Abstract
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system. It is an autoimmune disorder in which activated T cells cross the blood–brain barrier (BBB) to initiate an inflammatory response that leads to demyelination and axonal damage. The key mechanisms responsible for disease initiation are still unknown. We addressed this issue in experimental autoimmune encephalomyelitis (EAE), the animal model of MS. It is widely known that EAE manifests only in certain strains when immunized with myelin proteins or peptides. We studied the differential immune responses induced in two mouse strains that are susceptible or resistant to EAE induction when they are immunized with the 139–151 peptide of proteolipid protein, an encephalitogenic peptide capable of inducing EAE in the susceptible strain. The adequate combination of major histocompatibility complex alleles and myelin peptides triggered in susceptible mice a T helper type 17 (Th17) response capable of inducing the production of high-affinity anti-myelin immunoglobulin (Ig)G antibodies. These were not detected in resistant mice, despite immunization with the encephalitogenic peptide in junction with complete Freund's adjuvant and pertussis toxin, which mediate BBB disruption. These data show the pivotal role of Th17 responses and of high-affinity anti-myelin antibodies in EAE induction and that mechanisms that prevent their appearance can contribute to resistance to EAE.
Keywords: anti-myelin antibodies, experimental autoimmune encephalomyelitis, immune response, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a heterogeneous, chronic inflammatory disease of the central nervous system (CNS) that can induce severe disability. MS has been described as an autoimmune disorder because it is known that activated T cells cross the blood–brain barrier (BBB) to initiate an inflammatory response in the CNS that leads to demyelination and axonal damage. Of the different leucocyte subsets, T helper type 1 (Th1) and Th17 cells have been found to be important players in MS pathogenesis [1–8]. Moreover, the Th1/Th17 ratio is believed to be a key factor that may contribute to the heterogeneity of clinical forms of MS [8]. In addition, CD8+ T cells have been identified in the active lesions of MS patients [4] and B cells also play a role in the disease by contributing to intrathecal T cell activation [9] and by intrathecal synthesis of antibodies that associate with disease activity. The majority of MS patients present immunoglobulin (Ig)G oligoclonal bands (OCBs) in the cerebrospinal fluid (CSF) as a result of intrathecal IgG synthesis within the CNS [10], and a number of them also show IgM OCBs, which associate with a poor MS outcome [11].
The key mechanisms responsible for the initiation of MS are still unknown. It exhibits a complex physiopathology, which consists of interplay of genetic and environmental factors. Much evidence supports that certain major histocompatibility complex (MHC) antigens and exposure different infectious agents play a role in the breakdown of tolerance that conducts to MS, but the precise mechanisms determining susceptibility to the disease have not been ascertained fully.
To obtain further insights in disease susceptibility we used experimental autoimmune encephalomyelitis (EAE) model, which reproduces the clinical and histopathological features of multiple sclerosis [12]. EAE is induced only in certain strains of mice immunized with certain myelin proteins or peptides. We aimed to compare the differential immune responses induced in EAE-susceptible and resistant strains. We demonstrate here that susceptible mice developing EAE show a characteristic profile with induction of a helper Th17 response, and high affinity anti-proteolipidic protein (PLP) serum IgG antibodies. Further studies will demonstrate the role played by each of them in EAE and MS onset.
Material and methods
Mice
Eight-week-old female BALB/c (resistant strain) and SJL/J@RJ (susceptible strain) mice were purchased from Charles River (Barcelona, Spain). The mice were housed under standardized light- and climate-controlled conditions and were fed standard chow and water ad libitum. The experiments were performed according to the European Union (EU) regulations and were approved by our institutional Ethics Committee on Animal Experimentation (CEEA 07/10).
EAE induction and clinical follow-up
Mice from the susceptible (SJL/J@RJ, H-2s) and resistant (BALB/c, H-2d) strains were immunized under anaesthesia by subcutaneous injections of phosphate-buffered saline (PBS) containing 50 μg of the 139-151 PLP peptide (PLP139–151) emulsified in complete Freund's adjuvant (CFA) (Sigma, St Louis, MO, USA) containing 4 mg/ml of Mycobacterium tuberculosis H37RA (Difco Laboratories, Detroit, MI, USA). On days 0 and 2 post-immunization (p.i.), the mice received 200 ng of pertussis toxin (Sigma) intravenously. The PLP139–151 peptide was obtained from the Proteomics section of Universitat Pompeu Fabra in Barcelona, Spain. All the animals were weighed and examined daily in a blind manner for neurological signs using a six-point scale [13].
Splenocyte proliferative activity and cytokine production
The splenocytes that were obtained from eight mice per group from two independent experiments, which were euthanized on day 12 p.i., were seeded in 96-well plates at a cell density of 2 × 105 cells/well in Iscove's modified Dulbecco's medium (IMDM; PAA Laboratories GmbH, Pasching, Austria) supplemented with 10% HyClone®FetalClone I (Thermo Fisher Scientific, Waltham, MA, USA), 50 μmol/l of 2-mercaptoethanol (Sigma), 2 mmol/l of glutamine, 50 U/ml of penicillin and 50 mg/ml of streptomycin; the last three chemicals were obtained from Gibco BRL (Paisley, UK). For splenocyte activation, we used 5 μg/ml of PLP139–151 or 5 μg/ml of phytohaemagglutinin (PHA; Sigma). Cells that were cultured without any stimulus were used as baseline controls.
The supernatants (50 μl/well) were harvested after 48 h and stored at −80°C to further assess cytokine release. Then, 1 μCi/well of [3H]-thymidine (PerkinElmer Inc., Alameda, CA, USA) was added to the cells. The cultures were maintained under the same conditions for an additional 18 h, and the levels of incorporated radioactivity were determined using a beta-scintillation counter (Wallac, Turku, Finland). The stimulation index (SI) was expressed as the mean of the counts per minute (cpm) of five replicates from each mouse and culture condition divided by the mean cpm of the baseline control replicates. The results are expressed as the mean value [standard deviation (s.d.)] of the SI per group of mice.
The cytokine secretion pattern of three mice per group was determined in the supernatants by flow cytometry using the FlowCytomix Th1/Th2/Th17 10-plexkit (Bender MedSystems Inc., Burlingame, CA, USA), according to the manufacturer's instructions.
Immunophenotyping
The different subsets of lymphocytes in the splenocytes of 13 mice from three independent experiments were evaluated by flow cytometry. Anti-CD45-peridinin chlorophyll (PerCP)/cyanin 5·5 (Cy5·5), anti-CD8-phycoerythrin (PE), anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD25-allophycocyanin (APC) antibodies were used to analyse the T cell subpopulations and anti-CD45-PerCP/Cy5·5, anti-CD45R/B220-FITC, anti-CD1d-PE and anti-CD5-APC were used to study the B cell subsets. The frequencies of CD4+ and CD8+ regulatory T cells were analysed in five mice using anti-CD4-FITC, anti-CD8-PE, anti-CD25-PE/Cy7 and anti-forkhead box protein 3 (FoxP3)-APC. All the antibodies and their corresponding isotype controls were purchased from BD Pharmingen (San Jose, CA, USA). The anti-mouse FoxP3 Staining Set (eBioscience Ltd, Dublin, Ireland) was used to study the regulatory T subsets.
The percentages of T and B cell subpopulations were referenced to the total lymphocyte population (based on the CD45+ and SSC-A parameters). The frequency of regulatory B cells was defined as the percentage of CD5+CD1d+ cells within the B220+ cell population. The CD4+ and CD8+ regulatory T cell subsets were defined as the percentages of FoxP3+ cells within the CD4+CD25+ and CD8+CD25+ T cell populations, respectively. The data were analysed with a FacsCanto cytometer (BD) using FacsDiva software (BD Pharmingen).
Determination of anti-PLP139–151 antibody levels
At day 12 p.i., the mice were deeply anaesthetized. When the animals were unresponsive to plantar reflex stimulation in response to pressing the sole of the foot, a blood sample was obtained by cardiac puncture and collected in tubes without anti-coagulant to allow blood clot formation. The serum was obtained after centrifugation at 900 g for 15 min. Serum was stored at −80°C until assayed. The levels of anti-PLP139–151 IgG and IgM antibodies in the serum samples diluted 1/100 were measured by enzyme-linked immunosorbent assay (ELISA), as described previously [14]. The results are expressed as the numbers of units of optical density (OD) at a wavelength of 492 nm.
Statistical analysis
Student's t-test was performed to compare the mean values of two groups [15]. When required, equality of the variances was not assumed. Differences were considered statistically significant if P < 0·05. The data are expressed as the mean values (s.d.).
Results
Cellular responses
T cell responses
We explored differences between susceptible and resistant mice 12 days after immunization, when the immune response against the encephalitogenic antigen was established. Susceptible mice exhibited significantly higher amounts of naive (CD4+CD25−, P = 0·026), activated (CD4+CD25+, P < 0·001) and regulatory CD4+ T cells (CD4+CD25+FoxP3+, P = 0·026) (Fig. 1, Table 1). Conversely, we found no differences in the numbers of naive (CD8+CD25−), activated (CD8+CD25+) or regulatory CD8+ T cells (CD8+CD25+FoxP3+) (Table 1).
Fig. 1.
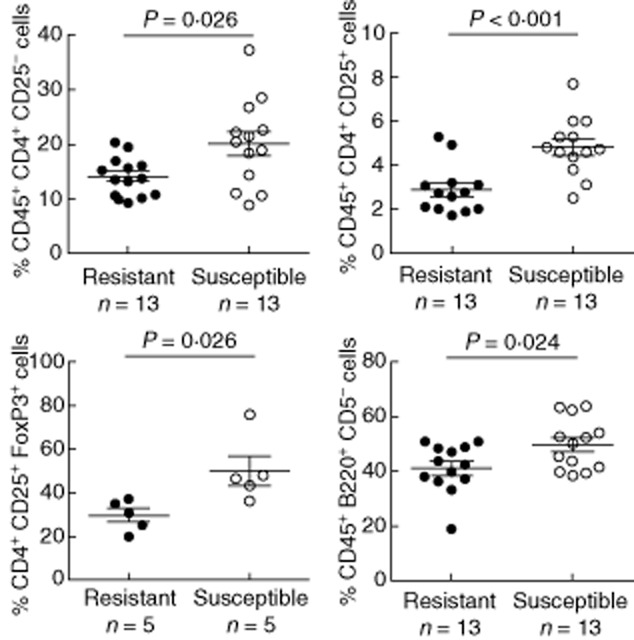
Differential expression of T and B cell subsets in susceptible and resistant mice immunized with the proteolipid protein (PLP)139–151 peptide. The frequencies of different T and B cell subsets were determined by flow cytometry. Susceptible mice (open circles) exhibit a higher percentage of naive CD4+ T cells (defined as CD45+CD4+CD25−), activated CD4+ T cells (CD45+CD4+CD25+), regulatory CD4+ T cells [CD4+CD25+forkhead box protein 3 (FoxP3)+] and T-dependent B cells (B2; CD45+B220+CD5−) compared with resistant mice (black circles). The percentages of naive and activated T cells and T-dependent B cells were defined as the frequencies of CD4+CD25−, CD4+CD25+ and B220+CD5− cells, respectively, within the total lymphocyte population (gated on CD45+ cells). The percentage of regulatory CD4+ T cells refers to the frequency of FoxP3+ cells within the CD4+CD25+ population.
Table 1.
T and B cell subpopulations in proteolipid protein (PLP)139–151-immunized susceptible and resistant mouse strains
| Cell population | Number of mice | Resistant strain (%) | Susceptible strain (%) |
|---|---|---|---|
| CD4+ naive T cells | 13 | 14·2 (3·5) | 20·1 (8·0)* |
| CD4+ activated T cells | 13 | 2·9 (1·1) | 4·8 (1·3)** |
| CD4+ regulatory T cells | 5 | 29·6 (6·8) | 49·9 (15·1)* |
| CD8+ naive T cells | 13 | 6·1 (2·7) | 7·7 (3·5) |
| CD8+ activated T cells | 13 | 0·2 (0·2) | 0·2 (0·1) |
| CD8+ regulatory T cells | 5 | 1·3 (2·4) | 4·1 (2·5) |
| B2 cells | 13 | 41·1 (8·8) | 49·6 (9·2)* |
| B1 cells | 13 | 2·6 (1·2) | 2·7 (0·8) |
| Regulatory B cells | 13 | 3·5 (1·8) | 3·2 (1·3) |
The data are expressed as the mean values ± standard deviation. The percentages of regulatory CD4+ and CD8+ T cells refer to the frequencies of forkhead box protein 3 (FoxP3)+ cells within the CD4+CD25+ and CD8+CD25+ populations, respectively. The frequency of regulatory B cells refers to the percentage of CD5+CD1d+ events within the B220+ population. The frequencies of the remaining T and B cell subsets are in reference to the total lymphocyte population.
* P < 0·05 and **P < 0·01.
We tested the proliferative capacity of splenocytes. Susceptible strain showed higher proliferative capacity upon non-specific (PHA) stimulation [stimulation index (SI): 20·1 (11·6)] compared with the resistant strain [SI: 9·5 (7·0)] (P = 0·045). PLP139–151-specific proliferation was detected only in susceptible mice [SI: 12·3 (9·6)], and not in resistant ones [SI: 1·9 (1·4)] (P = 0·018, Fig. 2a). This finding indicates the incapacity of resistant mice to develop a proper T cell response against the encephalitogenic peptide.
Fig. 2.
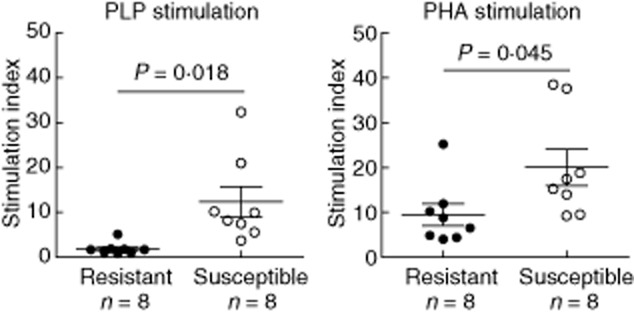
Different proliferative capacities of splenocytes from resistant and susceptible strains. The capabilities of splenocytes from the two studied strains (resistant and susceptible mice are represented by black and open circles, respectively) were measured by [3H]-thymidine incorporation. The splenocytes from susceptible mice stimulated with the proteolipid protein (PLP)139–151 peptide (specific stimulation) or phytohaemagglutinin (PHA) (non-specific stimulation) exhibited a higher proliferative capacity compared with that of resistant mice.
We next attempted to identify the specific T cell response that is induced in susceptible mice immunized with PLP139–151 by quantifying Th1, Th2 and Th17 cytokines. The studied cytokines were detected in the susceptible mice, with the only exception of interleukin (IL)-10 (Fig. 3). In contrast, resistant mice did not release IL-1α, IL-2, IL-5, IL-6, tumour necrosis factor (TNF)-α and granulocyte–macrophage colony-stimulating factor (GM-CSF), and only low levels of interferon (IFN)-γ and IL-17 were detected (Fig. 3), although we found significant differences only for IL-17 [123·6 (37·6) versus 7·0 (12·0) pg/ml, respectively; P = 0·007].
Fig. 3.
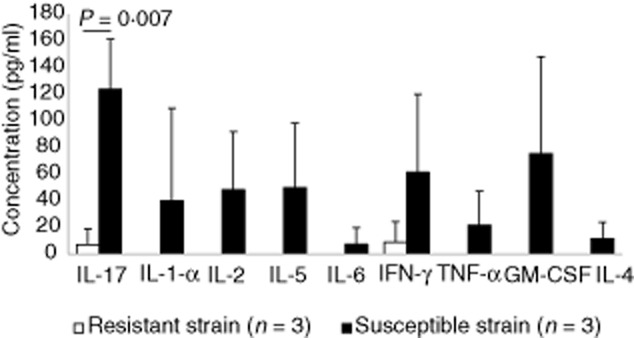
Lack of cytokine production by splenocytes from the resistant strain. The profile of T helper type 1 (Th1)/Th2/Th17 cytokine production in the supernatants of phytohaemagglutinin (PHA)-stimulated splenocytes was assessed by flow cytometry. Compared with susceptible mice (black bars), the majority of cytokines studied were not detected in the cells obtained from resistant mice (white bars). Interleukin (IL)-17 levels were statistically different between the groups. The error bars represent standard deviations (s.d.).
B cell subsets
We did not appreciate differences in the percentages of regulatory (B220+CD5+CD1d+) and T-independent B cells (B1; B220+CD5+) between susceptible and resistant mice (Table 1). However, the percentage of T-dependent B cells (B2; B220+CD5−) was increased significantly in susceptible mice immunized with PLP139–151 (P = 0·024, Fig. 1, Table 1).
Humoral responses
We then assayed the levels of anti-PLP139-151 IgG and IgM antibodies in serum. Both susceptible and resistant mice developed anti-PLP IgM antibodies upon immunization with PLP139–151, although the levels were higher in susceptible mice (P = 0·028). However, only susceptible mice were capable of producing anti-PLP139–151 IgG antibodies (Fig. 4). Despite immunization with the encephalitogenic peptide and with complete Freund's adjuvant, resistant mice did not show IgG antibodies anti-PLP.
Fig. 4.
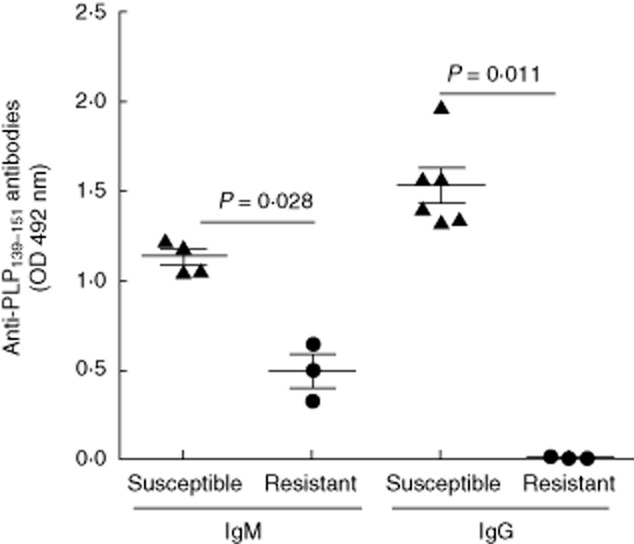
Antibody production. Levels of anti-proteolipid protein (PLP) immunoglobulin (Ig)M and IgG antibodies in 1/100-diluted serum samples obtained from six susceptible (triangles) and three resistant (circles) mice 12 days after immunization with PLP. The main differences were observed in the IgG titres, which were higher in the experimental autoimmune encephalomyelitis (EAE)-susceptible mice.
Discussion
EAE studies have yielded substantial progress towards understanding the pathogenesis of MS. However, the precise mechanisms implied in disease onset have not yet been identified. A direct role of MHC alleles in MS has been demonstrated recently by studying the relationship between EAE susceptibility and peptide presentation in transgenic mice expressing different human leucocyte antigen (HLA) class II molecules [16]. The mouse strains used in this study exhibit different susceptibilities to EAE induction and different MHC haplotypes. SJL/J mice (H-2s) are susceptible, while BALB/c mice (H-2d) are resistant to EAE induction with the PLP139–151 peptide. The differences in the H-2 haplotype conditioned a lack of proliferation of BALB/c lymphocytes against the encephalitogenic peptide, confirming the functional implications of MHC on EAE susceptibility.
We aimed to identify the immune mechanisms expanded differentially in resistant or susceptible mice in response to immunization with the encephalitogenic peptide. No differences were found in regulatory B cells or in CD8+ subsets, despite the effects they can have in MS and EAE [4,17–20]. This may be due to the time-point at which we made the study. It has been described recently that CD8+ T cells are involved in EAE initiation, being necessary to induce CD4+ Th17 cells [21] which seem to play an important role in EAE pathogenesis. We studied immune responses at a later disease stage, when CD4 T cell responses were already established. Our results suggest that the role of activated CD8+ T cells may be not so crucial at this point. By contrast, upon immunization with the PLP139–151 peptide, SJL susceptible mice showed a significant increase in the amount of activated CD4+ T cells, thus confirming a role for these cells in EAE susceptibility. To characterize this response further, we analysed cytokine secretion in splenocyte cultures. We found a significant increase of IL-17 in susceptible mice. It has been suggested that the Th17 subset is involved in CNS inflammatory events that lead ultimately to demyelination, including BBB disruption and the activation of microglia [5,6]. It has also been proposed that they have a key role in inducing autoantibody production [22]. We next evaluated the role of B cells and antibodies in EAE induction. We observed that only EAE mice were capable of producing anti-PLP139–151 IgG antibodies, which were associated previously with a severe EAE course [23]. Resistant mice, which failed to establish PLP-specific T helper responses, did not show anti-PLP IgG antibodies. Ineffective presentation of PLP peptide by MHC-II molecules of resistant mice may result in the lack of T–B cell co-operation and thus in an inhibition of isotype switch [24], and of anti-PLP IgG antibody production. Because T–B cell interaction is not so crucial for the primary antibody response, the synthesis of anti-PLP IgM was not abolished.
These data suggest strongly that IL-17 plays a pivotal role in the induction of relapsing EAE in SJL mice and that, directly or indirectly, it induces the production of high-affinity anti-myelin IgG antibodies. Future studies will demonstrate if they have a role in EAE/MS onset.
Acknowledgments
We thank the ‘Red Española de Esclerosis Múltiple (REEM)’ (RD07/0060; RD12/0032), which is sponsored by the Fondo de Investigación Sanitaria (FIS), the Instituto de Salud Carlos III, the Ministry of Economy and Competitiveness in Spain and the ‘Ajuts per donar Suport als Grups de Recerca de Catalunya (2009 SGR 0793)’, which is sponsored by the ‘Agència de Gestió d'Ajuts Universitarisi de Recerca’ (AGAUR) of the Generalitat de Catalunya in Spain. H. E. is supported by the ‘Sara Borrell’ programme (CD09/00363) of the FIS of the Ministry of Economy and Competitiveness of Spain. C. E. is partially supported by the ‘Miguel Servet’ programme (CP07/00146) from the FIS of the Ministry of Economy and Competitiveness of Spain.
Disclosures
The authors declare no financial conflicts of interest.
References
- 1.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLOS ONE. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 4.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppert J, Closhen D, Croxford A, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 6.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Berghmans N, Nuyts A, Uyttenhove C, Van Snick J, Opdenakker G, Heremans H. Interferon-gamma orchestrates the number and function of Th17 cells in experimental autoimmune encephalomyelitis. J Interferon Cytokine Res. 2011;31:575–587. doi: 10.1089/jir.2010.0137. [DOI] [PubMed] [Google Scholar]
- 8.Axtell RC, de Jong BA, Boniface K, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 10.Villar LM, Masjuan J, Sadaba MC, et al. Early differential diagnosis of multiple sclerosis using a new oligoclonal band test. Arch Neurol. 2005;62:574–577. doi: 10.1001/archneur.62.4.574. [DOI] [PubMed] [Google Scholar]
- 11.Villar LM, Sadaba MC, Roldan E, Masjuan J, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. 2005;115:187–194. doi: 10.1172/JCI22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29. doi: 10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espejo C, Carrasco J, Hidalgo J, et al. Differential expression of metallothioneins in the CNS of mice with experimental autoimmune encephalomyelitis. Neuroscience. 2001;105:1055–1065. doi: 10.1016/s0306-4522(01)00252-4. [DOI] [PubMed] [Google Scholar]
- 14.Kennel De March A, De Bouwerie M, Kolopp-Sarda MN, Faure GC, Bene MC, Bernard CC. Anti-myelin oligodendrocyte glycoprotein B-cell responses in multiple sclerosis. J Neuroimmunol. 2003;135:117–125. doi: 10.1016/s0165-5728(02)00434-4. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Analysis of continuous data from small samples. BMJ. 2009;338:a3166. doi: 10.1136/bmj.a3166. [DOI] [PubMed] [Google Scholar]
- 16.Luckey D, Bastakoty D, Mangalam AK. Role of HLA class II genes in susceptibility and resistance to multiple sclerosis: studies using HLA transgenic mice. J Autoimmun. 2011;37:122–128. doi: 10.1016/j.jaut.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rynda-Apple A, Huarte E, Maddaloni M, Callis G, Skyberg JA, Pascual DW. Active immunization using a single dose immunotherapeutic abates established EAE via IL-10 and regulatory T cells. Eur J Immunol. 2011;41:313–323. doi: 10.1002/eji.201041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun D, Whitaker JN, Huang Z, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 21.Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Z, Xu L, Xu W, Yin Z, Gao X, Xiong S. Interleukin-17 expression positively correlates with disease severity of lupus nephritis by increasing anti-double-stranded DNA antibody production in a lupus model induced by activated lymphocyte derived DNA. PLOS ONE. 2013;8:e58161. doi: 10.1371/journal.pone.0058161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtani S, Kohyama K, Matsumoto Y. Autoantibodies recognizing native MOG are closely associated with active demyelination but not with neuroinflammation in chronic EAE. Neuropathology. 2011;31:101–111. doi: 10.1111/j.1440-1789.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein JS, Hernandez SG, Craft J. T cells that promote B-cell maturation in systemic autoimmunity. Immunol Rev. 2012;247:160–171. doi: 10.1111/j.1600-065X.2012.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


