Abstract
Understanding the temperature sensitivity (Q10) of soil organic matter (SOM) decomposition is important for predicting soil carbon (C) sequestration in terrestrial ecosystems under warming scenarios. Whether Q10 varies predictably with ecosystem succession and the ways in which the stoichiometry of input SOM influences Q10 remain largely unknown. We investigate these issues using a grassland succession series from free-grazing to 31-year grazing-exclusion grasslands in Inner Mongolia, and an incubation experiment performed at six temperatures (0, 5, 10, 15, 20, and 25°C) and with four substrates: control (CK), glucose (GLU), mixed grass leaf (GRA), and Medicago falcata leaf (MED). The results showed that basal soil respiration (20°C) and microbial biomass C (MBC) logarithmically decreased with grassland succession. Q10 decreased logarithmically from 1.43 in free-grazing grasslands to 1.22 in 31-year grazing-exclusion grasslands. Q10 increased significantly with the addition of substrates, and the Q10 levels increased with increase in N:C ratios of substrate. Moreover, accumulated C mineralization was controlled by the N:C ratio of newly input SOM and by incubation temperature. Changes in Q10 with grassland ecosystem succession are controlled by the stoichiometry of newly input SOM, MBC, and SOM quality, and the combined effects of which could partially explain the mechanisms underlying soil C sequestration in the long-term grazing-exclusion grasslands in Inner Mongolia, China. The findings highlight the effect of substrate stoichiometry on Q10 which requires further study.
Keywords: Heterotrophic respiration, Q10, soil organic matter, stoichiometry, substrate, succession, warming
Introduction
It has become clear that the decomposition of soil organic matter (SOM) is positively correlated with temperature (Hartley and Ineson 2008; Zimmermann and Bird 2012; Zimmermann et al. 2012). The index of temperature sensitivity (Q10) has been widely used to depict the response of SOM decomposition to diurnal or seasonal temperature changes and increasingly to predict the response of soil carbon (C) sequestration in terrestrial ecosystems under warmer climate scenarios (Kirschbaum 1995; Luo et al. 2001; Knorr et al. 2005; Davidson and Janssens 2006; Conant et al. 2011).
However, the temperature sensitivity of SOM decomposition remains a highly debated topic, particularly in relation to the extent to which regional or global convergence of Q10 occurs (Zhou et al. 2009; Mahecha et al. 2010; Sierra 2012), and also regarding whether Q10 differs among soils and soil fractions (e.g., labile vs. recalcitrant) (Fang et al. 2005, 2006; Karhu et al. 2010a; Plante et al. 2010; Wetterstedt and Agren 2011; Tucker et al. 2013). Landscapes (e.g., grassland or forest) comprise mixed series of ecosystems in stage of succession or degradation. Understanding whether SOM decomposition and Q10 vary with ecosystem succession is important to evaluating soil C sequestration. To date, the variability of Q10 with ecosystem succession has not been extensively evaluated.
Inner Mongolian grasslands in China (about 150 million ha) have an enormous capacity to sequester atmospheric CO2 through rational land-use practices, especially through grazing exclusion (GE) (He et al. 2008, 2012). Soil C storage demonstrated an initial rapid increase following GE introduction and was maintained at a steady state after two decades of GE (He et al. 2008). Understanding the stability of accumulated SOM (decomposition rate and Q10) under long-term GE would be useful for accurately evaluating long-term soil C sequestration. The “carbon quality-temperature (CQT)” hypothesis predicts that the temperature sensitivity of SOM decomposition increases with biochemical recalcitrance, and this hypothesis has been tested on a variety of substrates and scales (Fierer et al. 2005; Conant et al. 2008b; Craine et al. 2010). Therefore, we assumed that Q10 should decrease with grassland succession as GE period increases because the C associated with sand and silt fractions in surface soil increase logarithmically (He et al. 2009). Moreover, the higher N:C ratio of SOM should enhance Q10 and soil C mineralization due to greater N limitation in grassland ecosystems (Billings and Ballantyne 2013).
Using a grassland succession series from free-grazing to 31-year GE, we conducted an 8-week incubation experiment using six temperatures and four substrates with differing N:C ratios. The main objectives of this study were to (1) investigate whether Q10 decreased predictably with grassland succession consistent with the CQT hypothesis, (2) determine the influence of the N:C stoichiometry of substrates on Q10 and soil C mineralization, and (3) discuss soil C sequestration mechanisms in Inner Mongolian grasslands in view of SOM decomposition.
Material and Methods
Study sites
The study was conducted in a typical steppe ecosystem on the Mongolian Plateau (43°33′N, 116°40′E). The area is a typical semi-arid continental climate with a mean annual temperature of 1.1°C and average annual precipitation of 345 mm (He et al. 2008). The soil is chestnut type, that is, Calcic kastanozems. The vegetation consists predominantly of grassland plants, such as Leymus chinensis, Stipa grandis, and Cleistogenes squarrosa.
Five grasslands, with similar vegetation and topography across in 2 km × 2 km in extent, comprised a successional series of grassland restoration, where plant communities and soil properties varied predictably after eliminating the large animal disturbance by fences (Table 1). The five plots were categorized as GE0, GE4, GE7, GE11, and GE31. Plot GE0 was subjected to long-term free-grazing and was in a slightly degraded condition in terms of the aboveground community and plant diversity. Plot GE4 was established in 2008 by fencing off a section of previously free-grazing grassland. Plots GE7, GE11, and GE31 were similarly established in 2004, 1999, and 1979, respectively (He et al. 2008). We assumed that changes in vegetation and soil properties among the five plots were mainly a result of grazing intensity and the length of GE, because the five experimental plots were floristically and topographically similar, and all were distributed in the same upper basalt platform.
Table 1.
Changes in vegetation and soil properties with grassland succession.
| Grassland type | Aboveground biomass (g m−2) | Soil organic carbon (g kg−1) | Soil total nitrogen (g kg−1) | Microbial biomass C (μg kg−1) | Soil pH | Land-use history |
|---|---|---|---|---|---|---|
| Free-grazing grassland (GE0) | 60.3 ± 20.6a* | 13.41 ± 0.46a | 1.43 ± 0.10a | 47.52 ± 0.46a | 8.17 ± 0.29a | Long-term free-grazing, good condition |
| 4-year grazing exclusion (GE4) | 162.3 ± 15.0b | 15.95 ± 0.55b | 1.60 ± 0.03a | 42.69 ± 1.73ab | 8.07 ± 0.11a | Grassland fenced since 2008, good condition |
| 7-year grazing exclusion (GE7) | 166.2 ± 13.3b | 16.32 ± 2.06bc | 1.64 ± 0.16a | 38.06 ± 1.59b | 7.92 ± 0.16ab | Grassland fenced since 2004, good condition |
| 11-year grazing exclusion (GE11) | 171.6 ± 9.6b | 18.19 ± 0.49c | 1.72 ± 0.10a | 39.53 ± 1.89b | 7.66 ± 0.19b | Grassland fenced since 1999, good condition |
| 31-year grazing exclusion (GE31) | 148.9 ± 41.3b | 17.73 ± 2.18bc | 1.48 ± 0.72a | 40.02 ± 0.66b | 7.19 ± 0.29c | Grassland fenced since 1979, good condition |
Data are represented as means ± 1 SD (n = 4). The same superscript letters within each column indicating no significant difference among grassland types at P < 0.05 level (ANOVA).
Field sampling
Field sampling was conducted in July 2011. In each plot, 4 sampling quadrats (1 m × 1 m) were established at 10-m intervals along a random transect. Aboveground biomass was clipped at ground level. Soil samples in the 0–20 cm soil layer were collected from 10 points in each quadrate. In the laboratory, we manually removed roots and visible organic debris from soil samples, and then measured soil water-holding capacity (WHC, %) and soil gravimetric moisture (%). Approximately 100 g of each soil samples was air-dried for analysis of soil properties (e.g., C, N, and pH). The remaining soil was stored at 4°C.
Laboratory incubation and analysis
The incubation experiment was conducted at six temperatures (0, 5, 10, 15, 20, and 25°C), using four substrates (control (CK), glucose (GLU), mixed grass leaf (GRA), and Medicago falcata leaf (MED). GRA and MED were collected from five plots and mixed evenly. The C concentration in GLU, GRA, and MED was 40.0%, 45.1%, and 44.0%, respectively, and the N concentration was 0%, 2.0%, and 5.2%, respectively. Thus, the N:C ratios of the substrates in increasing order were GLU (0) < GRA (0.043) < MED (0.117). We added 1% of the mass of the incubated soils as GLU, GRA, and MED, which approximated a 2-year input of new SOM from roots and litter in Inner Mongolian grasslands. In total, the incubation experiment comprised 480 samples, with 5 grassland types, 6 temperatures, 4 substrates, and 4 replicates for each treatment.
First, 40 g samples of fresh soils were put into incubation bottles, and the samples were adjusted to 60% WHC. Samples were incubated at 20°C and an instant 80% humidity for 4 days and then incubated at a treatment temperature (0, 5, 10, 15, 20, or 25°C) for 3 days prior to measurement of basal soil respiration. The substrates were subsequently added and mixed evenly. During the 56 days incubation experiment, soil respiration rates were measured 14 times, on days 1, 2, 3, 4, 5, 6, 7, 14, 21, 28, 35, 42, 49, and 56.
An automatic system for measuring soil respiration rates was developed through modification of the continuous gas-flow system reported by Cheng et al. (1993). The system consisted of a Li-COR CO2 analyzer (Li-7000), an electric water bath to control incubation temperature, an air-flow controller, soda-lime equipment to control the initial CO2 concentration, an auto-sampler on a turn-plate, automatic transformation valves to control the sample bottle, and a data collector (Fig. 1). In practice, the system was controlled by the data collector and first automatically lowered the CO2 concentration by using a bypass system of soda lime and then recorded the changes in CO2 concentration as it steadily increased. Soil respiration rates were calculated from the slope of the CO2 concentration as follows:
Figure 1.

Schematic representation of the configuration of the automatic measurement system for soil microbial respiration.
where R is soil respiration rate (μg CO2 g−1 h−1); C is the slope of the CO2 concentration; V is the volume of the incubation bottle and gas tube; m is the soil weight (g); α is the transformation coefficient of CO2 mass; and β is the transformation coefficient of time.
Soil organic C (SOC, %) was measured using a modified Mebius method (Nelson and Sommers 1982). Soil total N (%) was measured with a modified Kjeldahl wet-digestion procedure (Gallaher et al. 1976), using a 2300 Kjeltec Analyzer Unit (FOSS Tecator, Höganäs, Sweden). Soil pH was determined using a pH meter and a slurry of soil mixed with distilled water (1:2.5). Microbial biomass C (MBC) of fresh soil samples was analyzed using the fumigation – extraction method (Vance et al. 1987).
Calculations and statistical analysis
In this study, we selected the data from 1, 7, and 56 days incubations, respectively, to represent the instantaneous-term, short-term, and longer-term effects of the experimental treatments on soil C mineralization. Soil C mineralization at 20°C without substrate addition was used as the base soil respiration (Cmin-20°C).
The temperature sensitivity (Q10) of soil respiration in the 1- and 7-day incubations was calculated using the exponential equations, because 56-day incubation should be too longer to accurately evaluate Q10 due to the respiration rates declining faster at higher temperature with faster depletion of substrate.
where Y is the soil respiration rate (μgC g−1 h−1), T is the temperature (°C); A and B are the constants.
The stimulating effects (SEs) of soil respiration were then calculated to represent the sequestration capacity of grassland soils with fresh SOM input. SEs were calculated from the accumulated C mineralization under different substrates (GLU, GRA, or MED) divided by that of CK; higher values of SEs implied lower sequestration capacity.
One-way analysis of the variance (ANOVA) was used to investigate the differences in vegetation and soil properties among different grassland types. Univariate analysis of three factors (general linear model) was used to determine the effects of grassland types, incubation temperature, and substrates on soil C mineralization, Q10, and SEs. Logarithmic regression was used to identify the changing trend of Q10 and SEs with the duration of GE, MBC, and SOC. Data have been represented as means ± 1 standard deviation (n = 4). Differences were considered to be significant when P < 0.05. All analyses were conducted using SPSS statistical software (v. 13.0, SPSS, Chicago, IL, USA).
Results
Changes in soil properties
SOC increased significantly with increasing duration of GE (F = 9.18, P < 0.001); however, soil N content was not significantly different among grasslands. With grassland succession as a function of duration of GE, soil pH decreased from 8.17 (GE0) to 7.19 (GE31) (F = 15.57, P < 0.001) and MBC decreased logarithmically (F = 12.26, P = 0.004).
Changes in soil C mineralization
Cmin-20°C without the addition of external substrates differed significantly among various grasslands and decreased logarithmically with increasing duration of GE (R2 = 0.539, P < 0.001 for 1 day, R2 = 0.607, P < 0.001 for 7 days, and R2 = 0.596, P < 0.001 for 56 days). Cmin-20°C was linearly correlated with MBC (R2 = 0.597, P < 0.001 for 1 day, R2 = 0.377, P = 0.004 for 7 days, and R2 = 0.382, P = 0.004 for 56 days).
Grassland type, incubation temperature, and added substrates significantly influenced soil C mineralization, with notable interactive effects (Fig. 2, Table 2). Moreover, accumulated C mineralization in the CK, GLU, and GRA cases increased with temperature during the 8-week incubation in all grasslands. However, under MED conditions, accumulated C mineralization increased with temperature in the first week and then was highest at 15°C (Fig. 3).
Figure 2.
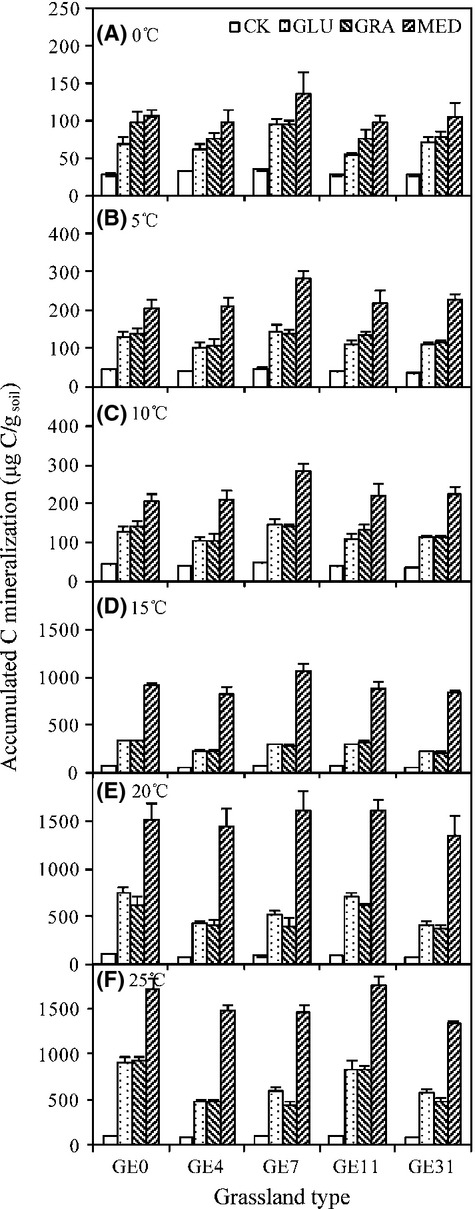
Changes in soil C mineralization with grazing-exclusion duration, substrate, and incubation temperature. CK, control; GLU, glucose; GRA, mixed grass leaf; MED, Medicago falcate leaf. Data were derived from 7-day incubation and represented as mean ± SD (n = 4).
Table 2.
Results of univariate analysis of accumulated C mineralization (Cmin) according to grassland type, incubation temperature, and substrate addition.
| Cmin-1 day | Cmin-7 day | Cmin-56 day | ||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Grassland type (G) | 52.69 | <0.0001 | 124.81 | <0.0001 | 286.78 | <0.0001 |
| Incubation temperature (T) | 587.31 | <0.0001 | 2501.55 | <0.0001 | 1253.31 | <0.0001 |
| Substrate addition (S) | 965.76 | <0.0001 | 6668.08 | <0.0001 | 4701.82 | <0.0001 |
| G × T | 6.75 | <0.0001 | 23.12 | <0.0001 | 26.57 | <0.0001 |
| G × S | 6.57 | <0.0001 | 20.86 | <0.0001 | 60.27 | <0.0001 |
| T × S | 144.25 | <0.0001 | 555.77 | <0.0001 | 220.02 | <0.0001 |
| G × T × S | 1.69 | 0.0020 | 3.77 | <0.0001 | 5.94 | <0.0001 |
Figure 3.

Changes in accumulated C mineralization with substrate addition (control [A], Glucose [B], Mixed grass leaf [C], and Medicago falcata leaf [D]) in 56-day incubation from 11-year grazing-exclusion grassland (GE11). Data were represented as mean ± SD (n = 4).
Changes in Q10
Grassland type and added substrate had significant effects on Q10 and again showed interactions with each other (Fig. 4, Table 3). Q10 values decreased significantly with the grassland succession (F = 3.87, P = 0.011 for 1 day; F = 6.32, P < 0.001 for 7 days). Q10 values increased significantly with increasing N:C stoichiometry of substrates (F = 5.84, P = 0.001 for 1 day; F = 7.66, P < 0.001 for 7 days). Q10 in the first day were ordered as follows: CK (1.305) < GRA (1.872) < GLU (2.263) < MED (3.222). In CK, GLU, and GRA, Q10 was correlated logarithmically with Cmin-20°C, MBC, SOC, and duration of GE (Fig. 5, Table 4). However, no significant correlation was found with MED addition.
Figure 4.

Changes in temperature sensitivity (Q10) of soil C mineralization with grassland type (A) and substrate (B). Data were represented as mean ± SD (n = 4).
Table 3.
Results of univariate analysis of Q10 according to grassland type and substrates.
| Q10-1 day | Q10-7 day | |||
|---|---|---|---|---|
| F | P | F | P | |
| Grassland type (G) | 29.37 | <0.0001 | 136.90 | <0.0001 |
| Substrate addition (A) | 510.44 | <0.0001 | 958.36 | <0.0001 |
| G × A | 3.65 | 0.0004 | 9.71 | <0.0001 |
Figure 5.
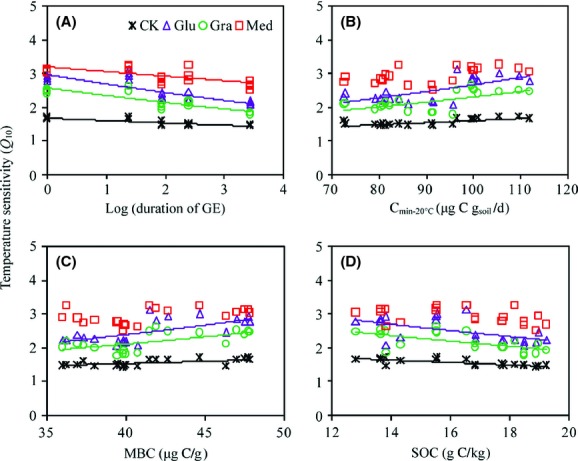
Relationship between temperature sensitivity (Q10) in 7-day incubation and duration of GE (A), Cmin-20°C (B), MBC (C), and SOC (D).
Table 4.
Relationships of Q10 with Cmin-20°C, MBC, SOC, and duration of GE.
| Cmin-20°C (μg C g−1)† | MBC (μg C g−1)‡ | SOC (g kg−1) | Duration of GE (year) | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | P | R2 | P | R2 | P | R2 | P | |
| 1-day | ||||||||
| Q10-CK* | 0.434§ | 0.002 | 0.510 | 0.001 | 0.413 | 0.003 | 0.693 | <0.001 |
| Q10-GLU | 0.416 | 0.002 | 0.212 | 0.041 | 0.107 | 0.160 | 0.253 | 0.024 |
| Q10-GRA | 0.422 | 0.003 | 0.253 | 0.028 | 0.281 | 0.020 | 0.674 | <0.001 |
| Q10-MED | 0.048 | 0.355 | 0.001 | 0.916 | 0.082 | 0.220 | 0.258 | 0.023 |
| 7-day | ||||||||
| Q10-CK | 0.608 | <0.001 | 0.408 | 0.002 | 0.541 | <0.001 | 0.684 | <0.001 |
| Q10-GLU | 0.555 | <0.001 | 0.455 | 0.001 | 0.310 | 0.011 | 0.646 | <0.001 |
| Q10-GRA | 0.426 | 0.001 | 0.374 | 0.004 | 0.375 | 0.004 | 0.726 | <0.001 |
| Q10-MED | 0.161 | 0.080 | 0.168 | 0.072 | 0.144 | 0.099 | 0.471 | 0.001 |
Q10 calculated for soil C mineralization in 1-day and 7-day incubations.
Cmin-20ºC is the soil respiration rate without substrate addition under 20°C after 1-day and 7-day incubation.
MBC is microbial biomass C measured using the fumigation-extraction method.
Logarithmic equations suited most situations, identified by the minimum Akaike Information Criterion (AIC).
Changes in SEs
Grassland type, incubation temperature, and substrate significantly influenced SEs, with interactions among their effects (Fig. 6, Table 5). Compared with temperature and grassland type, added substrates had the strongest effect on SEs, and the effects of MED were significantly higher than those of GLU and GRA (Fig. 6).
Figure 6.
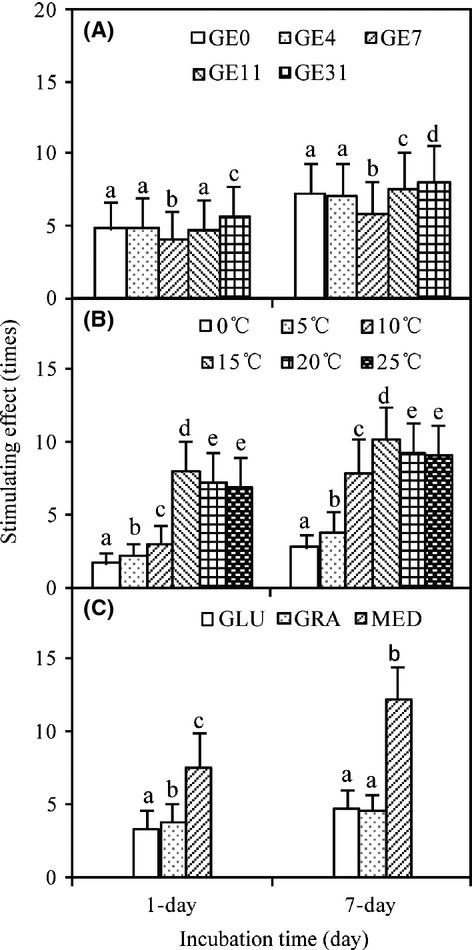
Stimulating effect of soil C mineralization according to grassland type (A), incubation temperature (B), and substrate type (C). Data with the same lowercase letters showed no significant difference at P = 0.05 level.
Table 5.
Univariate analysis of stimulating effects (SEs) according to grassland type, incubation temperature, and substrate addition.
| SE1 day* | SE7 day | SE56 day | ||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Grassland type (G) | 21.37 | <0.0001 | 188.44 | <0.0001 | 79.36 | <0.0001 |
| Temperature (T) | 417.12 | <0.0001 | 2979.44 | <0.0001 | 216.82 | <0.0001 |
| Substrate addition (S) | 553.31 | <0.0001 | 4740.99 | <0.0001 | 1042.76 | <0.0001 |
| G × T | 2.97 | <0.0001 | 94.07 | <0.0001 | 32.68 | <0.0001 |
| G × S | 5.04 | <0.0001 | 198.83 | <0.0001 | 79.40 | <0.0001 |
| T × S | 99.79 | <0.0001 | 1648.51 | <0.0001 | 355.05 | <0.0001 |
| G × T × S | 1.69 | 0.0082 | 87.02 | <0.0001 | 37.82 | <0.0001 |
SE1 day, SE7 day, and SE56 day represent the stimulating effect of substrates added in 1-, 7-, and 56-day incubations, respectively, which were calculated from the accumulated C mineralization for different substrates (GLU, GRA, and MED) divided by that of CK.
Discussion
Soil respiration with grassland succession
Soil respiration rates and MBC both decreased logarithmically with succession of grassland restoration and were significantly linearly correlated with that grazing grasslands had higher soil respiration rates, MBC, and dissolved organic C than did grazing-exclusion grasslands (Wu et al. 2012). The decrease in soil respiration with grazing exclusion mostly resulted from a decrease in MBC or from changes in microbial communities. Our results also supported the assumption that Inner Mongolian grasslands subjected to grazing exclusion have apparent C sequestration capacity as a result of depressed soil respiration (Zhou et al. 2007; He et al. 2008, 2012; Ingram et al. 2008).
These findings were not consistent with the assumption that soil respiration should increase to offset newly input SOM with grazing-exclusion grassland succession. One possible explanation is that inputs of new SOM act as a binding agents and promote the formation of soil aggregates in grazing-exclusion grasslands without large-animal trampling (Steffens et al. 2009; Wiesmeier et al. 2012; Zimmermann et al. 2012). Decreases in MBC and microbial activity and greater physical protection of SOM by increased soil aggregation (Plante et al. 2009) should be the mechanism underlying increased C sequestration in soils in these long-term grazing-exclusion grasslands in Inner Mongolia.
Temperature sensitivity with ecosystem succession
Q10 decreased logarithmically with grassland restoration succession and was negatively correlated with the content of SOC, which is in agreement with the “C quality-temperature” hypothesis, that Q10 should be inversely related to C quality (Conant et al. 2008a; Craine et al. 2010; Xu et al. 2012). The C associated with sand and silt fractions in the surface soil increased logarithmically as the period of GE increased (He et al. 2009), which implied greater instability of SOM and lower Q10. This issue is still highly debated, although a large number of studies have focused on Q10 from various soil types, soil profiles, land-use types, and latitude gradients (Pavelka et al. 2007; Vanhala et al. 2008; Karhu et al. 2010b; Schindlbacher et al. 2010; Jenkins and Adams 2011). Zhou et al. (2009) demonstrated that the global pattern of Q10 varied from 1.40 to 2.03. Mahecha et al. (2010) found Q10 to be globally convergent on 1.40, independent of mean annual temperature, and invariant among biomes.
Temperature sensitivity with different stoichiometry of input SOM
Stoichiometry of input SOM (N:C ratios) has a significant influence on the Q10 of SOM decomposition, which implies that the Michaelis–Menten equation 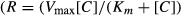 appropriately depicts the response of soil respiration after the addition of substrates because the canceling effect of Km on the apparent Q10 is greatly reduced by substrate saturation. Gershenson et al. (2009) reported that substrate availability had a significant positive effect on Q10. Compared with GLU and GRA, MED had a significantly greater effect on Q10, which implies that substrates with higher N:C will facilitate Q10 by reducing microbial N limitation in the process of SOM decomposition. Similarly, Billings and Ballantyne (2013) demonstrated that any reduction in the C:N flow ratio resulting from temperature increase could exacerbate extant C limitation or shift microbes from N limitation to C limitation. Our findings supported the assumption of Billings and Ballantyne (2013) and further highlight the importance of stoichiometry of substrate.
appropriately depicts the response of soil respiration after the addition of substrates because the canceling effect of Km on the apparent Q10 is greatly reduced by substrate saturation. Gershenson et al. (2009) reported that substrate availability had a significant positive effect on Q10. Compared with GLU and GRA, MED had a significantly greater effect on Q10, which implies that substrates with higher N:C will facilitate Q10 by reducing microbial N limitation in the process of SOM decomposition. Similarly, Billings and Ballantyne (2013) demonstrated that any reduction in the C:N flow ratio resulting from temperature increase could exacerbate extant C limitation or shift microbes from N limitation to C limitation. Our findings supported the assumption of Billings and Ballantyne (2013) and further highlight the importance of stoichiometry of substrate.
In theory, a negative respiratory response to warming should be observed as microbes experience greater relative C limitation, although such a response may be mediated by shifts in function within the microbial community (Luo et al. 2001). The N:C ratios of added substrates significantly affected Q10; however, it is still not clear whether Q10 is linearly related to the N:C ratio of these substrates. Therefore, changes in the plant community and in N:C ratios of new SOM inputs should influence the stability of intrinsic SOM and soil C sequestration potential, through regulation of Q10 by altering the stoichiometry of new inputs of SOM from root exudates in Inner Mongolian grasslands (Kong et al. 2011). If this holds true, achieving increased soil C storage by sowing legumes, as confirmed by many studies, should be re-evaluated under warming scenarios.
SEs with grassland succession and stoichiometry of input SOM
Grassland type, incubation temperature, and added substrates influenced SEs of CO2 emission after the addition of substrates. SEs were higher in GE0 and GE31, but lower in GE4, GE7, and GE11, which implied that the soils in GE0 and GE31 had lower C sequestration for a given input of SOM. This finding provides one of underlying mechanisms for an initial rapid increase in soil C storage following GE introduction followed by relatively stable levels after 2 decades of GE (He et al. 2008; Wu et al. 2008). Temperature influences the magnitude and duration of SEs after substrates are added. SEs increased with increasing temperature in the first stage and then reached a maximum at 15°C for GLU, GRA, and MED addition. Increase in the higher N:C ratio of the substrates added led to increased SEs in the first stage, but these were of shorter duration. The possible explanations for this are as follows: (1) addition of higher N:C substrates temporarily improves SOM quality and accelerates soil respiration, but this then leads to microbial N limitation when labile SOM is depleted (Billings and Ballantyne 2013), (2) temperature affects soil microbial enzyme activity, and 15°C should be the optimum temperature at which limitation of SOM quality after substrate addition does not occur.
In conclusion, the Q10 of SOM decomposition decreased logarithmically with grassland restoration succession and was negatively correlated with SOC content. The stoichiometry (N:C) of new SOM input had an apparent influence on soil C mineralization and temperature sensitivity, and higher N:C ratios led to higher Q10 levels and stronger soil C mineralization. Our findings imply that changes in the plant community and in the N:C ratios of new SOM input from litter, root, and roots exudates would influence the stability of intrinsic SOM and soil C sequestration potential through regulation of Q10. Overall, changes in Q10 with ecosystem succession are controlled by MBC, SOM quality, and the stoichiometry of new input SOM; these should be the underlying ecosystem-level mechanisms for soil C sequestration in grazing-exclusion grasslands.
Acknowledgments
Funding for this work came from The National Key Technology R&D Program (2012BAC01B08) and the Natural Science Foundation of China (31270519, 31070431).
Conflict of Interest
None declared.
References
- Billings SA, Ballantyne F. How interactions between microbial resource demands, soil organic matter stoichiometry, and substrate reactivity determine the direction and magnitude of soil respiratory responses to warming. Glob. Change Biol. 2013;19:90–102. doi: 10.1111/gcb.12029. [DOI] [PubMed] [Google Scholar]
- Cheng WX, Virginia RA. Measurement of microbial biomass in Arctic tundra soils using fumigation extraction and substrate-induced respiration procedures. Soil Biol. Biochem. 1993;25:135–141. [Google Scholar]
- Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF, et al. Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Change Biol. 2008a;14:868–877. [Google Scholar]
- Conant RT, Steinweg JM, Haddix ML, Paul EA, Plante AF, Six J. Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology. 2008b;89:2384–2391. doi: 10.1890/08-0137.1. [DOI] [PubMed] [Google Scholar]
- Conant RT, Ryan MG, Agren GI, Birge HE, Davidson EA, Eliasson PE, et al. Temperature and soil organic matter decomposition rates - synthesis of current knowledge and a way forward. Glob. Change Biol. 2011;17:3392–3404. [Google Scholar]
- Craine J, Spurr R, Mclauchlan K, Fierer N. Landscape-level variation in temperature sensitivity of soil organic carbon decomposition. Soil Biol. Biochem. 2010;42:373–375. [Google Scholar]
- Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- Fang CM, Smith P, Moncrieff JB, Smith JU. Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature. 2005;433:57–59. doi: 10.1038/nature03138. [DOI] [PubMed] [Google Scholar]
- Fang C, Smith P, Smith JU. Is resistant soil organic matter more sensitive to temperature than the labile organic matter? Biogeosciences. 2006;3:65–68. [Google Scholar]
- Fierer N, Craine JM, McLauchlan K, Schimel JP. Litter quality and the temperature sensitivity of decomposition. Ecology. 2005;86:320–326. [Google Scholar]
- Gallaher RN, Weldon CO, Boswell FC. A semi-automated procedure for total nitrogen in plant and soil samples. Soil Sci. Soc. Am. J. 1976;40:887–889. [Google Scholar]
- Gershenson A, Bader NE, Cheng WX. Effects of substrate availability on the temperature sensitivity of soil organic matter decomposition. Glob. Change Biol. 2009;15:176–183. [Google Scholar]
- Hartley IP, Ineson P. Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol. Biochem. 2008;40:1567–1574. [Google Scholar]
- He NP, Yu Q, Wu L, Wang YS, Han XG. Carbon and nitrogen store and storage potential as affected by land-use in a Leymus chinensis grassland of northern China. Soil Biol. Biochem. 2008;40:2952–2959. [Google Scholar]
- He NP, Wu L, Wang YS, Han XG. Changes in carbon and nitrogen in soil particle-size fractions along a grassland restoration chronosequence in northern China. Geoderma. 2009;150:302–308. [Google Scholar]
- He NP, Zhang YH, Dai JZ, Han XG, Baoyin TGT, Yu GR. Land-use impact on soil carbon and nitrogen sequestration in typical steppe ecosystems, Inner Mongolia. J. Geog. Sci. 2012;22:859–873. [Google Scholar]
- Ingram LJ, Stahl PD, Schuman GE, Buyer JS, Vance GF, Ganjegumte GK, et al. Grazing impacts on soil carbon and microbial communities in a mixed-grass ecosystem. Soil Sci. Soc. Am. J. 2008;72:939–948. [Google Scholar]
- Jenkins ME, Adams MA. Respiratory quotients and Q10 of soil respiration in sub-alpine Australia reflect influences of vegetation types. Soil Biol. Biochem. 2011;43:1266–1274. [Google Scholar]
- Karhu K, Fritze H, Hamalainen K, Vanhala P, Jungner H, Oinonen M, et al. Temperature sensitivity of soil carbon fractions in boreal forest soil. Ecology. 2010a;91:370–376. doi: 10.1890/09-0478.1. [DOI] [PubMed] [Google Scholar]
- Karhu K, Fritze H, Tuomi M, Vanhala P, Spetz P, Kitunen V, et al. Temperature sensitivity of organic matter decomposition in two boreal forest soil profiles. Soil Biol. Biochem. 2010b;42:72–82. [Google Scholar]
- Kirschbaum MUF. The temperature-dependence of soil organic-matter decomposition, and the effect of global warming on soil organic-C storage. Soil Biol. Biochem. 1995;27:753–760. [Google Scholar]
- Knorr W, Prentice IC, House JI, Holland EA. Long-term sensitivity of soil carbon turnover to warming. Nature. 2005;433:298–301. doi: 10.1038/nature03226. [DOI] [PubMed] [Google Scholar]
- Kong DL, Wu HF, Zeng H, Lü XT, Simmons M, Wang M, et al. Plant functional group removal alters root biomass and nutrient cycling in a typical steppe in Inner Mongolia, China. Plant Soil. 2011;346:133–144. [Google Scholar]
- Luo YQ, Wan SQ, Hui DF, Wallace LL. Acclimatization of soil respiration to warming in a tall grass prairie. Nature. 2001;413:622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- Mahecha MD, Reichstein M, Carvalhais N, Lasslop G, Lange H, Seneviratne SI, et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science. 2010;329:838–840. doi: 10.1126/science.1189587. [DOI] [PubMed] [Google Scholar]
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR, editors. Methods of soil analysis. Madison, WI: American Society of Agronomy and Soil Science Society of American; 1982. pp. 1–129. [Google Scholar]
- Pavelka M, Acosta M, Marek MV, Kutsch W, Janous D. Dependence of the Q10 values on the depth of the soil temperature measuring point. Plant Soil. 2007;292:171–179. [Google Scholar]
- Plante AF, Six J, Paul EA, Conant RT. Does physical protection of soil organic matter attenuate temperature sensitivity? Soil Sci. Soc. Am. J. 2009;73:1168–1172. [Google Scholar]
- Plante AF, Conant RT, Carlson J, Greenwood R, Shulman JM, Haddix ML, et al. Decomposition temperature sensitivity of isolated soil organic matter fractions. Soil Biol. Biochem. 2010;42:1991–1996. [Google Scholar]
- Schindlbacher A, Diaz-Pines C, De Gonzalo E, Gorria P, Matthews B, Inclan R, et al. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. J. Geophys. Res. Biogeosci. 2010;115:G03018. [Google Scholar]
- Sierra CA. Temperature sensitivity of organic matter decomposition in the Arrhenius equation: some theoretical considerations. Biogeochemistry. 2012;108:1–15. [Google Scholar]
- Steffens M, Kolbl A, Kögel-Knabner I. Alteration of soil organic matter pools and aggregation in semi-arid steppe topsoils as driven by organic matter input. Eur. J. Soil Sci. 2009;60:198–212. [Google Scholar]
- Tucker CL, Bell J, Pendall E, Ogle K. Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming? Glob. Change Biol. 2013;19:252–263. doi: 10.1111/gcb.12036. [DOI] [PubMed] [Google Scholar]
- Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987;19:703–707. [Google Scholar]
- Vanhala P, Karhu K, Tuomi M, Bjorklof K, Fritze H, Liski J. Temperature sensitivity of soil organic matter decomposition in southern and northern areas of the boreal forest zone. Soil Biol. Biochem. 2008;40:1758–1764. [Google Scholar]
- Wetterstedt JM, Agren GI. Quality or decomposer efficiency - which is most important in the temperature response of litter decomposition? A modelling study using the GLUE methodology. Biogeosciences. 2011;8:477–487. [Google Scholar]
- Wiesmeier M, Steffens M, Mueller CW, Kolbl A, Reszkowska A, Peth S, et al. Aggregate stability and physical protection of soil organic carbon in semi-arid steppe soils. Eur. J. Soil Sci. 2012;63:22–31. [Google Scholar]
- Wu L, He NP, Wang YS, Han XG. Storage and dynamics of carbon and nitrogen in soil following grazing exclusion in Leymus chinensis grasslands of northern China. J. Environ. Qual. 2008;37:663–668. doi: 10.2134/jeq2007.0196. [DOI] [PubMed] [Google Scholar]
- Wu HH, Wiesmeier M, Yu Q, Steffens M, Han XG, Kogel-Knabner I. Labile organic C and N mineralization of soil aggregate size classes in semiarid grasslands as affected by grazing management. Biol. Fertil. Soils. 2012;48:305–313. [Google Scholar]
- Xu X, Luo YQ, Zhou JZ. Carbon quality and the temperature sensitivity of soil organic carbon decomposition in a tallgrass prairie. Soil Biol. Biochem. 2012;50:142–148. [Google Scholar]
- Zhou ZY, Sun OJ, Huang JH, Li LH, Liu P, Han XG. Soil carbon and nitrogen stores and storage potential as affected by land-use in an agro-pastoral ecotone of northern China. Biogeochemistry. 2007;82:127–138. [Google Scholar]
- Zhou T, Shi PJ, Hui DF, Luo YQ. Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback. J. Geophys. Res. Biogeosci. 2009;114:G02016. doi: 10.1029/2008JG000850. [Google Scholar]
- Zimmermann M, Bird MI. Temperature sensitivity of tropical forest soil respiration increase along an altitudinal gradient with ongoing decomposition. Geoderma. 2012;187:8–15. [Google Scholar]
- Zimmermann M, Leifeld J, Conen F, Bird MI, Meir P. Can composition and physical protection of soil organic matter explain soil respiration temperature sensitivity? Biogeochemistry. 2012;107:423–436. [Google Scholar]


