Abstract
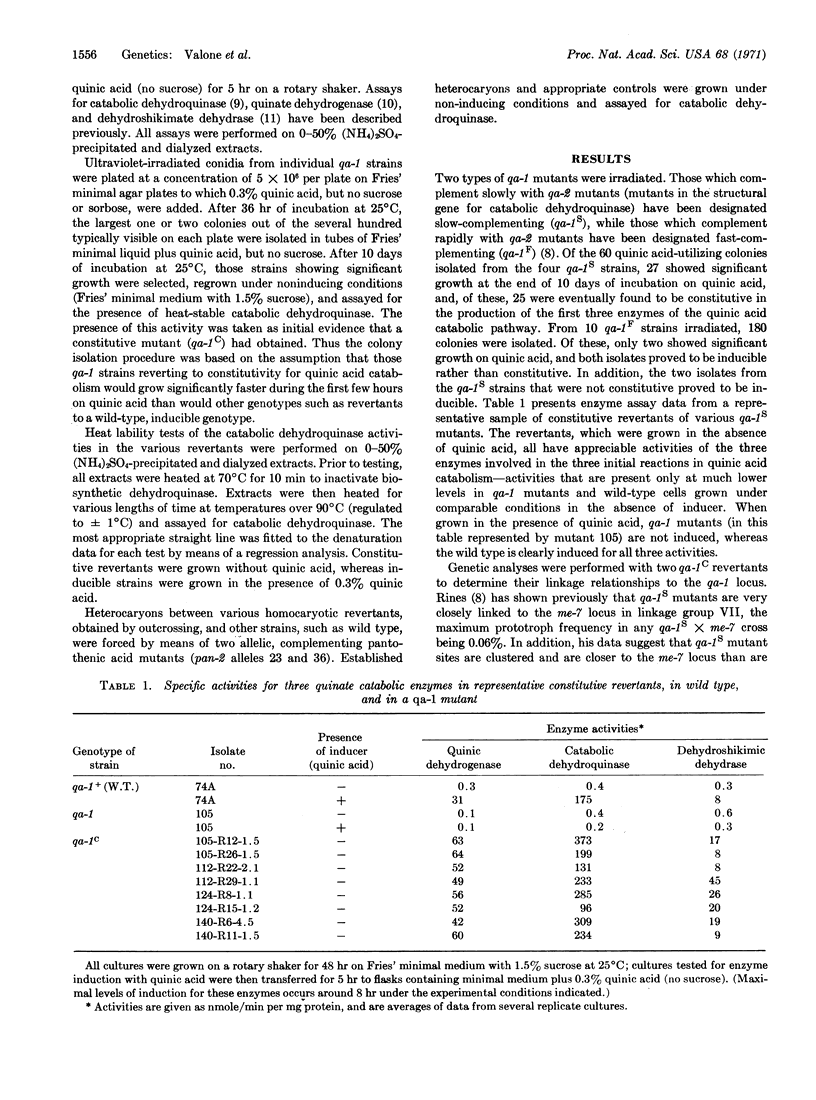
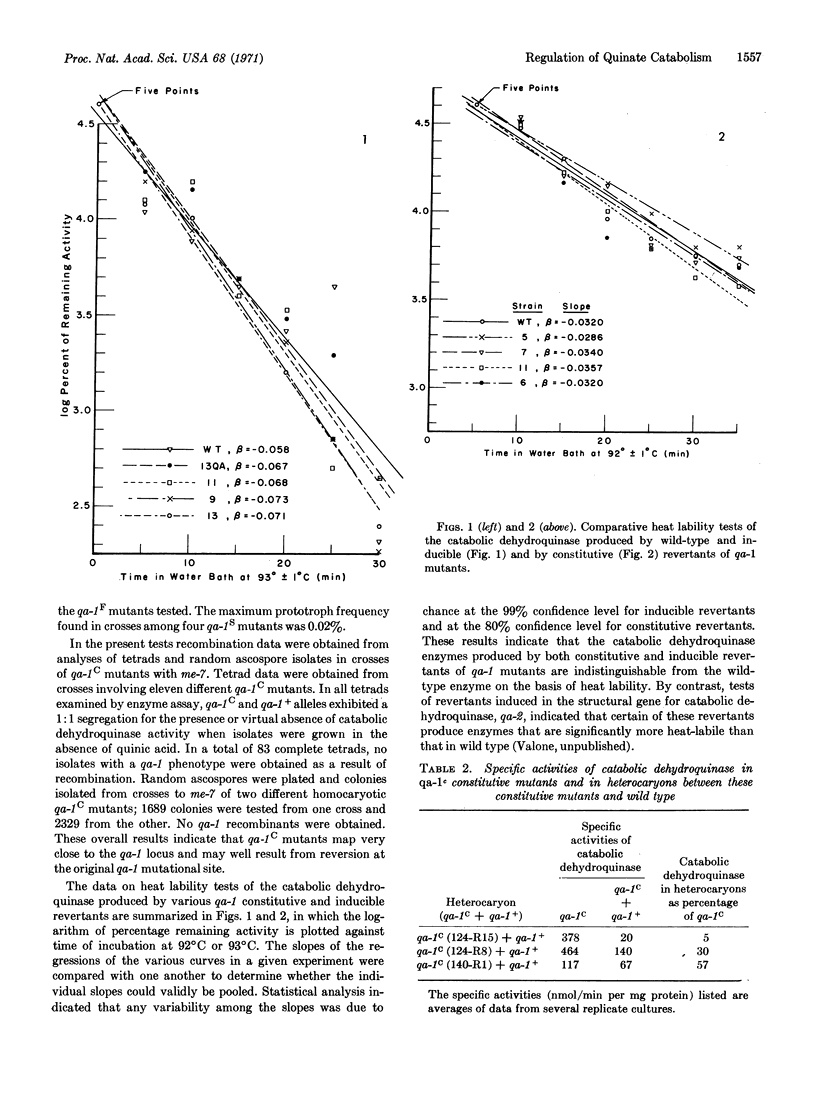
In Neurospora crassa, evidence has recently been obtained for a cluster of four closely-linked genes controlling the inducible enzymes catalyzing the first three reactions in the catabolism of quinic acid. Three of these genes appear to be the structural genes for the three enzymes. The fourth gene, designated qa-1, has been interpreted as having a regulatory function, since qa-1 mutants are pleiotropic types, are noninducible for the three enzymes, and form heterocaryons which complement mutants in the structural genes. The present studies were undertaken to elucidate further the nature of the regulatory role of the qa-1 locus. A number of constitutive (qa-1C) mutants have been obtained from certain qa-1 mutants as revertants selected for their ability to grow on quinic acid as a sole source of carbon. These qa-1C mutants produce high levels of all three enzymes in the absence of an inducer, map within (or very close to) the qa-1 locus, and produce a catabolic dehydroquinase (EC 4.2.1.10) which is indistinguishable, on the basis of thermolability tests, from that of wild type. In addition, when grown in the absence of an inducer, heterocaryons between wild-type (qa-1+) and different qa-1C mutants exhibit markedly different levels of constitutivity (from 5 to 50% of the particular parental qa-1C mutant) for catabolic dehydroquinase, one of the enzymes under qa-1 control. These overall results are interpreted as supporting the hypothesis that the qa-1+ gene product (presumably a multimeric protein) plays only a positive regulatory role in initiating synthesis of the three quinate catabolic enzymes.
Keywords: 5-dehydroquinate dehydratase, quinate dehydrogenase, 5-dehydroshikimate dehydrase, eucaryote
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cove D. J. Evidence for a near limiting intracellular concentration of a regulator substance. Nature. 1969 Oct 18;224(5216):272–273. doi: 10.1038/224272b0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS H. C., HAWTHORNE D. C. ENZYMATIC EXPRESSION AND GENETIC LINKAGE OF GENES CONTROLLING GALACTOSE UTILIZATION IN SACCHAROMYCES. Genetics. 1964 May;49:837–844. doi: 10.1093/genetics/49.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Jacob F. Genetic mapping of the regulator and operator genes of the lac operon. J Mol Biol. 1968 Sep 28;36(3):413–417. doi: 10.1016/0022-2836(68)90165-4. [DOI] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N. H., Partridge C. W., Ahmed S. I., Case M. E. The occurrence of two dehydroquinases in Neurospora crassa, one constitutive and one inducible. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1930–1937. doi: 10.1073/pnas.58.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]



