Abstract
Systemic bone loss is a hallmark of rheumatoid arthritis (RA). Inflammatory cytokines such as interleukin (IL)-6 promote bone resorption by osteoclasts. Sphingosine-1-phosphate (S1P) controls the migration of osteoclast precursor cells (OCPs) between the blood and bone marrow, in part via S1P receptors (S1PR1 and S1PR2) expressed on the surface of OCPs. OCPs (CD11b+Gr-1low+med) isolated from bone marrow of DBA/1J mice were stimulated with IL-6. S1P-directed chemotaxis of OCPs was evaluated using a transwell plate. mRNA expression of S1PR1 and S1PR2 was measured. DBA/1J mice were immunized with bovine type II collagen (days 0 and 21) and anti-mouse IL-6 receptor antibody (MR16-1) was administered on days 0 and/or 21. Trabecular bone volume was analysed using micro-computed tomography. The percentage of OCPs in tibial bone marrow and S1PR1 and S1PR2 mRNA expression in OCPs were measured. IL-6 stimulation significantly decreased S1P-directed chemotaxis of OCPs. IL-6 induced S1PR2 mRNA expression, but not S1PR1 mRNA expression, in OCPs. Bone volume was significantly lower in arthritic mice than in non-arthritic control mice on day 35. Treatment of immunized mice with MR16-1 significantly inhibited bone loss. In MR16-1-treated mice, the percentage of OCPs and expression of S1PR2 mRNA was each decreased compared with arthritic mice on day 14, but not on day 35. IL-6 increased the number of OCPs in tibial bone marrow via up-regulating S1PR2, thus playing a crucial role in systemic bone loss induced by inflammation.
Keywords: collagen-induced arthritis, interleukin-6, osteoclast, sphingosine-1-phosphate receptor 2
Introduction
Patients with rheumatoid arthritis (RA) often experience systemic bone loss during the early phase of the disease, and generalized osteoporosis is seen frequently in patients with longer disease duration [1]. Bone metabolism in patients with systemic osteoporosis is characterized by increased bone resorption that arises from chronic and systemic inflammatory processes.
The most extensively studied mechanisms of bone resorption concern how osteoclasts differentiate and how they become activated under inflammatory conditions. As a pathogenic mechanism of bone destruction, osteoclasts activated by inflammatory cytokines are thought to be responsible for systemic bone erosion. Receptor activator of nuclear factor (NF)-κB ligand (RANKL) is an essential factor for osteoclastogenesis [2,3]; it stimulates precursor cells of the myeloid lineage to differentiate into osteoclasts by binding to its signalling receptor, RANK [4,5]. RANKL also acts at all other stages of osteoclast generation and activity by stimulating osteoclast migration, fusion, activation and survival. It is reported that RANKL is produced by synovial fibroblasts, osteoblasts and lymphocytes [6].
More recently, the regulation of migration and positioning of osteoclast precursor cells (OCPs) has been attracting attention [7–15]. Ishii and colleagues have reported that highly organized migration of OCPs occurs between bone marrow and the bloodstream [16,17]. They showed that the mechanisms controlling the initial localization of OCPs into the bone space or counteracting the tendency of S1P to promote movement of OCPs between bone marrow and blood are in part via the S1P receptor 1 (S1PR1) and S1P receptor 2 (S1PR2) expressed on the surface of OCPs. S1PR1 mediates chemoattraction towards higher concentrations of S1P (from the bone marrow where S1P concentration is low towards the blood, where S1P concentration is high), whereas S1PR2 mediates chemorepulsion away from higher concentrations of S1P (from the blood where S1P concentration is high to the bone marrow, where S1P concentration is low). Inhibition of S1PR2 function changed the dynamics of OCP migration and relieved osteoporosis in a mouse model of bone loss [16,17].
Collagen-induced arthritis (CIA) is a widely studied model for understanding the pathogenesis of RA because its pathological features are similar to those of RA, with pannus formation, cartilage/bone erosion and systemic bone loss [18,19]. Moreover, it has been reported that raloxifene and zoledronic acid, which inhibit bone resorption and have a beneficial effect in the treatment of osteoporosis, also show therapeutic effects in the CIA model [18,19], which indicates that CIA is a useful model for evaluating the effect of drugs on the signs and symptoms of not only RA but also osteoporosis. We have reported that anti-interleukin (IL)-6 receptor (anti-IL-6R) antibody inhibits joint swelling in the CIA model [20]; however, it has not yet been evaluated whether intervention with anti-IL-6R antibody is effective against systemic bone loss.
In the present study, we investigated whether anti-IL-6R antibody inhibits systemic bone loss in the CIA model and the manner in which it exerts its effect. We found that anti-IL-6R antibody inhibited systemic bone loss and decreased the number of OCPs in tibial bone marrow via down-regulating S1PR2, which plays a crucial role in systemic bone loss.
Methods
Reagents
Rat anti-mouse IL-6R antibody (MR16-1) was prepared in our laboratories [21]. Macrophage colony-stimulating factor (M-CSF) and soluble RANKL (sRANKL) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Mouse IL-6 was purchased from Peprotech (Rocky Hill, NJ, USA) and mouse S1P was purchased from Sigma-Aldrich (St Louis, MO, USA).
Animals
Male DBA/1J mice were purchased from Charles River Japan (Yokohama, Japan). The specific pathogen-free mice were kept in cages in a room maintained at 20–26°C at a relative humidity of 55–75%. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Chugai Pharmaceutical Co., Ltd.
Induction of collagen-induced arthritis
CIA, which is thought to be due to anti-bovine type II collagen antibody, was induced as described previously, with modifications [20]. In brief, male DBA/1J mice (9 weeks old) were immunized intradermally at the base of the tail with 200 μg of bovine type II collagen (4 mg/ml; Collagen Research Center, Tokyo, Japan) emulsified with an equal volume of complete adjuvant H37Ra (Difco, Detroit, MI, USA). Three weeks later (day 21), mice received a booster immunization in the same manner. The clinical symptoms of arthritis in all four limbs were evaluated with a visual scoring system. Arthritic lesions were graded on a scale of 0–4, where: 0 = no change, 0·5 = swelling and erythema of 1 digit, 1 = swelling and erythema of 2 or more digits, 2 = mild swelling and erythema of the limb, 3 = gross swelling and erythema of the limb and 4 = gross deformity and inability to use the limb. The arthritis score for each mouse was the sum of the score of each of the four limbs, the maximum possible score thus being 16. The arthritis score was assessed from 21 days after the first immunization.
Treatment regimen
Mice in the non-arthritic control group did not receive any intervention treatment. Mice in the MR16-1-treated arthritic group were each injected intraperitoneally with 8 mg of MR16-1 in 800 μl of phosphate-buffered saline (PBS) before immunization on the day of first immunization (day 0) and/or before immunization 21 days later (day 21). Mice in the control arthritic group were injected intraperitoneally with PBS at the same times as the MR16-1-treated arthritic group. Each group consisted of eight to nine animals.
Structural analysis using micro-computed tomography (micro-CT)
Micro-CT is being used increasingly to measure the 3-dimensional (3D) bone structure of small animals because of its relative rapidity compared with conventional histology and its non-invasiveness and high spatial resolution [22–25]. In order to investigate the effects of MR16-1 treatment on the trabeculae in the cancellous tissue in the femur, 3D trabecular analysis was performed by scanning micro-CT (μCT 40; Scanco Medical, Zurich, Switzerland). The femur was scanned in 90 slices from the end of the growth plate. On the original 3D images, morphometric indices were determined directly from the binarized volume of interest (VOI) [26]. Probably the most known and most used output of micro-CT analyses of bone is the bone volume over total volume index (BV/TV, usually reported as a percentage value). It indicates the fraction of a given VOI that is occupied by mineralized bone (bone volume).
Analysis by fluorescence-activated cell sorter
Tibial bone marrow was isolated and passed through cell strainers to obtain single-cell suspensions. Single-cell suspensions were incubated with the Fc-receptor-blocking antibodies anti-CD16 and anti-CD32 (BD Biosciences, Franklin Lakes, NJ, USA) and then stained for 30 min with allophycocyanin (APC)-conjugated anti-CD11b antibody (BD Biosciences) and phycoerythrin (PE)-conjugated anti-Gr-1 antibody (BD Biosciences). A FACSCanto II Flow Cytometer (BD Biosciences) and FACSDiva software (BD Biosciences) were used for analysis.
In-vitro induction of osteoclasts from OCPs isolated from bone marrow
Bone marrow cells were isolated from male DBA/1J mice (9 weeks old). Cell suspensions from bone marrow were labelled with antibodies to CD11b and Gr-1 and were sorted into CD11b+Gr-1low+med cells (the OCP subset) and CD11b+Gr-1high cells with a fluorescence-activated cell sorter (FACSAria III; BD Biosciences). OCPs were seeded into 96-well plates (0·5 × 105 cells/well) and cultured for 5 days in α-modified Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS), M-CSF (30 ng/ml) and sRANKL (100 ng/ml). Cultured cells were fixed with 10% formalin in PBS for 10 min at room temperature. After treatment with ethanol/acetone (50:50 vol/vol) for 1 min, the well surface was air-dried and incubated for 30 min at room temperature with a tartrate-resistant acid phosphatase isoform (TRAP)-staining solution consisting of 0·1 M sodium acetate (pH 5·0) containing 0·01% naphthol AS-MX phosphate (Sigma-Aldrich) and 1% N, N-dimethylformamide as a substrate, and 0·06% fast red violet LB salt (Sigma-Aldrich) as a stain for the reaction product in the presence of 50 mM sodium tartrate. TRAP-positive multi-nuclear cells containing more than three nuclei were counted as osteoclasts.
Analysis of gene expression in OCPs from bone marrow
OCPs were isolated as described above. OCPs (1 × 105 cells/0·1 ml/well) were cultured with mouse IL-6 (1 or 10 ng/ml) for 24 h in RPMI-1640 supplemented with 10% FBS. Total RNA was extracted by using an RNeasy kit (Qiagen, Valencia, CA, USA), according to the kit manufacturer's protocol. cDNA was synthesized with an Omniscript RT kit (Qiagen) using random 9-mer primers (TaKaRa, Shiga, Japan), according to the kit manufacturer's protocol. Quantitative real-time polymerase chain reaction (PCR) was performed by running a TaqMan gene expression assay (Applied Biosystems, Foster City, CA, USA), targeting mouse S1PR1, S1PR2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on an ABI PRISM 7500 system (Applied Biosystems) according to the manufacturer's protocol.
Migration assay
Migration assays were performed according to the method described previously [27]. OCPs were incubated with IL-6 (10 ng/ml) in RPMI-1640 with 0·4 mg/ml fatty acid-free bovine serum albumin (BSA) (Calbiochem, La Jolla, CA, USA) for 24 h. OCPs (2 × 106 cells/0·1 ml/well) were added to the upper wells of 24-well, 5 μm pore, polycarbonate cell culture inserts (Costar, St Louis, MO, USA) with 0·6 ml of S1P (10−7 M) in the lower wells. The migration assays were conducted in RPMI-1640 with 0·4 mg/ml fatty acid-free BSA for 4 h. The numbers of OCPs at the start minus the number of OCPs at the end were counted as the number that migrated.
Statistical analysis
Statistical significances were estimated by Wilcoxon's test, Welch test, unpaired t-test and Dunnett's multiple comparison test using a statistical software package (SAS Institute Japan, Tokyo, Japan), with the significance level set to 5%.
Results
Effect of IL-6 on the migration of OCPs and on the expression of S1PR2 on OCPs
First, by using typical osteoclast markers, CD11b and Gr-1, we examined which type of cells differentiated into osteoclasts. Bone marrow cells were sorted into CD11b+Gr-1high and CD11b+Gr-1low+med fractions, and these cells were incubated with RANKL and M-CSF. CD11b+Gr-1low+med but not CD11b+Gr-1high cells differentiated into TRAP-positive osteoclasts (Fig. 1a). From these results, we defined CD11b+Gr-1low+med cells to be osteoclast precursor cells (OCPs).
Fig. 1.
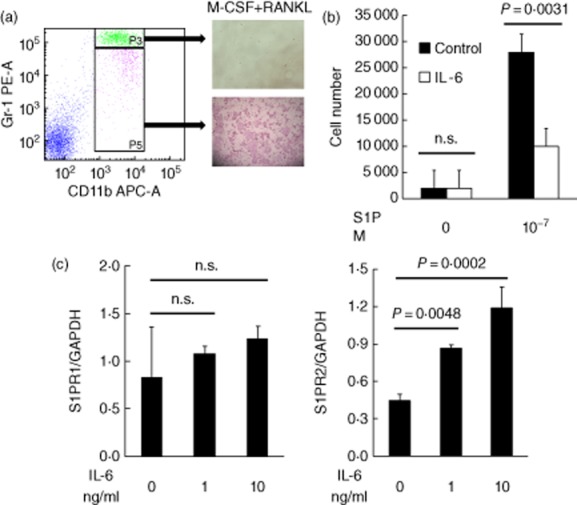
The effect of interleukin (IL)-6 on sphingosine-1-phosphate (S1P)-directed cell migration and S1P receptor (S1PR) expressions. (a) Bone marrow cells were isolated from male DBA/1J mice. Cells in the bone marrow cell suspension were sorted with a fluorescence-activated cell sorter (FACS) into CD11b+Gr-1high cells and CD11b+Gr-1low+med cells. CD11b+Gr-1high cells (upper image) and CD11b+Gr-1low+med cells (lower image) were cultured for 5 days with macrophage colony-stimulating factor (M-CSF) and soluble receptor activator of nuclear factor (NF)-κB ligand (sRANKL). (b) Osteoclast precursor cells (OCPs) (the CD11b+Gr-1low+med cells) were incubated with IL-6 (10 ng/ml) for 24 h. OCPs were added to the upper wells of 5-μm-pore cell culture inserts with 10−7 M S1P in the bottom wells. The migration assays were conducted for 4 h. The numbers of OCPs at the start minus the number of OCPs at the end were counted as the number that migrated. Each column indicates the mean and standard deviation of triplicate cultures. Statistical significance was analysed by unpaired t-test; n.s. indicates not significant. (c) OCPs were stimulated with IL-6 (1, 10 ng/ml) for 24 h, and S1PR1 and S1PR2 expressions were measured by real-time polymerase chain reaction (PCR). Each column indicates the mean and standard error of triplicate cultures. Statistical significance was analysed by Dunnett's multiple comparison test.
We used a cell culture insert to examine how IL-6 would affect migration of OCPs towards S1P. OCPs migrated towards the higher concentration of S1P (Fig. 1b). Conversely, treatment with IL-6 significantly inhibited the migration of OCPs.
OCPs express S1PR1 and S1PR2, each of which play a role in regulating the migration of OCPs towards S1P. To examine whether IL-6 regulated the expression of S1PR1 and S1PR2 and the migration of OCPs towards high concentrations of S1P, OCPs were sorted from bone marrow cells and incubated with IL-6. Stimulation with IL-6 induced expression of S1PR2 which affects migration away from S1P, but did not induce expression of S1PR1 which affects migration towards S1P (Fig. 1c).
Effect of MR16-1 on systemic bone loss in the CIA model
We have reported previously that in the mouse CIA model the concentration of IL-6 in serum increases dramatically the day after immunizations on days 0 and 21 [28]. Therefore, MR16-1 was administered on days 0 and 21 to inhibit IL-6 signalling. Injection of MR16-1 on days 0 and 21 significantly blocked the onset of arthritis (Fig. 2a). The incidence of arthritis in the arthritic control group was 100%, whereas the incidence of arthritis in the MR16-1-treated group was 50% on day 35. The serum level of IL-6, which is a marker of IL-6 signalling in CIA, was inhibited almost completely by MR16-1 treatment on day 35 (Supporting information, Fig. S1). To address the effect of MR16-1 on systemic bone loss, we used micro-computerized tomography (CT) to evaluate the distal ends of the right and left femur (Fig. 2b,c). In the CIA model on day 35, but not on day 14, the BV/TV ratio of the arthritic control group was reduced significantly compared with that of the non-arthritic control group. MR16-1 treatment increased the BV/TV ratio significantly with respect to that in the arthritic control group on day 35 (BV/TV = 0·056 ± 0·005 in the MR16-1 group versus BV/TV = 0·037 ± 0·004 in the arthritic group, P = 0·0064). The arthritis score and BV/TV on day 35 in both the arthritic group and the MR16-1 group was correlated significantly (Fig. 2d).
Fig. 2.
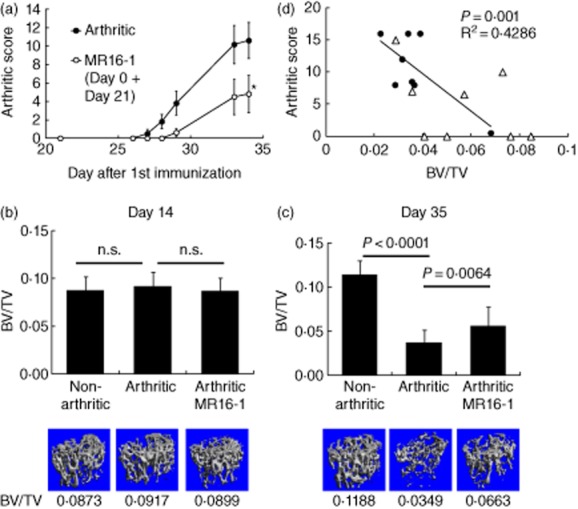
Effect of rat anti-mouse interleukin (IL)-6R antibody (MR16-1) injected on days 0 and 21 on trabecular bone volume in the collagen-induced arthritis (CIA) model. Mice were immunized with bovine type II collagen emulsified in complete adjuvant on days 0 and 21. Mice in the MR16-1-treated arthritic group were each injected intraperitoneally with 8 mg of MR16-1 in 800 μl of vehicle before immunization on the day of first immunization (day 0) and before immunization 21 days later (day 21). Bone samples were collected on days 14 or 35 after first immunization. Bone samples were analysed by micro-computed tomography (CT). (a) Arthritic score was assessed as described in the Methods section. Each symbol indicates the mean ± standard error of eight to nine animals. Statistical significance on day 34 was analysed by Wilcoxon's test (*P < 0·05). (b,c) Bone volume over total volume index (BV/TV) data were calculated from micro-computerized tomography (CT) images. Each column indicates the mean and standard deviation of eight to nine animals. Statistical significance on (b) day 14 and (c) day 35 was analysed by unpaired t-test; n.s. indicates not significant. Below each column is a representative micro-CT image of the distal femur for each group. (d) The correlation between arthritis score and BV/TV on day 35 was assessed by Pearson's correlation. Circles indicate the values of each animal in the arthritic control group, and triangles indicate the values of each animal in the MR16-1-treated group.
Effect of MR16-1 on the accumulation of OCPs in bone marrow
Next, we examined whether OCPs accumulated into bone marrow and how MR16-1 affected OCP numbers in bone marrow. The percentage of OCPs was significantly higher in bone marrow from mice with CIA than in bone marrow from non-arthritic control mice both on days 14 and 35 (Fig. 3a,b). MR16-1 treatment reduced significantly the percentage of OCPs on day 14, but not on day 35 (8·02 ± 0·7 in the MR16-1 group on day 14 versus 12·40 ± 0·88 in the arthritic group, P = 0·0012).
Fig. 3.
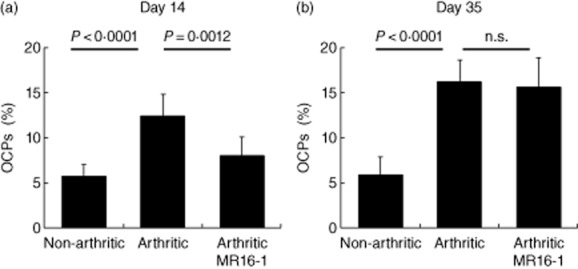
Effect of rat anti-mouse interleukin (IL)-6R antibody (MR16-1) on the number of osteoclast precursor cells (OCPs) in bone marrow. OCPs were collected from bone marrow on (a) day 14 and (b) day 35. Percentage of OCPs in bone marrow was measured by fluorescence-activated cell sorter (FACS). Each column indicates the mean and standard deviation of eight to nine animals. Statistical significance was analysed by unpaired t-test or Welch test; n.s. indicates not significant.
The expression level of S1PR2 in the arthritic group was increased compared with level in the non-arthritic control group (Fig. 4a,b). MR16-1 treatment reduced S1PR2 expression significantly on day 14, but not day 35 (1·38 ± 0·091 in the MR16-1 group on day 14 versus 2·77 ± 0·723 in the arthritic group, P = 0·0133). There was no difference in levels of S1PR1 between any of the groups (Fig. 4a,b).
Fig. 4.
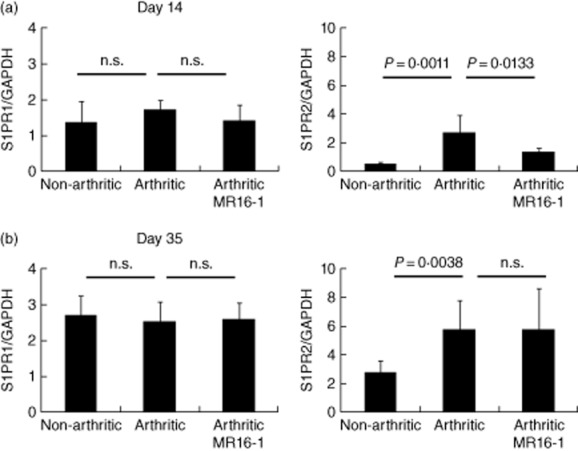
Effect of rat anti-mouse interleukin (IL)-6R antibody (MR16-1) on the expression of sphingosine-1-phosphate receptor (S1PR) in OCPs. OCPs were collected from bone marrow on (a) day 14 and (b) day 35. S1PR1 and S1PR2 expressions in bone marrow OCPs were measured by real-time polymerase chain reaction (PCR). Each column indicates the mean and standard deviation of eight to nine animals. Statistical significance was analysed by unpaired t-test or Welch test; n.s. indicates not significant.
Finally, we administered MR16-1 once on day 0 or once on day 21 to explore which injection was most useful for suppressing systemic bone loss (Fig. 5a,b). Treatment on day 0 inhibited arthritis score and systemic bone loss significantly on day 35, but treatment on day 21 did not.
Fig. 5.
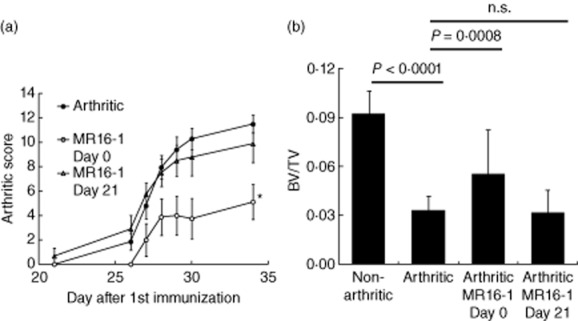
Effect of rat anti-mouse interleukin (IL)-6R antibody (MR16-1) on days 0 or 21 on trabecular bone volume in the collagen-induced arthritis (CIA) model. Mice in the MR16-1-treated arthritic group were each injected intraperitoneally with 8 mg of MR16-1 in 800 μl of vehicle on days 0 or 21. Bone samples were collected at day 35 after first immunization. Bone samples were analyzed by micro-computed tomography (CT). (a) Arthritis score was assessed as described in the Methods section. Each symbol indicates the mean ± standard error of eight to ten animals. Statistical significance on day 34 was analysed by Wilcoxon's test (*P < 0·05). (b) Bone volume over total volume index (BV/TV) data on day 35 was calculated from micro-CT images. Each column indicates the mean and standard deviation of eight to nine animals. Statistical significance was analysed by unpaired t-test or Welch test; n.s. indicates not significant.
Discussion
Several studies have demonstrated that the S1P–S1PR pathway operates to control egress of lymphocytes from primary and secondary lymphoid organs [16,27,29]. Moreover, it is now clear that osteoclast accumulation in bone marrow is also strictly controlled by the S1P–S1PR pathway [16,17]. Although it is not fully understood how the S1P–S1PR pathway is regulated in the course of systemic bone loss, it was recently reported that higher circulating S1P levels are associated with higher bone resorption and lower bone mineral density in women [30]. Conversely, the expression levels of S1PR2 in the course of systemic bone loss have not been examined. To our knowledge, the current study is the first to demonstrate that expression of S1PR2 on OCPs increased in CIA mice, resulting in the accumulation of OCPs in bone marrow.
We also found that S1PR2 on OCPs was up-regulated by IL-6 and that migration of OCPs towards S1P was reduced significantly in OCPs treated with IL-6 compared to OCPs not treated with IL-6. As reported previously, S1PR2 mediates chemorepulsion away from the blood, where the S1P concentration is higher than in bone marrow, and regulates the steady-state migration propensities of OCPs, constituting one part of a cycle that together with chemoattraction mediated by S1PR1 that may play a crucial role in the control of osteoclastogenesis and bone remodelling [17]. From these results, a possible mechanism of the systemic bone loss by IL-6 seen in this arthritis model is as follows: (1) S1PR2 on OCPs is up-regulated by IL-6; (2) up-regulated S1PR2 inhibits the egress of OCPs from bone marrow and induces; and (3) osteoclasts differentiated from OCPs promote bone destruction.
We defined CD11b+Gr-1low+med cells as OCPs because these cells differentiated into TRAP-positive osteoclasts when stimulated with M-CSF and RANKL. De Klerck et al. have reported that RANK is expressed only in CD11b+ cells and not in CD11b− cells, indicating that at least CD11b+ cells can differentiate into osteoclasts [31]. Gr-1 is a marker of bone marrow granulocytes, and the CD11b+Gr-1high population was located within the SSChigh FSCint–high phenotype which is a granulocyte fraction. Indeed, CD11b+Gr-1low+med cells but not CD11b+Gr-1high cells differentiated into TRAP-positive osteoclasts.
Inflammatory cytokines play a crucial role in the pathogenesis of CIA in mice. IL-6 and tumour necrosis factor (TNF)-α both induce osteoclastogenesis, and both IL-6- and TNF-α-over-expressing transgenic mice are observed to have osteopaenia [32,33]. However, there is an apparent difference between these cytokines in CIA mice. Although IL-6 and sIL-6R are abundant in the whole body, TNF-α is concentrated at sites of inflammation. Indeed, we found that IL-6 and sIL-6R were detectable in the serum of CIA mice and that the concentration of serum IL-6 was higher in CIA mice than in non-arthritic control mice; conversely, TNF-α was hardly detected in serum (Supporting information, Fig. S1). In RA patients, too, as with arthritic mice, levels of IL-6 and sIL-6R in the serum and synovial fluid are higher than levels in healthy individuals or in patients with osteoarthritis [34]. Therefore, IL-6-induced systemic osteopenia is very likely to occur in patients with RA. More recently, it has been reported that treatment with the humanized anti-IL-6R antibody tocilizumab reduced markers of bone resorption, telopeptide of type I collagen (CTX-I) and C-telopeptide pyridinoline cross-links of type I collagen (ICTP) and induced limited repair of bone erosions [35–37]. It is possible that these results can be explained partly by the inhibition of systemic bone loss resulting from tocilizumab inhibiting the IL-6 in RA.
MR16-1-mediated effects on S1PR2 expression and on infiltration of OCPs into bone marrow were detectable in arthritic mice on day 14; however, these effects were not detectable on day 35, despite the mice receiving a second MR16-1 injection on day 21. From the serum IL-6 level on day 35 (Supporting information, Fig. S1), we confirmed that MR16-1 inhibited IL-6 signalling. Therefore, it seems that another factor might have induced S1PR2 expression on day 35. It has been reported that lipopolysaccharides induce S1PR2 expression in human microvascular endothelial cells [38]; therefore, it is possible that a factor other than IL-6 induced OCP accumulation via S1PR2 expression.
This raises the question of whether MR16-1 treatment reduces arthritis-associated osteoporosis in a direct or indirect manner. It is possible that the beneficial effects of MR16-1 on osteoporosis seen in vivo are due mainly to a reduction in overall severity of arthritis, which then leads to lower systemic concentrations of proinflammatory mediators; therefore, MR16-1 possibly acts only indirectly. A correlation between arthritis score and BV/TV was observed in both the arthritic group and the MR16-1-treated arthritic group. Although this result suggests that the inhibitory effect of MR16-1 on arthritis severity coincides with reduced systemic bone loss, it is not clear whether there is a direct effect on OCP accumulation or an indirect effect via reducing systemic concentrations of proinflammatory mediators. Further studies in RA patients with respect to the relationship between the expression of S1PR2 and the clinical response after anti-IL-6 therapy is necessary to clarify whether MR16-1 treatment acts directly or indirectly to reduce arthritis-associated systemic bone loss.
In the present study, we demonstrated for the first time that S1PR2 expression on OCPs was increased in an arthritis model, resulting in the accumulation of OCPs in bone marrow. We also showed that IL-6 increases the number of OCPs in tibial bone marrow via up-regulating S1PR2, and plays a crucial role in systemic bone loss induced by inflammation.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website:
Fig. S1. The serum concentration of cytokine. Cytokine levels in sera were measured by ELISA. Sera were obtained on Day 35. Each column indicates the mean and SD of 8–9 animals.
References
- 1.Shibuya K, Hagino H, Morio Y, Teshima R. Cross-sectional and longitudinal study of osteoporosis in patients with rheumatoid arthritis. Clin Rheumatol. 2002;21:150–158. doi: 10.1007/s10067-002-8274-7. [DOI] [PubMed] [Google Scholar]
- 2.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 3.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 4.Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Sarosi I, Yan XQ, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Huang Y, Collin-Osdoby P, et al. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- 8.Wright LM, Maloney W, Yu X, et al. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36:840–853. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi K, Saitoh Y, Minami T, et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol. 2009;183:7825–7831. doi: 10.4049/jimmunol.0803627. [DOI] [PubMed] [Google Scholar]
- 10.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 11.Choi SJ, Cruz JC, Craig F, et al. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- 12.Lean JM, Murphy C, Fuller K, et al. CCL9/MIP-1gamma and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem. 2002;87:386–393. doi: 10.1002/jcb.10319. [DOI] [PubMed] [Google Scholar]
- 13.Ha J, Choi HS, Lee Y, et al. CXC chemokine ligand 2 induced by receptor activator of NF-kappa B ligand enhances osteoclastogenesis. J Immunol. 2010;184:4717–4724. doi: 10.4049/jimmunol.0902444. [DOI] [PubMed] [Google Scholar]
- 14.Kwak HB, Ha H, Kim HN, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008;58:1332–1342. doi: 10.1002/art.23372. [DOI] [PubMed] [Google Scholar]
- 15.Binder NB, Niederreiter B, Hoffmann O, et al. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15:417–424. doi: 10.1038/nm.1945. [DOI] [PubMed] [Google Scholar]
- 16.Ishii M, Egen JG, Klauschen F, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii M, Kikuta J, Shimazu Y, et al. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207:2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Goff B, Soltner E, Charrier C, et al. A combination of methotrexate and zoledronic acid prevents bone erosions and systemic bone mass loss in collagen induced arthritis. Arthritis Res Ther. 2009;11:R185. doi: 10.1186/ar2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi I, Hagino H, Okano T, et al. Effect of raloxifene on arthritis and bone mineral density in rats with collagen-induced arthritis. Calcif Tissue Int. 2011;88:87–95. doi: 10.1007/s00223-010-9432-6. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Hashizume M, Mihara M. IL-6 blockade preferentially inhibits Th17 differentiation in collagen-induced arthritis. Rheumatol Int. 2011;31:127–131. doi: 10.1007/s00296-010-1552-9. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki M, Yamada Y, Nishimoto N, et al. Characterization of anti-mouse interleukin-6 receptor antibody. Immunol Lett. 2002;84:231–240. doi: 10.1016/s0165-2478(02)00202-x. [DOI] [PubMed] [Google Scholar]
- 22.Barou O, Valentin D, Vico L, Tirode C, Barbier A, et al. High-resolution three-dimensional micro-computed tomography detects bone loss and changes in trabecular architecture early: comparison with DEXA and bone histomorphometry in a rat model of disuse osteoporosis. Invest Radiol. 2002;37:40–46. doi: 10.1097/00004424-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Ruegsegger P, Koller B, Muller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996;58:24–29. doi: 10.1007/BF02509542. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama T, Tanizawa T, Muramatsu H, Endo N, Takahashi HE, et al. A morphometric comparison of trabecular structure of human ilium between microcomputed tomography and conventional histomorphometry. Calcif Tissue Int. 1997;61:493–498. doi: 10.1007/s002239900373. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Pham SM, Crabbe DL. High-resolution micro-CT evaluation of mid- to long-term effects of estrogen deficiency on rat trabecular bone. Acad Radiol. 2003;10:1153–1158. doi: 10.1016/s1076-6332(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher JC, Jerpbak CM, Jee WS, et al. 1,25-Dihydroxyvitamin D3: short- and long-term effects on bone and calcium metabolism in patients with postmenopausal osteoporosis. Proc Natl Acad Sci USA. 1982;79:3325–3329. doi: 10.1073/pnas.79.10.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda Y, Matsuyuki H, Shimano K, et al. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- 28.Mihara M, Yoshida H. The involvement of interleukin-6 in the augmentation of immune response by immunologic adjuvants. In: Benvenuto A, editor. Immunologic adjuvant research. New York: Nova Science Publishers; 2009. pp. 105–112. [Google Scholar]
- 29.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Lee SY, Lee YS, et al. Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J Clin Endocrinol Metab. 2012;97:E1421–E1428. doi: 10.1210/jc.2012-1044. [DOI] [PubMed] [Google Scholar]
- 31.De Klerck B, Carpentier I, Lories RJ, et al. Enhanced osteoclast development in collagen-induced arthritis in interferon-gamma receptor knock-out mice as related to increased splenic CD11b+ myelopoiesis. Arthritis Res Ther. 2004;6:R220–R231. doi: 10.1186/ar1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Benedetti F, Rucci N, Del Fattore A, et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006;54:3551–3563. doi: 10.1002/art.22175. [DOI] [PubMed] [Google Scholar]
- 33.Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotake S, Sato K, Kim KJ, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 35.Garnero P, Thompson E, Woodworth T, et al. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 2010;62:33–43. doi: 10.1002/art.25053. [DOI] [PubMed] [Google Scholar]
- 36.Karsdal MA, Schett G, Emery P, et al. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RADIATE study ( NCT00106522) Semin Arthritis Rheum. 2012;42:131–139. doi: 10.1016/j.semarthrit.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Finzel S, Rech J, Schmidt S, et al. Interleukin-6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: a micro CT study. Ann Rheum Dis. 2013;72:396–400. doi: 10.1136/annrheumdis-2011-201075. [DOI] [PubMed] [Google Scholar]
- 38.Du J, Zeng C, Li Q, et al. LPS and TNF-α induce expression of sphingosine-1-phosphate receptor-2 in human microvascular endothelial cells. Pathol Res Pract. 2012;208:82–88. doi: 10.1016/j.prp.2011.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


