Abstract
Objective
This study was carried out to determine relative survival rates and trends in outcomes in patients who underwent resection of periampullary adenocarcinomas (PACs) with curative intent at a single institution over the last three decades.
Methods
From 1980 to 2011, 2564 pancreaticoduodenectomies (PDs) were performed for PACs. Pathological diagnosis, therapy and survival were retrospectively analysed.
Results
The primary sites included the pancreas (66%), ampulla (16%), bile duct (12%) and duodenum (6%). Operation volume increased from 11 per year in the 1980s to 135 per year in the 2000s (P < 0.001). Patients in the 1980s were younger (median age: 64 years; range: 33–90 years) than those in the 1990s (median age: 68 years; range: 31–103 years) and 2000s (median age: 68 years; range: 24–93 years) (P < 0.001). Over time, the frequency of a diagnosis of pancreatic cancer arising from intraductal papillary mucinous neoplasm increased from 2% in the 1980s to 8% in the 2000s (P < 0.001). The rate of 30-day mortality after surgery in the 1980s was 2%, which was similar to rates in the 1990s (1%) and 2000s (1%). Survival in each type of PAC did not change over time. Pancreatic cancer was associated with the worst survival (median survival: 19 months) compared with adenocarcinomas of the ampulla (median survival: 47 months), bile duct (median survival: 23 months) and duodenum (median survival: 54 months) (P < 0.001).
Conclusions
There are significant differences among PACs in longterm survival following PD. Although the numbers of patients undergoing safe resection have increased, overall longterm outcomes have not improved significantly.
Introduction
Periampullary adenocarcinomas (PACs) are biologically distinct neoplasms that arise in the epithelia of the pancreatic duct, distal bile duct, ampulla or duodenum.1 Despite their similar histological appearance and close proximity in tissue of origin, longterm survival following resection of each of the different PACs varies greatly.2 Pancreatic adenocarcinoma is the most common of all periampullary cancers, accounting for approximately 44 000 new patients each year in the USA, followed by ampullary cancer, distal cholangiocarcinoma and duodenal cancer.3–9 Many patients with periampullary cancers are found to have locally advanced or metastatic disease at diagnosis that precludes surgical resection and are thus unlikely to be curable. In patients with localized periampullary cancer, pancreaticoduodenectomy (PD) is the only treatment modality to offer a chance for cure.10,11 Historically, 5-year estimated survival in patients undergoing resection of periampullary cancers has been poor and ranges from approximately 20% in pancreatic adenocarcinoma to 50% in duodenal adenocarcinoma.2
Although studies have reported outcomes for the individual PACs,2,12–14 relative outcomes in patients undergoing resection for PACs during the contemporary period are not well characterized. Moreover, recent trends in survival and outcomes following resection of PACs over time have not been well documented. Over the past three decades, numerous studies have reported improvements in operative mortality,5,13,15 preoperative staging and selection, and adjuvant therapy16–18 for periampullary cancers. With these presumed advances, the hypothesis of this study was that outcomes in patients undergoing potentially curative resections of periampullary cancers might have improved since the 1980s.
The purpose of this study was to determine relative survival and trends in outcome using a large cohort of patients who underwent resection of a PAC with curative intent at a single institution over the last three decades.
Materials and methods
Inclusion criteria and data collection
All patients undergoing PD for a PAC between 1980 and 2011 at the Johns Hopkins Hospital were identified from a prospectively maintained database. Periampullary adenocarcinomas were defined as adenocarcinomas originating in the epithelia of the ampulla of Vater, distal bile duct (distal cholangiocarcinoma), the proximal–mid-duodenum, or the head of pancreas. All histological variants of pancreatic adenocarcinoma such as tubular, intraductal papillary mucinous neoplasm (IPMN)-associated colloid and IPMN-associated tubular adenocarcinoma were included. Patients undergoing resection of other pancreatic neoplasms, such as neuroendocrine tumours, were excluded from this analysis.
Patient demographics, tumour pathological staging, surgical treatment-related variables and follow-up data were retrieved from a prospectively maintained database. Survival data were also confirmed from the social security death index.
Patient factors evaluated included age and gender. Treatment factors included the type of operation, necessity for blood vessel reconstruction, and complications. Postoperative pancreatic fistula (POPF) was defined according to the International Study Group on Pancreatic Fistula (ISGPF) definition.19 In brief, drain amylase was checked when the patient was on regular diet. Postoperative PF was diagnosed when drain amylase was three times higher than the upper limit of normal serum at any volume on or after postoperative day 3. Delayed gastric emptying (DGE) was defined as an inability to return to standard diet by the end of the first postoperative week following PD or the reinsertion of a nasogastric tube prior to this period.20 Ninety-day mortality was defined as death within 90 days of surgery.
Pathological factors evaluated included location of tumour, tumour–node–metastasis (TNM) stage, margin status, and histological differentiation (good, moderate, poor). The primary pathological diagnosis and the extent of disease were determined using the standard approach as detailed previously.21 In brief, the location of the tumour was determined grossly and microscopically. All four periampullary components were evaluated for in situ carcinoma. Margins assessed included the pancreatic neck resection margin, biliary margin, bowel margins and uncinate margin. Maximal tumour size was determined and defined as the maximum diameter at pathological examination. The lymph node ratio (LNR) was defined as the number of nodes involved divided by the number of lymph nodes examined. Microvascular invasion was defined as microscopic tumour invasion identified in the microvascular structure of the surrounding normal tissue, which was contiguous to the tumour.
For the trend analysis, the cohort was divided into three groups by decade (1980–1989, 1990–1999 and 2000–2011).
The study was approved by the institutional internal review board.
Statistical analysis
spss Version 16.0 (SPSS, Inc., Chicago, IL, USA) was used to analyse and calculate data. Survival curves were estimated using the Kaplan–Meier method and compared using the Breslow test. The interquartile range (IQR) is equal to the difference between the upper and lower quartiles. Continuous variables were expressed as medians and ranges and compared using the Mann–Whitney test. Categorical variables were compared using a chi-squared test (or Fisher's exact test). Overall survival (OS) was computed from the time of operative resection to the date of last follow-up. A P-value of <0.05 was considered to indicate statistical significance.
Multivariate analysis was undertaken using variables from univariate analysis most likely to impact survival (i.e. P < 0.05) using the Cox proportional hazards method. For this multivariate analysis, death within 30 days of the operation was excluded to allow the Cox regression model to accurately identify predictors of survival related to the malignancy.
Results
Comparisons and trends in patient and operative characteristics
Between 1980 and 2011, 2564 PDs were performed for PACs at the Johns Hopkins Hospital. These included 1688 adenocarcinomas of the head of the pancreas (66%), 401 of the ampulla of Vater (16%), 317 of the distal common bile duct (12%), and 158 of the duodenum (6%). Patient demographic data are shown in Table 1. The median age of the entire cohort was 67 years (range: 24–103 years) and 55% were male. Distal bile duct adenocarcinomas occurred significantly more often in male (62%) than in female patients (P = 0.006).
Table 1.
Demographic data for patients with periampullary adenocarcinomas (n = 2564)
| Ampullary | Bile duct | Duodenal | Pancreatic | P-value | |
|---|---|---|---|---|---|
| (n = 401) | (n = 317) | (n = 158) | (n = 1688) | ||
| Age, years, median (range) | 68 (29–90) | 68 (34–92) | 67 (24–103) | 67 (31–93) | 0.49 |
| Male gender, n (%) | 230 (57%) | 196 (62%) | 87 (55%) | 899 (53%) | 0.33 |
| Tumour size, cm, median (range) | 2.1 (0–6.6) | 2.1 (0–7) | 3.7 (0.2–13) | 3.1 (0–17) | |
| Differentiation, n (%) | |||||
| Good | 18 (4%) | 13 (4%) | 4 (3%) | 68 (4%) | |
| Moderate | 218 (54%) | 166 (52%) | 87 (55%) | 898 (53%) | |
| Poor or anaplastic | 138 (34%) | 149 (47%) | 52 (33%) | 653 (39%) | |
| Unknown | 26 (6%) | 19 (6%) | 15 (9%) | 69 (4%) | |
| Microvascular invasion, n (%) | 129 (32%) | 93 (29%) | 45 (28%) | 671 (40%)a | <0.001 |
| Perineural invasion, n (%) | 124 (31%) | 190 (60%) | 47 (30%) | 1164 (69%) | |
| Lymph node ratio, median | 0.07 | 0.08 | 0.07 | 0.14a | <0.001 |
Patients with pancreatic adenocarcinoma were more likely to have microvascular invasion than patients with other types of periampullary adenocarcinoma (P < 0.001). Perineural invasion was also more common in pancreatic adenocarcinoma than in cholangiocarcinoma (P = 0.0016) and ampullary and duodenal adenocarcinoma (P < 0.001).
Patients in the 1980s were younger (median age: 64 years; range: 33–90 years) than those in the 1990s (median age: 68 years; range: 31–103 years) (P < 0.001) and 2000s (median age: 68 years; range: 24–93 years) (P < 0.001). Over time, the frequency of PD for pancreatic adenocarcinoma arising from IPMN increased from 2% (two patients) in the 1980s to 6% (101 patients) in the 2000s (P < 0.001).
With the growth in the volume of PDs (Fig. 1), surgical complexity also increased. The increase in venous resection and reconstruction was related to the increase in the number of patients undergoing resection of pancreatic adenocarcinoma rather than other periampullary cancers (P < 0.01) (Table 2).
Figure 1.
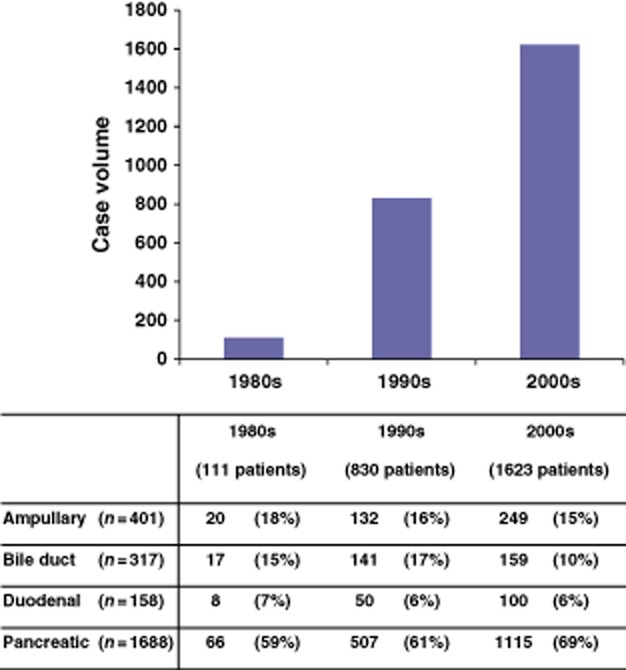
Rates of pancreaticoduodenectomy in patients with periampullary adenocarcinomas during 1980–2011. Case volume increased progressively during each of the decades (P < 0.001). Patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma increased significantly not only in number (P < 0.001), but also in proportion (P = 0.003)
Table 2.
Incidences of major venous resection during pancreaticoduodenectomy (PD) in patients with periampullary adenocarcinomas
| Ampulla | Distal bile duct | Duodenum | Head of pancreas | Total | |
|---|---|---|---|---|---|
| (n = 401) | (n = 317) | (n = 158) | (n = 1688) | (n = 2564) | |
| 1980s (n = 111), n (%) | 0 | 0 | 1 (2%) | 0 | 1 (1%) |
| 1990s (n = 830), n (%) | 0 | 0 | 0 | 15 (3%) | 15 (2%) |
| 2000s (n = 1623), n (%) | 3 (1%) | 8 (5%) | 1 (2%) | 66 (6%) | 78 (5%) |
| Total | 3 (1%) | 8 (3%) | 2 (1%) | 81 (5%) | |
More vein resections were performed during PD in the 2000s than in the previous two decades (P < 0.001). Pancreaticoduodenectomy in pancreatic cancer involved more vein resection than PD in ampullary or duodenal cancer. (P < 0.01).
Comparisons and trends in operative complications
The overall complication rate in the entire cohort was 52%. The most common complications included DGE (16%), POPF (9%) and wound-related complications (12%). Relative frequencies of complications differed among the various PACs (Table 3).
Table 3.
Rates of postoperative pancreatic fistula, delayed gastric emptying and wound-related complications in patients undergoing pancreaticoduodenectomy for periampullary adenocarcinomas during 1980–2011 by disease type and decade
| Ampulla (n = 401) | Distal bile duct (n = 317) | Duodenum (n = 158) | Head of pancreas (n = 1688) | Total (n = 2564) | |
|---|---|---|---|---|---|
| Rates of postoperative pancreatic fistula, n (%) | |||||
| 1980s (n = 111) | 1 (5%) | 2 (12%) | 0 | 0 | 3 (3%) |
| 1990s (n = 830) | 21 (16%) | 17 (12%) | 5 (10%) | 18 (4%) | 61 (7%) |
| 2000s (n = 1623) | 35 (14%) | 22 (14%) | 22 (22%) | 75 (7%) | 154 (9%) |
| Total | 57 (14%) | 41 (13%) | 27 (17%) | 93 (6%) | 218 (9%) |
| Rates of delayed gastric emptying, n (%) | |||||
| 1980s (n = 111) | 3 (15%) | 1 (6%) | 2 (25%) | 2 (3%) | 8 (7%) |
| 1990s (n = 830) | 20 (15%) | 20 (14%) | 9 (18%) | 74 (15%) | 123 (15%) |
| 2000s (n = 1623) | 46 (18%) | 18 (11%) | 14 (14%) | 190 (17%) | 268 (17%) |
| Total | 69 (17%) | 39 (12%) | 25 (16%) | 266 (16%) | 399 (16%) |
| Rates of wound-related complications, n (%) | |||||
| 1980s (n = 111) | 4 (20%) | 1 (6%) | 0 | 1 (2%) | 6 (5%) |
| 1990s (n = 830) | 14 (11%) | 18 (13%) | 5 (10%) | 33 (7%) | 70 (8%) |
| 2000s (n = 1623) | 37 (15%) | 26 (16%) | 16 (16%) | 151 (14%) | 230 (14%) |
| Total | 55 (14%) | 45 (14%) | 21 (13%) | 185 (11%) | 306 (12%) |
Non-pancreatic adenocarcinomas were associated with a significantly higher incidence of postoperative pancreatic fistula (POPF) than pancreatic adenocarcinoma (P < 0.001). Comparisons by decade showed a higher incidence of POPF in the 2000s than in the 1980s (P < 0.05).
Despite differences in complication rates among the PACs, each cohort had similar rates of 30-day (P = 0.89) and 90-day (P = 0.47) mortality. The rate of 30-day mortality after PD in the 1980s was 2% and thus did not differ significantly from the rates of 1% reported in both the 1990s and 2000s (P = 0.79). The rate of 90-day mortality after PD was 4% in the 1980s, and remained at that level in the 1990s and 2000s.
Comparisons and trends in pathological data
The median tumour size of all pathological types was 2.5 cm (range: 0–17 cm) (Table 1) and tumour size was similar in ampullary cancer and bile duct cancer. The median tumour size in duodenal cancer was larger than that in ampullary and bile duct cancer (P < 0.001), and larger than that in pancreatic cancer (P < 0.001).
No difference in the grade of tumour differentiation was identified among all types of PAC (Table 1). The overall rate of margin positivity for all tumour types was 21%, with the majority of cases classified as of R1 status. Margin status is summarized in Table 4. The rate of margin positivity varied significantly based on type of PAC. The frequency of positive margin resection did not change over the years for any of the PACs. Data on lymph node metastasis status are summarized in Table 5. Patients with pancreatic adenocarcinoma were more likely to have regional lymph node metastases (P < 0.001).
Table 4.
Positive margin status in patients undergoing pancreaticoduodenectomy for periampullary adenocarcinomas during 1980–2011 by disease type
| Margin status | Ampulla (n = 401) | Distal bile duct (n = 317) | Duodenum (n = 158) | Head of pancreas (n = 1688) | Total (n = 2564) |
|---|---|---|---|---|---|
| Negative, n (%) | 368 (92%) | 252 (79%) | 134 (85%) | 1054 (62%) | 1807 (71%) |
| Tumour within 1 mm, n (%) | 12 (3%) | 16 (5%) | 9 (6%) | 185 (11%)a | 222 (9%) |
| Positive, n (%) | 13 (3%) | 47 (15%) | 10 (6%) | 440 (26%)a | 510 (21%) |
| Unknown, n (%) | 9 (2%) | 3 (1%) | 5 (3%) | 9 (1%) | 26 (1%) |
Positive resection margins were significantly higher in pancreatic adenocarcinoma (26%) than in adenocarcinomas of the bile duct (15%), ampulla (3%) or duodenum (6%) (P < 0.001). Pancreatic adenocarcinoma also had a significantly higher proportion of carcinoma within 1 mm of the margin compared with the other periampullary adenocarcinomas (P < 0.001).
Table 5.
Positive lymph node metastasis in patients undergoing pancreaticoduodenectomy for periampullary adenocarcinomas during 1980–2011 by disease type and decade
| Ampulla (n = 401) | Distal bile duct (n = 317) | Duodenum (n = 158) | Head of pancreas (n = 1688) | |
|---|---|---|---|---|
| 1980s (n = 111) | 5 (25%) | 5 (29%) | 4 (50%) | 41 (62%) |
| 1990s (n = 830) | 66 (50%) | 92 (65%) | 31 (62%) | 374 (74%) |
| 2000s (n = 1623) | 159 (64%) | 108 (68%) | 66 (66%) | 861 (77%) |
| Total | 230 (57%) | 205 (65%) | 101 (64%) | 1275 (76%) |
Patients with pancreatic adenocarcinoma were more likely to have regional lymph node metastases (P < 0.001). The rate of lymph node metastasis increased over the decades in patients with pancreatic adenocarcinoma (62% in the 1980s versus 77% in the 2000s; P = 0.008).
Comparisons and trends in survival
The estimated median survival and 5-year OS rate for the entire cohort were 22 months and 26%, respectively. These values varied significantly among the various PAC types. Pancreatic adenocarcinoma was associated with the worst median OS (19 months) compared with ampullary adenocarcinoma (47 months), distal cholangiocarcinoma (23 months) and duodenal adenocarcinoma (54 months) (Fig. 2). Similarly, estimated 5-year survival was lowest in pancreatic adenocarcinoma (18%) compared with other PACs of the ampulla (45%), distal bile duct (27%) and duodenum (49%) (Fig. 2). Overall survival in each type of PAC did not change over time (Fig. 3).
Figure 2.
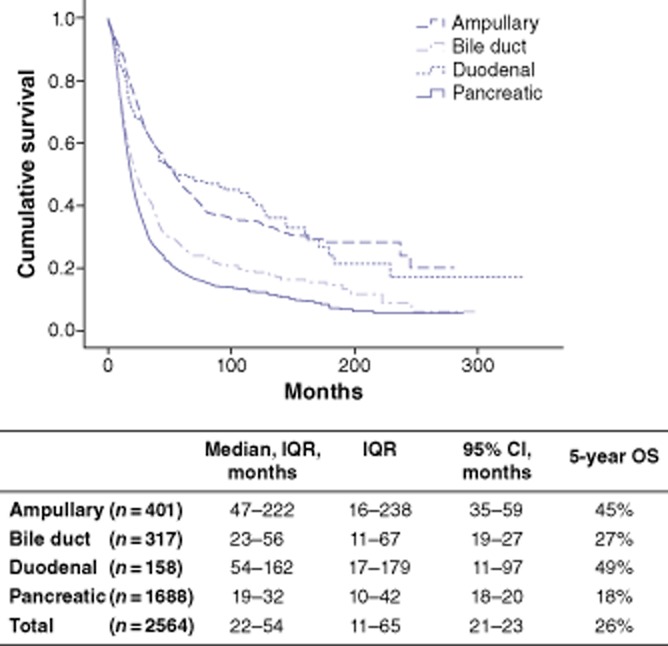
Overall survival (OS) in patients with periampullary adenocarcinomas undergoing pancreaticoduodenectomy during 1980–2011. IQR, interquartile range; 95% CI, 95% confidence interval
Figure 3.
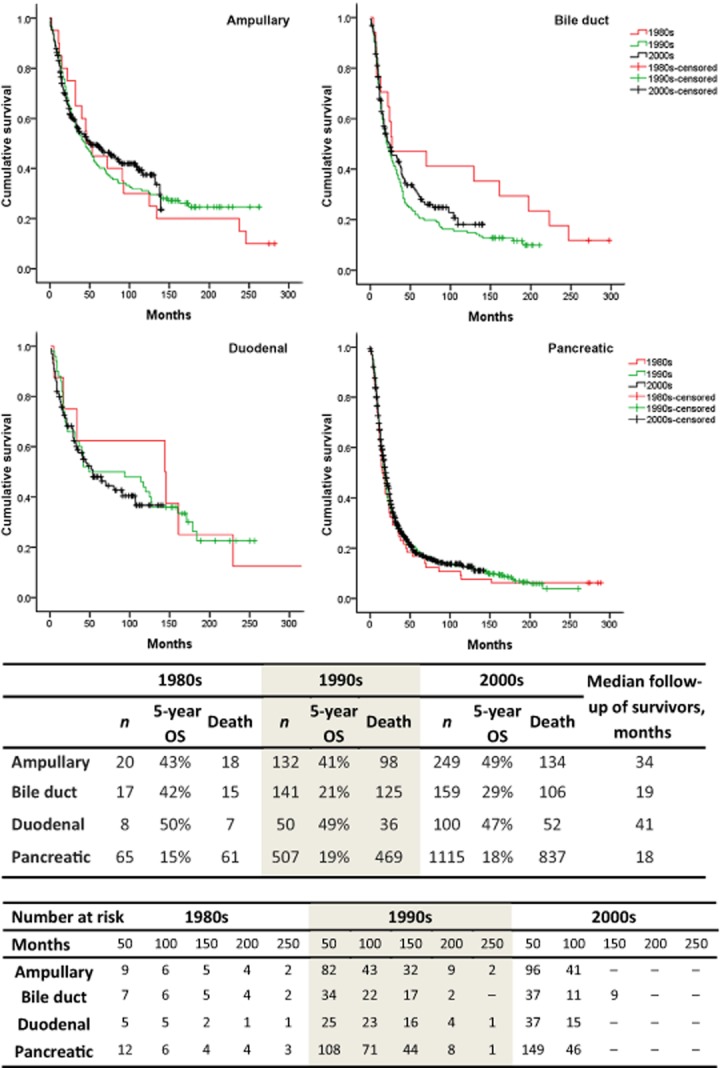
Survival analysis by disease type and decade in patients with periampullary adenocarcinomas undergoing pancreaticoduodenectomy during 1980–2011, showing the number of deaths at the end of the study for each time period and median follow-up in survivors of each tumour type. OS, overall survival
In univariate analysis, tumour type, preoperative jaundice, pain, weight loss, vein resection during surgery, positive margin, nodal metastasis, and occurrence of POPF were associated with worse OS. In a Cox proportional hazards multivariate regression model, tumour type, vein resection, positive margin and nodal metastasis were strongly associated with OS. Nodal metastasis had the strongest association with OS [hazard ratio (HR) 1.61; P < 0.001], whereas vein resection had the weakest association with OS (HR = 1.25; P = 0.02) (Table 6).
Table 6.
Univariate and multivariate analyses of predictors of overall survival in patients undergoing pancreaticoduodenectomy for periampullary adenocarcinomas during 1980–2011
| Variables | P-value, univariate analysis | P-value, multivariate analysis | HR | 95% CI |
|---|---|---|---|---|
| Tumour type | <0.001 | <0.001 | 1.15 | 1.10–1.20 |
| Vein resection | <0.001 | 0.020 | 1.25 | 1.04–1.51 |
| Positive margins | <0.001 | <0.001 | 0.66 | 0.60–0.73 |
| Nodal metastasis | <0.001 | <0.001 | 1.61 | 1.45–1.79 |
| POPF | 0.001 | 0.240 | 1.10 | 0.94–1.29 |
| Jaundice | 0.005 | 0.080 | 0.91 | 0.82–1.01 |
| Pain | 0.038 | 0.56 | 0.97 | 0.88–1.07 |
| Weight loss | 0.002 | 0.22 | 0.94 | 0.84–1.04 |
HR, hazard ratio; 95% CI, 95% confidence interval; POPF, postoperative pancreatic fistula.
Discussion
The primary goal of this study was to evaluate trends in clinicopathological features and outcomes among the various PACs. A cohort of 2564 patients submitted to resection of PACs was studied. The study spans a period during which significant management changes occurred, which allows for a meaningful analysis of trends in outcomes. This work provides a perspective of changing experience in the management of PACs at a single institution. Pancreatic cancer was confirmed to have the most aggressive features, including the highest rates of regional lymph node involvement and positive margins, and poorest longterm survival.13 The best OS was seen in both ampullary and duodenal cancer, both of which had 5-year survival rates over twice that found in pancreatic cancer. It should be noted, however, that as a group periampullary cancers are among the most lethal of gastrointestinal neoplasms.
This study shows a trend towards an increase in the number of PDs performed at a single institution over the past three decades. The relative distribution of periampullary cancers subjected to resection was unchanged over this period. Significant increases were apparent in the number and proportion of patients undergoing PD for IPMN-associated cancers. Over the three decades, 30-day mortality remained stable at about 1–2%, despite a trend towards increased age in patients undergoing resection and an increase in the complexity of the operation as greater numbers of patients underwent vein resection.
Based on the premise that refinements in staging, systemic therapy and radiotherapy have been made over the past three decades, the present study sought to determine if these changes were associated with improvements in survival over this period. Unfortunately, no trends that would suggest improved longterm outcomes over time were identified. A decade-by-decade comparison demonstrated no improvement in survival since the 1980s. In particular, in pancreatic cancer, in the setting of which significant evolution in the provision of adjuvant chemotherapy and chemoradiotherapy has occurred,22 no trend towards improved survival was found.
As pancreatic cancer is the most common PAC and studies often combine the various PACs, there may be a perception that all of these neoplasms are uniformly associated with a poor prognosis.18,23 The current study demonstrates that periampullary cancers are associated with significantly different longterm survival rates. This finding is interesting in light of the fact that these adenocarcinomas originate in tissues that are separated by only millimetres, have nearly identical venous and lymphatic drainage on the gross anatomic level, and are often indistinguishable on standard histopathological assessment; yet they are associated with very different outcomes. This work, along with that of others, confirms that the unique intrinsic characteristics of each PAC, often described as its ‘biological behaviour’, remain the most important prognostic indicators in periampullary cancers amenable to resection.24
Similarly to this study, others have shown that OS in duodenal and ampullary adenocarcinomas is much better than in distal cholangiocarcinoma and pancreatic cancer. One explanation for this observation is that duodenal and ampullary adenocarcinomas are diagnosed at an earlier stage than cholangiocarcinoma or pancreatic carcinoma. Indeed, in the current study, more patients undergoing resection for pancreatic cancer were diagnosed at a more advanced stage and demonstrated higher rates of positive lymph nodes compared with patients diagnosed with other types of PAC (P < 0.001). However, an alternative explanation is that the lower stage at diagnosis of the non-pancreatic PAC reflects an inherent lower propensity towards metastasis of these tumours. Which of these theories is correct cannot be concluded from the current data. However, the suggestion that the unique biology of each PAC has a role to play is supported by recent reports that have shed light on the genetic underpinnings of these tumours.25–29 Histology and genetic association have suggested that ampullary cancer is closer to intestinal cancer than to pancreatobiliary cancer.30,31 The pancreatobiliary type of differentiation independently predicts a poor prognosis after PD in PACs.32,33
Several published studies have evaluated predictors of outcomes in periampullary cancers after resection. Positive lymph node status and LNR were associated with worse survival rates among those with no evidence of distant metastatic disease.24,34 Perineural growth and angioinvasion were also shown to be important risk factors for survival in periampullary cancers after resection.35 These studies were restricted by relatively small numbers of patients, which prohibited robust multivariate analysis. Data derived from the Surveillance, Epidemiology and End Results (SEER) database have been used to achieve sufficient statistical power. However, these data lack detail on the characteristics of the treatment regimen. In this study, multivariate analysis in a relatively large population of patients linked to detailed data showed tumour type, vein resection rate, margin status and nodal status to be associated with worse OS in PACs.
The limitations of this study include: (i) its status as a retrospective analysis, which has the potential to be influenced by selection bias; (ii) relatively few patients were operated during the 1980s in comparison with the more recent decades; (iii) the study includes no data on the actual rate and composition of neoadjuvant and adjuvant chemoradiation therapy, and (iv) the fact that no data on disease-specific survival were available.
Conclusions
Despite a presumed improvement in disease staging and the provision of adjunct therapies over time, there has been no measurable improvement in longterm survival in any of the PACs. Periampullary adenocarcinomas are associated with distinct outcomes. The best OS was seen in both ampullary and duodenal cancer and the worst in pancreatic cancer.
Conflicts of interest
None declared.
References
- 1.Sarmiento JM, Nagomey DM, Sarr MG, Farnell MB. Periampullary cancers: are there differences? Surg Clin North Am. 2001;81:543–555. doi: 10.1016/s0039-6109(05)70142-0. [DOI] [PubMed] [Google Scholar]
- 2.Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928–1935. doi: 10.1245/s10434-011-2168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB. 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 7.Sarela AI, Brennan MF, Karpeh MS, Klimstra D, Conlon KC. Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol. 2004;11:380–386. doi: 10.1245/ASO.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg. 1998;228:87–94. doi: 10.1097/00000658-199807000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712–1715. doi: 10.1002/bjs.1800831217. [DOI] [PubMed] [Google Scholar]
- 10.Cameron JL. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg. 2005;140:708. doi: 10.1001/archsurg.140.7.708. [DOI] [PubMed] [Google Scholar]
- 11.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falconi M, Crippa S, Dominguez I, Barugola G, Capelli P, Marcucci S, et al. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol. 2008;15:3178–3186. doi: 10.1245/s10434-008-0099-4. [DOI] [PubMed] [Google Scholar]
- 13.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 14.Glazer ES, Liu P, Abdalla EK, Vauthey JN, Curley SA. Neither neoadjuvant nor adjuvant therapy increases survival after biliary tract cancer resection with wide negative margins. J Gastrointest Surg. 2012;16:1666–1671. doi: 10.1007/s11605-012-1935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter JM, Cameron JL, Lillemoe KD, Campbell KA, Chang D, Riall TS, et al. Periampullary and pancreatic incidentaloma: a single institution's experience with an increasingly common diagnosis. Ann Surg. 2006;243:673–680. doi: 10.1097/01.sla.0000216763.27673.97. discussion 680–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs. observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 17.Morak MJ, van der Gaast A, Incrocci L, van Dekken H, Hermans JJ, Jeekel J, et al. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008;248:1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- 18.Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, et al. Longterm survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: longterm results of EORTC trial 40891. Ann Surg. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 19.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nugent FW, Stuart K. Adjuvant and neoadjuvant therapy in curable pancreatic cancer. Surg Clin North Am. 2010;90:323–339. doi: 10.1016/j.suc.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Jarufe NP, Coldham C, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Favourable prognostic factors in a large UK experience of adenocarcinoma of the head of the pancreas and periampullary region. Dig Surg. 2004;21:202–209. doi: 10.1159/000079346. [DOI] [PubMed] [Google Scholar]
- 24.Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehehalt F, Rummele P, Kersting S, Lang-Schwarz C, Ruckert F, Hartmann A, et al. Hepatocyte nuclear factor (HNF) 4alpha expression distinguishes ampullary cancer subtypes and prognosis after resection. Ann Surg. 2011;254:302–310. doi: 10.1097/SLA.0b013e31821994a8. [DOI] [PubMed] [Google Scholar]
- 26.Embuscado EE, Laheru D, Ricci F, Yun KJ, de Boom Witzel S, Seigel A, et al. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther. 2005;4:548–554. doi: 10.4161/cbt.4.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modelling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura K, Meckel KF, Laird LS, Chia CY, Park JJ, Olino KL, et al. Integrin alpha2 mediates selective metastasis to the liver. Cancer Res. 2009;69:7320–7328. doi: 10.1158/0008-5472.CAN-09-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone G, Santini D, Zagami M, Vincenzi B, Verzi A, Morini S, et al. COX-2 expression of ampullary carcinoma: correlation with different histotypes and clinicopathological parameters. Virchows Arch. 2006;449:334–340. doi: 10.1007/s00428-006-0255-9. [DOI] [PubMed] [Google Scholar]
- 31.Ruemmele P, Dietmaier W, Terracciano L, Tornillo L, Bataille F, Kaiser A, et al. Histopathologic features and microsatellite instability of cancers of the papilla of Vater and their precursor lesions. Am J Surg Pathol. 2009;33:691–704. doi: 10.1097/PAS.0b013e3181983ef7. [DOI] [PubMed] [Google Scholar]
- 32.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. doi: 10.1186/1471-2407-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301–309. doi: 10.1007/s00534-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 34.Hurtuk MG, Hughes C, Shoup M, Aranha GV. Does lymph node ratio impact survival in resected periampullary malignancies? Am J Surg. 2009;197:348–352. doi: 10.1016/j.amjsurg.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 35.van Roest MH, Gouw AS, Peeters PM, Porte RJ, Slooff MJ, Fidler V, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: perineural growth more important prognostic factor than tumour localization. Ann Surg. 2008;248:97–103. doi: 10.1097/SLA.0b013e31817b6609. [DOI] [PubMed] [Google Scholar]


