. However, a pneumothorax can occur during surgery or in the immediate post-operative period. To avoid a lung lesion, we recommend inserting the trocar at the end of the expiratory phase and, if a tension pneumothorax appears, the anaesthesiologist can, if necessary, increase the positive end-expiratory pressure concomitantly to limit the haemodynamic effects of the insufflated CO246.
In conclusion, LLR is safe and feasible for the resection of all liver segments, with a favourable outcome and a low morbidity. The need for conversion is not abolished by the learning curve and remains a continuous challenge, especially when approaching P-S segments. Indeed, even in expert hands the need for conversion remains likely because as their experience grows surgeons are pushing the limits towards more complex resections.28 Further efforts should be addressed at reducing the conversion rate.
Conflicts of interest
None declared.
References
- 1.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 3.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 4.Ardito F, Tayar C, Laurent A, Karoui M, Loriau J, Cherqui D. Laparoscopic liver resection for benign disease. Arch Surg. 2007;142:1188–1193. doi: 10.1001/archsurg.142.12.1188. discussion 1193. [DOI] [PubMed] [Google Scholar]
- 5.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392. doi: 10.1097/SLA.0b013e318146996c. discussion 392–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22:38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 7.Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–486. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 8.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. 1992;6:97–98. [Google Scholar]
- 9.Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 10.Belli G, Limongelli P, Fantini C, D'Agostino A, Cioffi L, Belli A, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041–1048. doi: 10.1002/bjs.6680. [DOI] [PubMed] [Google Scholar]
- 11.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 12.Kazaryan AM, Marangos IP, Rosok BI, Rosseland AR, Villanger O, Fosse E, et al. Laparoscopic resection of colorectal liver metastases: surgical and long-term oncologic outcome. Ann Surg. 2010;252:1005–1012. doi: 10.1097/SLA.0b013e3181f66954. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 14.O'Rourke N, Shaw I, Nathanson L, Martin I, Fielding G. Laparoscopic resection of hepatic colorectal metastases. HPB. 2004;6:230–235. doi: 10.1080/13651820410023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki A, Nitta H, Otsuka K, Takahara T, Nishizuka S, Wakabayashi G. Ten-year experience of totally laparoscopic liver resection in a single institution. Br J Surg. 2009;96:274–279. doi: 10.1002/bjs.6472. [DOI] [PubMed] [Google Scholar]
- 16.Soubrane O, Cherqui D, Scatton O, Stenard F, Bernard D, Branchereau S, et al. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg. 2006;244:815–820. doi: 10.1097/01.sla.0000218059.31231.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker TB, Jay CL, Ladner DP, Preczewski LB, Clark L, Holl J, et al. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery. 2009;146:817–823. doi: 10.1016/j.surg.2009.05.022. discussion 823–815. [DOI] [PubMed] [Google Scholar]
- 18.Koffron AJ, Kung RD, Auffenberg GB, Abecassis MM. Laparoscopic liver surgery for everyone: the hybrid method. Surgery. 2007;142:463–468. doi: 10.1016/j.surg.2007.08.006. discussion 468 e461–462. [DOI] [PubMed] [Google Scholar]
- 19.Edwin B, Nordin A, Kazaryan AM. Laparoscopic liver surgery: new frontiers. Scand J Surg. 2011;100:54–65. doi: 10.1177/145749691110000110. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis CL. What's in a name, AKI? Liver Transpl. 2009;15:455–456. doi: 10.1002/lt.21754. [DOI] [PubMed] [Google Scholar]
- 22.Makdissi FF, Surjan RC, Machado MA. Laparoscopic enucleation of liver tumors. Corkscrew technique revisited. J Surg Oncol. 2009;99:166–168. doi: 10.1002/jso.21206. [DOI] [PubMed] [Google Scholar]
- 23.Troisi RI, Van Huysse J, Berrevoet F, Vandenbossche B, Sainz-Barriga M, Vinci A, et al. Evolution of laparoscopic left lateral sectionectomy without the Pringle maneuver: through resection of benign and malignant tumors to living liver donation. Surg Endosc. 2011;25:79–87. doi: 10.1007/s00464-010-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, et al. Laparoscopic liver resection of benign liver tumors. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 25.Costi R, Scatton O, Haddad L, Randone B, Andraus W, Massault PP, et al. Lessons learned from the first 100 laparoscopic liver resections: not delaying conversion may allow reduced blood loss and operative time. J Laparoendosc Adv Surg Tech A. 2012;22:425–431. doi: 10.1089/lap.2011.0334. [DOI] [PubMed] [Google Scholar]
- 26.Abu Hilal M, Underwood T, Taylor MG, Hamdan K, Elberm H, Pearce NW. Bleeding and hemostasis in laparoscopic liver surgery. Surg Endosc. 2010;24:572–577. doi: 10.1007/s00464-009-0597-x. [DOI] [PubMed] [Google Scholar]
- 27.Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250:772–782. doi: 10.1097/SLA.0b013e3181bd93b2. [DOI] [PubMed] [Google Scholar]
- 28.Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery. 2008;144:32–38. doi: 10.1016/j.surg.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, et al. Safe and feasible inflow occlusion in laparoscopic liver resection. Surg Endosc. 2009;23:906–908. doi: 10.1007/s00464-008-0257-6. [DOI] [PubMed] [Google Scholar]
- 30.Gumbs AA, Gayet B, Gagner M. Laparoscopic liver resection: when to use the laparoscopic stapler device. HPB. 2008;10:296–303. doi: 10.1080/13651820802166773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirski MA, Lele AV, Fitzsimmons L, Toung TJ. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106:164–177. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Valenza F, Chevallard G, Fossali T, Salice V, Pizzocri M, Gattinoni L. Management of mechanical ventilation during laparoscopic surgery. Best Pract Res Clin Anaesthesiol. 2010;24:227–241. doi: 10.1016/j.bpa.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Saner FH, Cicinnati VR, Sotiropoulos G, Beckebaum S. Strategies to prevent or reduce acute and chronic kidney injury in liver transplantation. Liver Int. 2012;32:179–188. doi: 10.1111/j.1478-3231.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- 34.Bazin JE, Gillart T, Rasson P, Conio N, Aigouy L, Schoeffler P. Haemodynamic conditions enhancing gas embolism after venous injury during laparoscopy: a study in pigs. Br J Anaesth. 1997;78:570–575. doi: 10.1093/bja/78.5.570. [DOI] [PubMed] [Google Scholar]
- 35.Jayaraman S, Khakhar A, Yang H, Bainbridge D, Quan D. The association between central venous pressure, pneumoperitoneum, and venous carbon dioxide embolism in laparoscopic hepatectomy. Surg Endosc. 2009;23:2369–2373. doi: 10.1007/s00464-009-0359-9. [DOI] [PubMed] [Google Scholar]
- 36.Poultsides G, Brown M, Orlando R., 3rd Hand-assisted laparoscopic management of liver tumors. Surg Endosc. 2007;21:1275–1279. doi: 10.1007/s00464-006-9174-8. [DOI] [PubMed] [Google Scholar]
- 37.Eiriksson K, Fors D, Rubertsson S, Arvidsson D. High intra-abdominal pressure during experimental laparoscopic liver resection reduces bleeding but increases the risk of gas embolism. Br J Surg. 2011;98:845–852. doi: 10.1002/bjs.7457. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt SC, Schumacher G, Klage N, Chopra S, Neuhaus P, Neumann U. The impact of carbon dioxide pneumoperitoneum on liver regeneration after liver resection in a rat model. Surg Endosc. 2010;24:1–8. doi: 10.1007/s00464-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 39.Boggi U, Moretto C, Vistoli F, D'Imporzano S, Mosca F. Robotic suture of a large caval injury caused by endo-GIA stapler malfunction during laparoscopic wedge resection of liver segments VII and VIII en-bloc with the right hepatic vein. Minim Invasive Ther Allied Technol. 2009;18:306–310. doi: 10.1080/13645700903201001. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen KT, Geller DA. Outcomes of laparoscopic hepatic resection for colorectal cancer metastases. J Surg Oncol. 2010;102:975–977. doi: 10.1002/jso.21655. [DOI] [PubMed] [Google Scholar]
- 42.Martin RC, 2nd, Reuter NP, Woodall C. Intra-operative contrast-enhanced ultrasound improves image enhancement in the evaluation of liver tumors. J Surg Oncol. 2010;101:370–375. doi: 10.1002/jso.21511. [DOI] [PubMed] [Google Scholar]
- 43.Cash DM, Miga MI, Glasgow SC, Dawant BM, Clements LW, Cao Z, et al. Concepts and preliminary data toward the realization of image-guided liver surgery. J Gastrointest Surg. 2007;11:844–859. doi: 10.1007/s11605-007-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marvik R, Lango T, Tangen GA, Andersen JO, Kaspersen JH, Ystgaard B, et al. Laparoscopic navigation pointer for three-dimensional image-guided surgery. Surg Endosc. 2004;18:1242–1248. doi: 10.1007/s00464-003-9190-x. [DOI] [PubMed] [Google Scholar]
- 45.Rauth TP, Bao PQ, Galloway RL, Bieszczad J, Friets EM, Knaus DA, et al. Laparoscopic surface scanning and subsurface targeting: implications for image-guided laparoscopic liver surgery. Surgery. 2007;142:207–214. doi: 10.1016/j.surg.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Fellahi JL, Caille V, Charron C, Daccache G, Vieillard-Baron A. Hemodynamic effects of positive end-expiratory pressure during abdominal hyperpression: a preliminary study in healthy volunteers. J Crit Care. 2012;27:33–36. doi: 10.1016/j.jcrc.2011.03.003. [DOI] [PubMed] [Google Scholar]


