Abstract
Background and Purpose
While studies have linked types of fatty acids with coronary heart disease, data on individual fatty acids and risk of ischemic stroke are limited. We aimed to examine the associations between serum fatty acid concentrations and incidence of ischemic stroke and its subtypes.
Methods
We conducted a prospective case-control study nested in the Women's Health Initiative Observational Study cohort of postmenopausal US women aged 50 to 79 years. Between 1993 and 2003, incident cases of ischemic stroke were matched 1:1 to controls on age, race, and length of follow-up (964 matched pairs). Conditional logistic regression was used to estimate odds ratios (OR) and 99.9% confidence intervals (CI) for ischemic stroke and its subtypes.
Results
The multivariable adjusted OR and 99.9% CI of ischemic stroke associated with a 1-standard deviation (SD) increment in serum fatty acid concentration was 1.38 (99.9% CI, 1.05-1.83) for linelaidic acid (18:2tt, SD=0.04%); 1.27 (99.9% CI, 1.06-1.51) for palmitic acid (16:0, SD=2.74%); 1.20 (99.9% CI, 1.01-1.43) for oleic acid (18:1n9, SD=2.32%); 0.72 (99.9% CI, 0.59-0.87) for docosapentaenoic acid (22:5n3, SD=0.18%); 0.72 (99.9% CI, 0.59-0.87) for docosahexaenoic acid (22:6n3, SD=0.91%); and 0.81 (99.9% CI, 0.67-0.98) for arachidonic acid (20:4n6, SD=2.02%). These associations were generally consistent for atherothrombotic and lacunar stroke, but not cardioembolic stroke.
Conclusions
These findings suggest that individual serum trans, saturated, and monounsaturated fatty acids are positively associated with particular ischemic stroke subtypes, while individual n3 and n6 polyunsaturated fatty acids are inversely associated.
Introduction
Few epidemiologic studies have examined the associations between serum fatty acids, a biomarker of fatty acid intakes, and ischemic stroke risk.1 Thus far, the results have been inconsistent. One study conducted in Japan found that serum saturated fatty acids were associated with increased risk of ischemic stroke, while serum linoleic acid (an n6 polyunsaturated fatty acid) was associated with decreased risk.2 Another study conducted in Finland found similar associations as the Japan study, but additionally reported that serum monounsaturated and n3 polyunsaturated fatty acids were associated with increased risk of ischemic stroke.3 However, a third study found no significant associations for serum saturated or monounsaturated fatty acids.4
Two possibilities have been raised that could explain these inconsistent findings. First, the associations between serum fatty acids and ischemic stroke may not be consistent for all ischemic stroke subtypes. In the Japan study described previously, the results were driven by lacunar stroke, this subtype comprising 95 of the 122 ischemic strokes in that study.2 Importantly, this study did not have sufficient power to examine associations for atherothrombotic stroke, the subtype of ischemic stroke directly related to blood cholesterol.5 Combining atherothrombotic stroke with lacunar and cardioembolic strokes would dilute expected associations. However, to our knowledge, only this Japan study has examined serum fatty acids in relation to atherothrombotic stroke and other ischemic stroke subtypes.
Second, the inconsistent findings could be due to individual fatty acids having different effects on ischemic stroke. In the Japan study described previously, despite finding that serum myristic and palmitic, but not stearic, saturated fatty acids were positively associated with total ischemic stroke in unadjusted analysis, investigators combined these three fatty acids in multivariable analysis.2 Depending on the relative contribution of each fatty acid, it is possible to find inconsistent associations for total serum saturated fatty acids. However, few studies have examined serum fatty acids individually while controlling for potential confounders.
To clarify these possibilities, we conducted a prospective case-control study nested in a large cohort of postmenopausal US women to examine the associations between 25 individual serum fatty acid concentrations from stored serum samples collected at baseline and the incidence of ischemic stroke and ischemic stroke subtypes. We hypothesize that individual serum trans and saturated fatty acids would be positively associated with atherothrombotic stroke, whereas individual serum monounsaturated and polyunsaturated fatty acids would be inversely associated.
Methods
Study Population
We conducted a case-control study of 964 incident ischemic stroke cases and 964 matched control subjects nested within the Women's Health Initiative Observational Study (WHI-OS).6 Briefly, the WHI was conducted in 40 clinical centers across the US to examine the impact of a number of factors on the major causes of morbidity and mortality in postmenopausal women.7 Eligible women were 50 to 79 years of age at baseline, postmenopausal, had no medical conditions associated with a predicted survival of <3 years, and provided informed consent to be a part of the study as approved by the institutional review boards. The WHI-OS enrolled 93,676 women between October 1993 and December 1998. There were 972 confirmed incident ischemic stroke cases between 1993 and 2003. Control subjects were individually matched 1:1 to the cases on age and race at the time of ischemic stroke. Blood quantity was not sufficient to analyze fatty acid content for 9 participants, leaving a total of 964 case-control pairs.
Case identification and classification
Incident ischemic strokes during the follow-up period were identified through self-report during annual medical history updates (annual response rate 94%).8 Using additional details from medical charts, brain imaging, or death certificates, the potential outcomes were subject to local adjudication by physicians, then central adjudication by trained neurologists, according to standard criteria. Over 95% of participant-reported stroke cases were classified based on CT or MRI findings.9
Central adjudicators further classified ischemic strokes by subtypes according to the Trial of ORG 10172 Acute Stroke Trial (TOAST) Classification.10 The TOAST classification focuses on the presumed underlying stroke mechanism; requires detailed investigations (such as brain computed tomography, magnetic resonance imaging, angiography, carotid ultrasound, and echocardiography); and distinguishes 5 categories of stroke, which include large artery atherothrombotic stroke, small vessel lacunar stroke, cardioembolic stroke, other, and undetermined mechanism.11 Probable and possible subtypes determinations were combined in this report. Transient ischemic attack, hemorrhagic stroke, strokes not requiring hospitalization, and strokes not confirmed by central adjudication were not included as a stroke outcome.
Control selection
Controls were sampled from women at risk at the time ischemic stroke events occurred during the follow-up period and matched 1:1 to case subjects on age at baseline (±2 years), race/ethnicity (white, black, Hispanic, Asian, American Indian, or other/unspecified), date of study enrollment (±3 months), and follow-up time.
Data collection
Fasting blood samples were collected from all WHI-OS participants by clinic staff members following a standardized protocol for venipuncture at baseline.12 Samples were centrifuged, separated by layers, frozen onsite at −70°C, and shipped to the central WHI repository for long-term storage.
Serum fatty acids were analyzed at Dr. Rhobert Evans' lab at the University of Pittsburgh. Fatty acids were extracted according to the general technique of Bligh and Dyer using 1, 2-dinonadecanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Inc. Alabaster, AL) (50 μg of 19:0) as an internal standard.13 The lipid extracts were resuspended in 1.5 ml 14% boron trifluoride methanol and derivatized according to the procedure of Morrison and Smith.14 The extracts were analyzed by capillary GC:column (SP-2380, 105 m × 53 mm ID, 0.20 um film thickness). The gas chromatograph was a Perkin Elmer Clarus 500 equipped with a flame ionization detector. Operating conditions: the oven temperature was 140°C for 35 min, then ramped at 8°C/min to 220°C, and held for 12 min. Injector and detector temperatures were both at 260°C and helium, the carrier gas, was at 15 psi. Identification of components was by comparison of retention times with those of authentic standards (Sigma Chemical Co., St. Louis, MO). Duplicate samples and control pools were analyzed with each batch of samples. The coefficients of variation of fatty acids ranged from 3.1% to 13.2%. The Spearman correlation coefficients between dietary fatty acid intake (% of total fat intake assessed by a FFQ) and serum fatty acid concentrations among non-cases (both measured at baseline) were 0.53 for docosahexaenoic acid (DHA), 0.42 for eicosapentaenoic acid (EPA), 0.18 for docosapentaenoic acid (DPA), 0.16 for palmitic acid, 0.15 for linelaidic acid, 0.07 for arachidonic acid, and 0.03 for oleic acid.
Participants completed sociodemographic and lifestyle questionnaires during baseline visits to a WHI clinical center.12 Physical activity was assessed based on participant report about the frequency, intensity, and duration of walking, exercise, or recreational activity and summarized into total metabolic equivalent task (MET)-hours per week. Certified clinic staff measured participant height, weight, and blood pressure. Height and weight were measured without shoes or heavy clothing and with pocket contents removed. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Following standardized procedures, blood pressure was measured twice after a 5-minute seated rest with a 30-second rest in between. The average of two blood pressure measurements was used in analysis. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or a diastolic blood pressure ≥ 90 mm Hg or self-report of antihypertensive medication use. Diabetes was defined as being on treatment for diabetes by self-report or having a fasting glucose level ≥ 126 mg/dL. Participants reported their history of smoking, diabetes, atrial fibrillation, angina, or revascularization (coronary bypass surgery or angioplasty of the coronary arteries), as well as current use of medications.
Lipoprotein profiles were assayed at Liposcience using nuclear magnetic resonance. Low-density lipoprotein (LDL) cholesterol was calculated from high-density lipoprotein (HDL) cholesterol, total cholesterol, and triglyceride concentrations among women who had a triglyceride value <400 mg/dL using the Friedewald equation.15 LDL cholesterol values were set to missing for those women whose triglyceride value was >400 mg/dL (n=35) or who were missing HDL cholesterol, total cholesterol, or triglyceride values (n=7).
Statistical analysis
The McNemar's chi-square test was used to compare covariate proportions between ischemic stroke cases and controls. The Wilcoxon signed-rank test was used to compare serum fatty acid concentration and covariate means between ischemic stroke cases and controls. The distributions of the serum fatty acids were highly skewed to the right. Therefore, we used the median as the measure of central tendency for descriptive statistics.
Conditional logistic regression was used to estimate odds ratios (OR) and 99.9% confidence intervals (CI) for ischemic stroke and its subtypes, conditioning on matching factors in all models. We divided women into quintiles based on distribution of serum fatty acid concentrations in the case-control study population. We estimated multivariable adjusted ORs and 99.9% CIs comparing the odds of ischemic stroke among women in the higher quintiles of serum fatty acids with the odds of ischemic stroke in the lowest quintile. Linear trend tests were performed by assigning the median value of the quintile of the fatty acid to each category and entering this variable into the model as a continuous variable. Since trends were generally linear, multivariable adjusted ORs were calculated per 1-standard deviation (SD) increment in each fatty acid. Our primary model was adjusted for BMI, smoking status, diabetes, and aspirin use. Subsequent models were adjusted for blood pressure and blood cholesterol variables, since these factors are the likely mediators of the association between serum fatty acids and ischemic stroke. Two-sided P ≤ 0.001 was considered significant to account for multiple comparisons and to decrease the risk of detecting a false positive association (Type I error). All analyses were performed using SAS statistical software (Version 9.2, SAS Institute Inc., Cary, NC, USA).
Results
Study Population
Between 1993 and 2003, we identified 964 cases of incident ischemic stroke. Of these, 96 were atherothrombotic strokes, 250 were lacunar strokes, 209 were cardioembolic strokes, 42 were ischemic strokes of other determined etiology, 366 were ischemic strokes of undetermined etiology, and 1 was missing subtype information. The mean age at baseline of women with ischemic stroke was 68.7 (SD 6.4) years (Table 1). Women with incident ischemic stroke were more likely to be overweight or obese, be a current smoker, have diabetes, and to use aspirin. We accounted for these differences by adjusting for these variables in multivariable adjusted analysis.
Table 1.
Baseline characteristics of the study population by ischemic stroke case and control status.
| Non-case | Case | |
|---|---|---|
| N | 964 | 964 |
| Age, year | 68.7 (6.4) | 68.7 (6.4) |
| Race | ||
| African American | 79 (8) | 79 (8) |
| Native American | 5 (1) | 5 (1) |
| Asian | 21 (2) | 21 (2) |
| Hispanic | 20 (2) | 20 (2) |
| White | 826 (86) | 826 (86) |
| Other | 10 (1) | 10 (1) |
| Education | ||
| ≤ High school/GED | 226 (24) | 235 (25) |
| Some college/ training after high school | 358 (37) | 391 (41) |
| College graduate | 377 (39) | 330 (35) |
| Family income | ||
| <$35,000 | 427 (48) | 479 (54) |
| $35,000 to $74,999 | 332 (38) | 302 (34) |
| ≥$75,000 | 126 (14) | 114 (13) |
| Moderate/strenuous activities ≥20 minutes* | ||
| None | 121 (13) | 149 (16) |
| Some | 356 (37) | 419 (44) |
| 2-4 episodes/wk | 198 (21) | 160 (17) |
| >4 episodes/wk | 282 (29) | 225 (24) |
| Alcohol intake | ||
| Non-drinker | 111 (12) | 117 (12) |
| Past drinker | 177 (18) | 211 (22) |
| <7 drinks/wk | 559 (58) | 511 (53) |
| ≥7 drinks/wk | 116 (12) | 122 (13) |
| Blood pressure, mm Hg | ||
| Systolic* | 130 (18) | 137 (20) |
| Diastolic* | 74 (10) | 75 (10) |
| Hypertensiona* | 450 (47) | 647 (67) |
| Serum lipids, mg/dL | ||
| Total cholesterol | 231 (38) | 233 (39) |
| HDL-C* | 60 (16) | 57 (16) |
| LDL-C | 139 (37) | 141 (37) |
| Triglycerides* | 161 (80) | 180 (90) |
| Total cholesterol to HDL-C ratio* | 4.2 (1.4) | 4.4 (1.5) |
| BMI, kg/m2* | ||
| Mean | 27.0 (5.3) | 27.7 (5.9) |
| < 25.0 | 388 (41) | 330 (35) |
| 25.0 to 29.9 | 342 (36) | 365 (38) |
| ≥30.0 | 221 (23) | 260 (27) |
| Smoking status* | ||
| Never | 523 (55) | 501 (53) |
| Past | 397 (42) | 374 (39) |
| Current | 36 (4) | 78 (8) |
| Comorbidities | ||
| Diabetesb* | 72 (7) | 153 (16) |
| Atrial fibrillation* | 55 (6) | 92 (10) |
| Angina* | 53 (6) | 90 (9) |
| Revascularization* | 11 (1) | 38 (4) |
| Medications | ||
| Aspirin* | 238 (25) | 295 (31) |
| Antihypertensive* | 319 (33) | 449 (47) |
| Lipid-lowering | 157 (16) | 182 (19) |
| Statins | 83 (9) | 92 (10) |
| HRT | 363 (38) | 381 (40) |
GED, General Equivalency Diploma; BMI, body mass index; HRT, hormone replacement therapy
McNemar's chi-square or paired Student's t test P ≤ 0.05, as appropriate.
Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or a diastolic blood pressure ≥ 90 mm Hg or self-report of antihypertensive medication use.
Diabetes was defined as having a fasting glucose level ≥ 126 mg/dL or being on treatment for diabetes by self-report.
Distribution of serum fatty acids
Twenty-five individual serum fatty acids were examined (Table 2). The major contributors (median >5%) to the composition of serum fatty acids were linoleic (27%), palmitic (24%), oleic (18%), stearic (9%), and arachidonic (8%) fatty acids. The serum concentrations of 18:1t and linelaidic trans fatty acids, myristic and palmitic saturated fatty acids, and 7-hexadecenoic, oleic, and eicosenoic monounsaturated fatty acids were higher among ischemic stroke cases than controls. The concentrations of alpha-linolenic, eicosatetraenoic, EPA, DPA, DHA n3 polyunsaturated fatty acids and linoleic, arachidonic, adrenic, and 22:5n6 n6 polyunsaturated fatty acids were lower among ischemic stroke cases than controls.
Table 2. Distribution of serum fatty acid concentrationsa.
| Non-case | Case | |||||||
|---|---|---|---|---|---|---|---|---|
| Common name | Q1 | Median | Q3 | Q1 | Median | Q3 | Diffb | |
| 18:2n6 | Linoleic | 24.22 | 27.12 | 30.40 | 23.78 | 26.73 | 29.70 | *-0.55 |
| 16:0 | Palmitic | 22.07 | 23.77 | 25.77 | 22.56 | 24.37 | 26.44 | * 0.65 |
| 18:1n9 | Oleic | 16.12 | 17.59 | 19.07 | 16.66 | 18.17 | 19.68 | * 0.46 |
| 18:0 | Stearic | 7.83 | 8.54 | 9.18 | 7.67 | 8.42 | 9.20 | -0.06 |
| 20:4n6 | Arachidonic | 7.02 | 8.27 | 9.67 | 6.63 | 8.01 | 9.43 | *-0.31 |
| 16:1n7 | 7-Hexadecenoic | 1.65 | 2.23 | 2.96 | 1.79 | 2.34 | 3.09 | * 0.15 |
| 20:3n6 | Dihomo-gamma-linolenic | 1.71 | 2.05 | 2.40 | 1.75 | 2.03 | 2.34 | -0.01 |
| 22:6n3 | Docosahexaenoic | 1.50 | 1.97 | 2.65 | 1.34 | 1.75 | 2.32 | *-0.26 |
| 18:1n7 | Vaccenic | 1.29 | 1.47 | 1.69 | 1.31 | 1.49 | 1.68 | 0.01 |
| 14:0 | Myristic | 0.64 | 0.84 | 1.09 | 0.66 | 0.89 | 1.14 | * 0.06 |
| 18:1t | (no common name) | 0.56 | 0.75 | 1.03 | 0.60 | 0.80 | 1.05 | * 0.05 |
| 20:5n3 | Eicosapentaenoic | 0.44 | 0.60 | 0.85 | 0.40 | 0.56 | 0.76 | *-0.09 |
| 22:5n3 | Docosapentaenoic | 0.45 | 0.56 | 0.68 | 0.41 | 0.51 | 0.63 | *-0.05 |
| 18:3n3 | Alpha-linolenic | 0.44 | 0.55 | 0.68 | 0.42 | 0.53 | 0.66 | *-0.02 |
| 18:3n6 | Gamma-linolenic | 0.32 | 0.41 | 0.53 | 0.31 | 0.41 | 0.52 | -0.01 |
| 16:1t | Palmitelaidic | 0.27 | 0.33 | 0.38 | 0.28 | 0.33 | 0.39 | 0.001 |
| 22:4n6 | Adrenic | 0.15 | 0.30 | 0.43 | 0.15 | 0.29 | 0.42 | *-0.005 |
| 17:0 | Margaric | 0.25 | 0.28 | 0.32 | 0.24 | 0.28 | 0.32 | -0.003 |
| 20:2n6 | Eicosadienoic | 0.22 | 0.26 | 0.30 | 0.22 | 0.25 | 0.30 | -0.004 |
| 22:5n6 | (no common name) | 0.19 | 0.25 | 0.32 | 0.19 | 0.24 | 0.31 | *-0.012 |
| 15:0 | Pentanoic | 0.15 | 0.19 | 0.23 | 0.15 | 0.19 | 0.23 | 0.003 |
| 20:1n9 | Eicosenoic | 0.09 | 0.14 | 0.24 | 0.10 | 0.14 | 0.26 | *0.004 |
| 20:4n3 | Eicosatetraenoic | 0.05 | 0.08 | 0.11 | 0.05 | 0.07 | 0.10 | *-0.004 |
| 18:2tt | Linelaidic | 0.02 | 0.03 | 0.06 | 0.02 | 0.04 | 0.07 | * 0.005 |
| 24:1n9 | Nervonic | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.05 | -0.004 |
Q, quartile; Diff, difference; t, trans.
Fatty acids are shown in order of median concentration of fatty acids among non-cases.
The average of the differences between case and control serum fatty acid concentration.
Wilcoxon signed-rank test P ≤ 0.05.
Total ischemic stroke
With adjustment for BMI status, smoking status, diabetes, and aspirin use and accounting for the matching factors, serum linelaidic acid (18:2tt, SD=0.04%, OR=1.38, 99.9%CI, 1.05-1.83), palmitic acid (16:0, SD=2.74%, OR=1.27, 99.9%CI, 1.06-1.51), and oleic acid (18:1n9, SD=2.32%, OR=1.20, 99.9%CI, 1.01-1.43) were associated with increased incidence of ischemic stroke. Serum DPA (22:5n3, SD=0.18%, OR=0.72, 99.9%CI, 0.59-0.87), DHA (22:6n3, SD=0.91%, OR=0.72, 99.9%CI, 0.59-0.87) and arachidonic acid (20:4n6, SD=2.02%, OR=0.81, 99.9%CI, 0.67-0.98) were associated with decreased incidence (Figure 1, eTable 1). Further adjustment for blood pressure and blood cholesterol did not change estimates by more than 10%.Neither did further adjustment for physical activity, angina, atrial fibrillation, or revascularization change estimates by more than 10% (data not shown).
Figure 1.
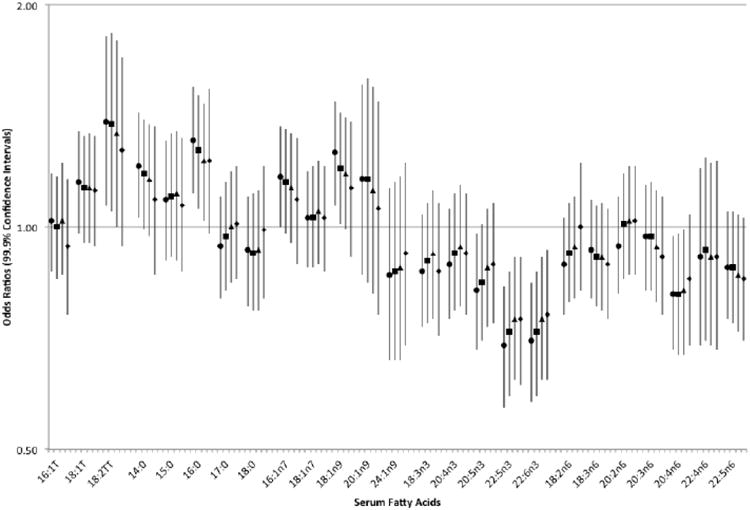
Odds ratios and 99.9% confidence intervals for total ischemic stroke associated with a 1-standard deviation increment in individual serum fatty acids.
To examine the possibility that the positive association for serum oleic acid was due to its correlations with serum arachidonic acid or serum DHA (Spearman rank correlation coefficients -0.43 and -0.31, respectively), we additionally adjusted for these fatty acids using the residual method.16 The multivariable adjusted OR for total ischemic stroke associated with a 1-SD increment in oleic acid was attenuated by 9% after adjustment for serum arachidonic acid, by 10% after adjustment for serum DHA, and completely attenuated after adjustment for serum total polyunsaturated fatty acids (OR=1.00, 99.9% CI, 0.84-1.19).
Ischemic stroke subtypes
We further examined the fatty acids that were significantly associated with total ischemic stroke (serum linelaidic acid, palmitic acid, oleic acid, DPA, DHA, and arachidonic acid) in relation to ischemic stroke subtypes. The associations between these serum fatty acids and total ischemic stroke were generally consistent for atherothrombotic stroke, lacunar stroke, and ischemic stroke of undetermined etiology, except that we did not observe a positive association between palmitic acid and atherothrombotic stroke (Figure 2, eTable 2).We did observe an inverse association between serum DHA and cardioembolic stroke, making DHA the only serum fatty acid to be consistently inversely associated with all subtypes of ischemic stroke. We did not have sufficient statistical power to examine associations for ischemic strokes of other determined etiology (42 matched pairs).
Figure 2.
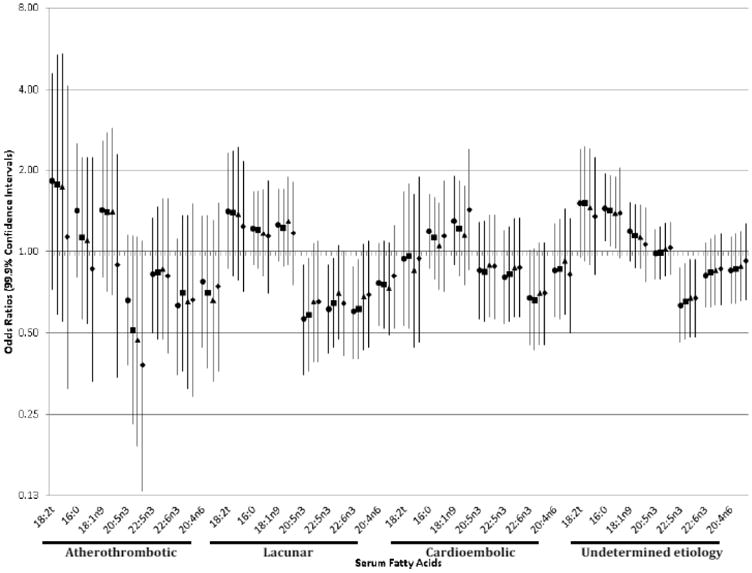
Odds ratios and 99.9% confidence intervals for ischemic stroke subtypes associated with a 1-standard deviation increment in individual serum fatty acids.
Further adjustment for blood pressure attenuated associations for lacunar stroke (e.g., by 10% for DHA), but not atherothrombotic stroke. Further adjustment for blood cholesterol attenuated associations for lacunar stroke (e.g., by 37% for linelaidic fatty acid) and atherothrombotic stroke (e.g., by 82% for linelaidic fatty acid, and reversed the associations for palmitic and oleic fatty acids).
Discussion
In this study, the largest case-control study of ischemic strokes among postmenopausal US women, serum linelaidic (trans) acid, palmitic (saturated) acid, and oleic (monounsaturated) acid were associated with increased incidence of ischemic stroke. Serum DPA, and DHA (n3 polyunsaturated) acids and arachidonic (n6 polyunsaturated) acid were associated with decreased incidence. These associations were generally consistent for atherothrombotic and lacunar stroke, but not cardioembolic stroke.
Our results suggest that individual serum fatty acids have different associations with ischemic stroke. However, large prospective cohort studies have not been able to examine fatty acids individually. These studies assessed fatty acid intake through participant report of diet (e.g. FFQ), which can only distinguish major types of fat (e.g., saturated fat). Two prospective studies reported that saturated fat intake and animal fat intake (mostly saturated fat) were associated with decreased risk of ischemic stroke and cerebral infarction death, respectively.17, 18 However, four prospective studies failed to find associations between saturated fat intake and ischemic stroke.19-22 One possible explanation for these inconsistent results is that individual fatty acids, even those of the same type, may have different effects on cardiovascular disease risk. For example, myristic (14:0) and palmitic (16:0) acids tend to increase plasma total and LDL-cholesterol concentrations compared with oleic acid, whereas stearic acid (18:0) does not.23 It is possible that examining general types of fat intake masked the associations of individual fatty acids and ischemic stroke. However, individual fatty acids used in studies of dietary intake tend to be highly correlated. These high correlations make distinguishing their individual relations with ischemic stroke risk difficult.24
In contrast to the prospective studies using fatty acids intake derived from participant report of intake, case-control studies using biomarkers of fatty acid intake – serum, erythrocytes, and adipose tissue – are able to examine fatty acids individually (e.g., palmitic acid). In the largest of these previous studies, Iso and colleagues found that serum myristic and palmitic acids, but not stearic acid, were associated with increased risk of ischemic stroke, even though all three are saturated fatty acids.2 In addition, serum linoleic acid, but not other polyunsaturated fatty acids, was associated with decreased risk of ischemic stroke. Smaller case-control studies also suggest that individual fatty acids, even those of the same type, may have different effects on ischemic stroke risk.4, 25-27 These studies highlight the importance of examining individual fatty acids in relation to ischemic stroke risk.
Our results also suggest that the associations between serum fatty acids and ischemic stroke may not be consistent across all ischemic stroke subtypes. To our knowledge, only two studies have reported associations for ischemic stroke subtypes. In these studies, the associations between individual fatty acids and total ischemic stroke were consistent for lacunar stroke, but not atherothrombotic or cardioembolic stroke. Iso and colleagues reported that the associations between serum saturated and linoleic fatty acids and ischemic stroke were driven by lacunar stroke, this subtype comprising 95 of the 122 ischemic strokes in that study.2 In a recent longitudinal study using data from the full WHI-OS cohort, we found that the positive association between dietary trans fat intake and ischemic stroke was consistent for lacunar stroke, but in that study, we were not able to examine the associations for individual fatty acids and the statistic power was insufficient for examining atherothrombotic strokes.22 Nevertheless, these prior studies highlight the importance of examining ischemic stroke subtypes separately.
The associations between fatty acids and ischemic stroke may not be consistent across all subtypes because ischemic stroke is a heterogeneous condition. There is evidence that blood cholesterol may play a role in the development of some ischemic stroke subtypes, but not others.5 Kuller and Reisler examined the incidence of ischemic stroke subtypes in populations with varying blood cholesterol levels.28 Based on this analysis, they hypothesized that blood cholesterol may play a role in atherothrombotic stroke, the ischemic stroke subtype with an atherogenic basis. Fatty acid intakes have a powerful effect on blood cholesterol concentrations, and they were hypothesized to play a role in the development of atherothrombotic stroke, as well. One of the challenges in studying atherothrombotic stroke, the least prevalent ischemic stroke subtype, is low statistical power. However, we had sufficient power to detect general trends in atherothrombotic stroke incidence according to serum fatty acid concentration. Consistent with Kuller and Reisler's hypothesis, linelaidic, oleic, DPA, DHA, and arachidonic acids were associated with atherothrombotic stroke.
While the effect of fatty acid intake on blood cholesterol likely explains the associations for atherothrombotic stroke, the explanation for the apparent associations for lacunar stroke is not clear. However, a few possibilities can be proposed. First, intake of DHA may lower blood pressure. A meta-analysis of 36 randomized trials reported that fish oil supplementation lowered systolic and diastolic blood pressures by 3.5 and 2.4 mm Hg, respectively.29 Importantly, serum DHA, which may have a stronger blood pressure lowering effect than EPA,30 was the only serum fatty acid that was consistently associated with all subtypes of ischemic stroke in the present study. Second, intake of DHA inhibits thrombosis through their anti-platelet effects.31 In the present study, serum DHA was inversely correlated with d-dimer concentration, which is a measure of thrombotic disease such as lacunar stroke. Third, intake of trans fat and n3 polyunsaturated fatty acids have pro- and anti-inflammatory effects, respectively.32, 33 High concentrations of inflammatory markers have been associated with greater neurological deterioration in lacunar strokes.34 Finally, there is limited evidence suggesting that trans fat intake may adversely affect glucose tolerance, which may lead to development of diabetes, which is a major risk factor for lacunar stroke. Most observational studies report a positive association between trans fat intake and diabetes, however these results have not been supported by the results of controlled feeding studies.35
Our study has some limitations. One concern is the stability of serum fatty acids during storage and freeze/thaw procedures. However, serum storage for up to 12 years at −80°C protected polyunsaturated fatty acids, the most susceptible fatty acids to storage and freeze-thaw, from oxidation.36 We conducted an analysis of 15 samples analyzed originally in 2005, one year later in 2006, and then after an additional freeze/thaw procedure. No statistically significant differences were detected between the mean values of the fatty acids from the original and one-year or freeze/thaw samples. In addition, serum fatty acids may reflect diet only over a short period of time, and only one measure of serum was done in the WHI. We make the assumption that the concentration of fatty acids in serum remains relatively stable over time.
When these ischemic strokes were classified, TOAST was the best currently available with moderate to good inter-observer reliability with training11. The field of ischemic stroke classification has been evolving to address the large number of cases that are classified as having “undetermined etiology.” In the newer ASCO Phenotypic System, cases can be characterized by all 4 phenotypes based on the level of diagnostic certainty37. Using the ASCO classification could reduce the proportion of ischemic stroke cases with underdetermined etiology and increase statistical power.
Another concern is that the TOAST classification uses a history of diabetes mellitus and hypertension in its lacunar stroke definition.38 This classification bias could, in part, explain the association between serum fatty acid and lacunar stroke, assuming that serum fatty acids act through hypertension to increase risk of ischemic stroke. More recent studies have advocated the use of a “risk factor-free classification” to avoid classification bias.5 However, the prevalence of hypertension among the ischemic stroke subtypes was relatively uniform (72% of atherothrombotic, 73% of lacunar, and 68% of cardioembolic stroke cases) suggesting that this misclassification was not a major source of bias in this study.
Finally, there is evidence that associations between fatty acid biomarkers and ischemic stroke may vary by the age of the study population and type of biomarker used.3 The results derived from this study population of predominately white postmenopausal women may not be generalizable to other populations.
In conclusion, we report that serum linolaidic trans fatty acid, palmitic saturated fatty acid, and oleic monounsaturated fatty acid were positively associated with incidence of ischemic stroke, while serum DPA and DHA n3 polyunsaturated fatty acids and arachidonic n6 polyunsaturated fatty acid were inversely associated with incidence of ischemic stroke. These associations were generally consistent for atherothrombotic and lacunar strokes. These findings highlight the importance of individual fatty acids in the development of particular subtypes of ischemic stroke.
Supplementary Material
Acknowledgments
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
We gratefully acknowledge the expert fatty acid analyses of Ms. Brandi Duffy and Ms. Rona de la Vega.
Sources of Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. This study was supported by a grant from the National Institute of Neurological Disorders and Stroke to Dr. Ka He (R21NS056445). Dr. Yaemsiri was supported by the American Heart Association Mid-Atlantic Predoctoral Fellowship and the National Heart, Lung, and Blood Institute National Research Service Award Training Grant (5-T32-HL007055-30).
Footnotes
Author contributions: KH designed research and concepts. LFT, RWE, SWS collected and provided access to the data. SY analyzed the data and drafted the manuscript. SY had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the interpretation of the data and critical revision of the manuscript and take public responsibility for the whole content of the manuscript.
Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He K, Xu Y, Van Horn L. The puzzle of dietary fat intake and risk of ischemic stroke: A brief review of epidemiologic data. J Am Diet Assoc. 2007;107:287–295. doi: 10.1016/j.jada.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, et al. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 3.Tilvis RS, Erkinjuntti T, Sulkava R, Farkkila M, Miettinen TA. Serum lipids and fatty acids in ischemic strokes. Am Heart J. 1987;113:615–619. doi: 10.1016/0002-8703(87)90642-9. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen TA, Huttunen JK, Naukkarinen V. Cholestanol and fatty acids of serum lipids as risk factors of stroke. Monogr Atheroscler. 1986;14:19–25. [PubMed] [Google Scholar]
- 5.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk factors for ischemic stroke subtypes: The atherosclerosis risk in communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 6.Wassertheil-Smoller S, Kooperberg C, McGinn AP, Kaplan RC, Hsia J, Hendrix SL, et al. Lipoprotein-associated phospholipase a2, hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51:1115–1122. doi: 10.1161/HYPERTENSIONAHA.107.103721. [DOI] [PubMed] [Google Scholar]
- 7.Prentice RL, Rossouw JE, Furberg C, Johnson S, Henderson M, Cummings S, et al. Design of the women's health initiative clinical trial and observational study. The women's health initiative study group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the women's health initiative. Ann Epidemiol. 2003;13:S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen JC, Brunner RL, Ren H, Wassertheil-Smoller S, Larson JC, Levine DW, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–3192. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein LB, Jones MR, Matchar DB, Edwards LJ, Hoff J, Chilukuri V, et al. Improving the reliability of stroke subgroup classification using the trial of org 10172 in acute stroke treatment (toast) criteria. Stroke. 2001;32:1091–1098. doi: 10.1161/01.str.32.5.1091. [DOI] [PubMed] [Google Scholar]
- 11.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The women's health initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the women's health initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 14.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride--methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults;. Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 16.Willett W. Nutritional epidemiology. Oxford University Press; 1998. [Google Scholar]
- 17.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA. 1997;278:2145–2150. [PubMed] [Google Scholar]
- 18.Sauvaget C, Nagano J, Hayashi M, Yamada M. Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke. 2004;35:1531–1537. doi: 10.1161/01.STR.0000130426.52064.09. [DOI] [PubMed] [Google Scholar]
- 19.Seino F, Date C, Nakayama T, Yoshiike N, Yokoyama T, Yamaguchi M, et al. Dietary lipids and incidence of cerebral infarction in a japanese rural community. J Nutr Sci Vitaminol (Tokyo) 1997;43:83–99. doi: 10.3177/jnsv.43.83. [DOI] [PubMed] [Google Scholar]
- 20.He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, et al. Dietary fat intake and risk of stroke in male us healthcare professionals: 14 year prospective cohort study. BMJ. 2003;327:777–782. doi: 10.1136/bmj.327.7418.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu F, Hennekens CH, et al. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation. 2001;103:856–863. doi: 10.1161/01.cir.103.6.856. [DOI] [PubMed] [Google Scholar]
- 22.Yaemsiri S, Sen S, Tinker L, Rosamond W, Wassertheil-Smoller S, He K. Trans fat intake, aspirin, and ischemic stroke among postmenopausal women. Ann Neurol. 2012 doi: 10.1002/ana.23555. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: Human studies. Am J Clin Nutr. 1997;65:1628S–1644S. doi: 10.1093/ajcn/65.5.1628S. [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: A critical review. J Am Coll Nutr. 2001;20:5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 25.Ricci S, Celani MG, Righetti E, Caruso A, De Medio G, Trovarelli G, et al. Fatty acid dietary intake and the risk of ischaemic stroke: A multicentre case-control study. Ufa study group. J Neurol. 1997;244:360–364. doi: 10.1007/s004150050102. [DOI] [PubMed] [Google Scholar]
- 26.Ricci S, Patoia L, Berrettini M, Binaglia L, Scarcella MG, Bucaneve G, et al. Fatty acid pattern of red blood cell membranes and risk of ischemic brain infarction: A case-control study. Stroke. 1987;18:575–578. doi: 10.1161/01.str.18.3.575. [DOI] [PubMed] [Google Scholar]
- 27.Bucalossi A, Mori S. Fatty acid composition of adipose tissue in ischemic heart disease and stroke. Gerontol Clin (Basel) 1972;14:339–345. doi: 10.1159/000245419. [DOI] [PubMed] [Google Scholar]
- 28.Kuller L, Reisler DM. An explanation for variations in distribution of stroke and arteriosclerotic heart disease among populations and racial groups. Am J Epidemiol. 1971;93:1–9. doi: 10.1093/oxfordjournals.aje.a121223. [DOI] [PubMed] [Google Scholar]
- 29.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 31.Agren JJ, Vaisanen S, Hanninen O, Muller AD, Hornstra G. Hemostatic factors and platelet aggregation after a fish-enriched diet or fish oil or docosahexaenoic acid supplementation. Prostaglandins Leukot Essent Fatty Acids. 1997;57:419–421. doi: 10.1016/s0952-3278(97)90421-x. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 34.Castellanos M, Castillo J, Garcia MM, Leira R, Serena J, Chamorro A, et al. Inflammation-mediated damage in progressing lacunar infarctions: A potential therapeutic target. Stroke. 2002;33:982–987. doi: 10.1161/hs0402.105339. [DOI] [PubMed] [Google Scholar]
- 35.Thompson AK, Minihane AM, Williams CM. Trans fatty acids, insulin resistance and diabetes. Eur J Clin Nutr. 2011;65:553–564. doi: 10.1038/ejcn.2010.240. [DOI] [PubMed] [Google Scholar]
- 36.Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr. 2000;54:367–372. doi: 10.1038/sj.ejcn.1600964. [DOI] [PubMed] [Google Scholar]
- 37.Shang W, Liu J. Stroke subtype classification: A comparative study of asco and modified toast. J Neurol Sci. 2012;314:66–70. doi: 10.1016/j.jns.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


