Abstract
An increase in interleukin (IL)-17A-producing cells, particularly at sites of tissue inflammation, is observed frequently, yet the mechanism is not fully understood. This study aims to dissect the role of IL-17 in autoimmunity-mediated neuroinflammation. The cytokine milieu containing elevated IL-17, which often appears in active states of autoimmunity, was mimicked in vitro by a supernatant obtained from rat peripheral blood monocytes stimulated with phorbol mystistate acetate (PMA)/ionomycin. The application of such inflammatory media on only primary cultured cerebellar granule neurones resulted in significant apoptosis, but the presence of astrocytes largely prevented the effect. The supernatants of the stimulated astrocytes, especially those that contained the highest level of IL-17, achieved the best protection, and this effect could be blocked by anti-IL-17 antibodies. Protein IL-17 inhibited intracellular calcium increase and protected the neurones under inflammatory attack from apoptosis. IL-17, but not interferon (IFN)-γ, in the inflammatory media contributed to astrocyte secretion of IL-17, which depended on the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway activation. The astrocytes that were treated with IL-17 alone or with prolonged treatment of the inflammatory media failed to produce sufficient levels of IL-17. Moreover, confirmatory data were obtained in vivo in a monophasic experimental autoimmune uveitis (EAU) in Lewis rats; in this preparation, the high-level IL-17-containing the cytokine milieu was demonstrated, along with IL-17 secretion by the resident neural cells. The antagonism of IL-17 at a late stage disturbed the disease resolution and resulted in significant neural apoptosis. Our data show a dynamic role of IL-17 in the maintenance of homeostasis and neuroprotection in active neuroinflammation.
Keywords: autoimmune response, experimental autoimmune uveitis, interleukin-17, neuroinflammation, neuroprotection, primary neurone culture
Introduction
Elevated levels of proinflammatory cytokines, including interleukin (IL)-17A, interferon (IFN)-γ, tumour necrosis factor (TNF-α), IL-6, etc. are often correlated with active states of the autoimmune diseases, including those affecting the central nervous system (CNS) such as multiple sclerosis (MS) and uveitis. Generated mainly by immune cells that are recruited to the site of the inflammation, these cytokines may enhance the local inflammatory environment via the induction of chemokines and adhesion molecules and recruitment of additional immune cells [1]. Conversely, in response to inflammatory stimuli, the resident microglia and astrocytes become activated to produce cytokines and trophic factors that can exert damaging or protective effects, and thus may contribute to disease resolution or deterioration [2]. Because most patients with CNS autoimmunity have the relapsing–remitting form, which often progresses to more debilitating disease, the persistence of such an inflammatory environment is deleterious to neuronal function [3]. However, in an acute disease where a high inflammatory environment operates for a short time followed by clinical recovery, the CNS activity remains mainly unaffected. Therefore, elucidating how certain components and dynamics of the local environment may affect activities of the CNS-resident cells and disease outcome is important for the therapy.
The cytokine IL-17 (IL-17A) is the founding member of the IL-17 family of cytokines, which includes five other members, designated IL-17A-F [4]. Of these members, IL-17F shares the highest homology (55%) with IL-17, and these two cytokines may function as homodimeric or heterodimeric secreted protein with similar activities [5]. The IL-17 receptor is expressed ubiquitously, therefore most cells can potentially respond to this cytokine [6]. Furthermore, although IL-17 alone is a weak inducer of target genes, it has been shown to synergize with IL-1β, IL-22, IFN-γ, TNF-α and other cytokines in vivo [6]. Notably, IL-17 is vigorously involved in mediating proinflammatory responses via the induction of many other cytokines, including IL-6, granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-1β, TGF-β, TNF-α and chemokines, including IL-8 and monocyte chemotactic protein-1 (MCP-1), from many cell types [7]. Moreover, IL-17 function is essential to T helper type 17 (Th17) cells [8]. As a result of these functions, the IL-17 family has been linked to many autoimmune-related diseases, including rheumatoid arthritis, asthma and CNS autoimmunity.
As a consequence of a dominant Th17 response, a high-level IL-17-containing environment is a frequent phenomenon observed in sera or biopsies of active diseases, including MS, uveitis and their respective animal models, experimental autoimmune encephalomyelitis (EAE) and experimental autoimmune uveitis (EAU) [3]. Both models share many mechanisms of inflammatory tissue damage. Other effector Th subsets, such as IFN-γ-producing Th1, co-exist and play less pathogenic or regulatory roles [9,10]. Apart from Th17, other cells, including the resident astrocytes [11], the infiltrating γδ T cells [12] and natural killer (NK) T cells [13], may also produce IL-17 in the local environment. Reactive astrocytes are part of neuroinflammation and most probably contribute to a high-level IL-17-containing cytokine milieu in vivo. For example, astrocytes can act as ‘non-professional’ antigen-presenting cells in MS by the expression of major histocompatibility complex (MHC) class II and co-stimulatory molecule B7, both of which are essential for the development of effector Th cells. In addition, among a wide array of mediators, astrocytes produce cytokines, including IL-1β, IL-6, TNF-α, IL-10, and TGF-β, during inflammation [14]. In addition to their roles in the induction of active immune responses and the neuroprotective and/or neurotoxic processes [14], certain of these cytokines are shown to act in synergy or promote mutual generation with IL-17 in vivo [6,15]. Taken together, as different cytokines tend to work in network rather than an individual action it is unclear thus far, however, whether the existence of a high-level IL-17-containing environment for active diseases indicates a direct pathogenic role for IL-17 or whether it is a result of feedback mechanisms.
Nevertheless, IL-17 may play a role in multiple cell types and conditions, as suggested by ubiquitous IL-17R expression [6]. Recent data show the neurotrophic features of the cytokine [16,17]. An anti-inflammatory role of IL-17 is revealed in an acute EAU in mice [18]. Here, we report a novel neuroprotective role of IL-17 that is produced by astrocytes under the short-term stimulation of a high-level IL-17 cytokine milieu in vitro; the finding was also confirmed in an acute EAU model in vivo. Therefore, given that IL-17 is both a key component and a neurotrophic factor in active neuroinflammation similar to cytokine IL-6 or TGF-β, both of which can be produced by reactive astrocytes [19,20], our results suggest that a targeted therapy with IL-17 in acute neuroinflammation should be taken with caution.
Materials and methods
Primary neurone and astrocyte cultures
The primary cultures of cortical astrocytes or cerebellar granule neurones (CGNs) were obtained from naive newborn or 3-day-old Wistar rats [21–23]. Briefly, under cold sterile conditions, the cerebral cortex or the cerebellum was isolated, minced and digested with 0·25% trypsin/0·53 mM ethylenediamine tetraacetic acid (EDTA) at 37°C for 25 min, followed by adding Dulbecco's modified Eagle's medium (Gibco Life Technologies, Paisley, UK) supplemented with 10% fetal bovine serum (Gibco), 100 U/ml penicillin and 0·1 mg/ml streptomycin. The resultant cells were collected by centrifugation and further dissociated by titration through a Pasteur pipette. The astrocytes were resuspended in complete medium on six-well plates at 5 × 105 cells/well, passaged every 2–3 days and used in experiments after the fifth passage. The CGNs were plated at 4 × 105 cells/well in 12-well plates coated with 25 μg/ml poly-D-lysine (Sigma, St Louis, MO, USA) and cultivated in basal Eagle's medium (Gibco), supplemented with 25 mM KCl, 2 mM glutamine (Gibco), antibiotics and 2% B27 (Gibco). 1-β-D-Arabinofuranosylcytosine (2 μg/ml; Sigma) was added to the culture 24 h after plating to limit the growth of non-neuronal cells [23]. The CGNs were used for experiments after 5 days of culture. The purities of the primary-cultured astrocytes and CGNs were greater than 90% by immunofluorescent microscopy with an anti-glial fibrillary acidic protein (GFAP) antibody (Biosynthesis Biotechnology Co. Ltd, Peking, China) or a neurone-specific class III beta-tubulin antibody (Tuj1) (Covance Inc., Princeton, NJ, USA) (Supporting information, Fig. S1a). In addition, CD11b-positive microglia culture was excluded from the the primary astrocyte culture (Supporting information, Fig. S1b). For co-culture of the CGNs and astrocytes, the harvested astrocytes were added to the CGN culture for 6 h before the co-cultures were exposed to the inflammatory stimuli for further experiments.
Preparation of the inflammatory media with supernatants of stimulated PBMC
PBMC from Wistar rats were cultured in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (FBS) and antibiotics; 3 × 106 cells were stimulated with one of the three stimulants, 50 ng/ml phorbol myristate acetate (PMA) plus 1 μg/ml ionomycin (Sigma), 100 ng/ml Escherichia coli 0111: B4 of lipopolysaccharide (LPS, Sigma) or 5 μg/ml concanavalin A (ConA; Sigma) for varied time-periods before being harvested and analysed by real-time reverse transcription–polymerase chain reaction (RT–PCR), and the supernatant of the stimulated PBMC by enzyme-linked immunosorbent assay (ELISA). The supernatant of the PBMC stimulated by PMA/ionomycin for 6 h was used and named after the Inflammatory Media (Infl. Med.). A full Infl. Med. was used to stimulate the primary-cultured astrocytes, whereas a half-diluted Infl. Med. was used by the neuronal culture media to stimulate the CGNs to allow further experiments (Supporting information, Fig. S2).
Recombinant rat proteins, neutralizing antibodies and inhibitor drugs
The recombinant rat proteins IL-17 and IFN-γ were from ProSpec (Ness-Ziona, Israel). The polyclonal antibodies against the rat cytokine IL-17 (Acris Antibodies, San Diego, CA, USA) and monoclonal antibodies against rat IFN-γ (Biosource Int. Inc., Camarillo, CA, USA) were used to neutralize or block activity of the respective cytokines. The Janus kinase 2 (JAK)2 inhibitors AG490, the nuclear factor (NF)-κB inhibitors pyrrolidine dithiocarbamate (PDTC) and the mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitors U0126 were purchased from Sigma.
Induction and treatment of the acute experimental autoimmune uveitis (EAU)
Female Lewis rats (160–180 g, 5–6 weeks; Peking Vital River Laboratory Animal Ltd, Beijing, China) were maintained in specified pathogen-free conditions according to the guidelines for Care and Use of Laboratory Animals by the China National Institute of Health. All the experimental procedures adhered to the Association for Research in Vision and Ophthalmology Statement for the use of the animals.
The induction of an acute EAU by immunization with peptide interphotoreceptor retinoid-binding protein (IRBP) 1177–1191 (R16) has been described previously [24]. Briefly, the EAU rat was injected in each of its footpads with 0·1 ml of an emulsion containing 50 μg R16 and 2·5 mg/ml of Mycobacterium tuberculosis H37Ra in Freund's complete adjuvant (CFA; Sigma); the control animal received equivalent injections at the same position with phosphate-buffered saline (PBS) emulsified with CFA. Concurrently, both groups of animals were injected intraperitoneally with 1 μg of pertussis toxin (Sigma). All animals were monitored daily. Clinical signs were scored by slit-lamp biomicroscopy using the following criteria: 0, the eye is translucent and reflects light; 0·5, dilated blood vessels in the iris; 1·0, engorged blood vessels in iris; abnormal pupil contraction; 2·0, hazy anterior chamber; decreased red reflex; 3·0, moderately opaque anterior chamber, but pupil still visible; dull red reflex; and 4·0, opaque anterior chamber and obscured pupil; red reflex absent; proptosis [25]. The specified tissues were collected at the end of each week until 35 days post-immunization (dpi). Three batches of immunization were accomplished, each including 35 EAU and 15 control animals. For the EAU treatment group, an additional seven rats from each batch were injected intravenously with 20 μg of anti-IL-17 neutralizing antibodies per animal at 17, 19, 21 and 23 dpi, respectively; the PBS-injected animals were used as non-treated controls. The treated versus control animals were killed on 28 dpi.
Quantitative real-time RT–PCR (qRT–PCR)
The relative expressions of multiple genes at the mRNA level were detected in the relevant tissues of the animals and the stimulated cells by qRT–PCR with an ABI STEPONE real-time PCR System (Applied Biosystems, Foster City, CA, USA). The total RNA was extracted by Trizol reagent (Invitrogen Ltd, Shanghai, China), and 1 μg of RNA was reverse-transcribed by high-capacity cDNA reverse transcription kits (Applied Biosystems). The gene expression analysis was performed by the Power SYBR Green PCR Master Mix (Applied Biosystems). The PCR conditions for all genes were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min and fluorescence determination at the melting temperature of the product for 20 s. The relative expression of each gene of interest at the mRNA level was calculated by delta-delta-2-CT methods [24], and the values were normalized as fold changes in relation to the expression of the relevant housekeeping genes. Analysis of the tissues was performed by an adapted method that we have reported previously [24]. Briefly, for each batch immunization, the whole retinal tissues from animals with the same clinical scores of matched disease stage were used for analysis to avoid variations from different batches of immunizations and varied rats. Three housekeeping genes (Ldha, RGD, Rp113a) were used for the normalization of the retinal samples to ensure stringent comparison. The sequences of the specific primers (Sangon Ltd, Shanghai, China) for the tested genes are available by request. Each experiment was performed in duplicate and repeated two to three times; the standard error of the mean (s.e.m.) among repeated experiments were stringently less than 2% when analysed by Student's t-test (data not shown).
TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labelling) and lactate dehydrogenase (LDH) release assays on neural cells
For detection of apoptosis in vitro, TUNEL assays were performed with the one-step TUNEL fluorescent kit (Beyotime, Jiangsu, China). Briefly, the CGNs or astrocytes stimulated with or without the Infl. Med. were permeabilized with 0·1% Triton X-100, followed with fluorescein isothiocyanate (FITC)-labelled TUNEL staining for 1 h at 37°C. The TUNEL-positive cells were imaged under a fluorescent microscope (Nikon 80i, Tokyo, Japan) equipped with a CCD camera (Nikon DS-Ri1), and quantified as the number of green spots in each photograph (×100); 10 photographs were counted. The lactate dehydrogenase (LDH) release assays (Sigma) were used for the necrosis analysis. The LDH activity in the supernatants of the CGNs with or without stimulation was detected by an enzymatic test, in which the formation of the formazan product of the idonitrotetrazolium dye was examined colorimetrically. Briefly, 15 μl of lactate solution (36 mg/ml), an equal volume of p-iodonitrotetrazolium violet solution (2 mg/ml) and NAD+/diaphorase solution were added in order to 100 μl of the supernatant of the CGNs in 96-well plates. The optical densities (OD) were measured by a microplate reader (M5; Molecular Devices Inc., Sunnyvale, CA, USA) with a 490-nm filter. The data were expressed as the percentage of dead cells, for which the 100% value is obtained from neurones completely lysed by 1% Triton X-100.
Direct and indirect immunofluorescent microcopy
CGNs, 2 × 104 cells/well, or astrocytes with or without stimulation were seeded on glass coverslips in 12-well plates. The harvested cells or 7-μm retinal tissue frozen sections from the animals were fixed in 4% paraformaldehyde and blocked in blocking buffer. The incubation of the respective primary antibodies including anti-rat Tuj1 (Covance Inc.), anti-rat IL-17 or CD11b (Abcam Inc., Cambridge, MA, USA), or anti-rat GFAP antibody (Cell Signaling Technology, Danvers, MA, USA) was used in 1% goat serum and 0·1 % bovine serum albumin (BSA) at 4°C overnight before the application of the specific secondary antibodies, including FITC-conjugated goat (BD Bioscience, San Jose, CA, USA; IL-17) and the Alexa Fluor®-555-conjugated goat anti-mouse secondary antibody (Cell Signaling Technology; red, against GFAP or CD11b). The cells or tissue sections were subsequently incubated with 2-(4- amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Roche, Basel, Switzerland) for the cell nuclei staining before the slides were mounted. For the neural cells to direct immunofluorescent microscopy, the FITC-conjugated antibody against GFAP (Biosynthesis Biotechnology) was applied, and DAPI was used to stain the cell nuclei. The fluorescent images were taken by a fluorescence microscope (Nikon 80i) with a cold CCD camera (Nikon DS-Ri1) by the NIS-Elements F version 3.0 software. The double-positive staining of GFAP and IL-17 on reactive astrocytes was quantified by measuring the merged fluorescence intensity in the retina using the NIS-Elements BR version 4.00.00 software (Nikon). The intensity was averaged from 10 fields of the view [26].
Immunohistochemistry
Two-μm sections of the eyeball from the EAU, treated and control animals or the primary cultured neural cells were fixed in 4% paraformaldehyde and blocked in blocking buffer. For the cytokine IL-17-positive staining, the slice of tissues or cells was incubated with either an anti-rat IL-17 rabbit polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or non-immune rabbit immunoglobulin (Ig)G. The biotinylated secondary antibody, avidin–biotinylated enzyme complex (ABC) and 3,3′-diaminobenzidine (DAB) substrate were used as the detecting reagents (Zhongshan Goldenbridge Biotechnology, Beijing, China). For TUNEL staining on detection of apoptotic cells, the retinal tissue sections were incubated with 20 μg/ml proteinase K in 10 mM Tris-HCL for 20 min at room temperature. Non-specific peroxidase staining was eliminated with 3% H2O2. The sections were then reacted with the TUNEL reaction mixture (In Situ Cell Death Detection Kit; Roche) for 60 min before incubation with horseradish peroxidase (HRP) for 30 min at 37°C, and visualized with a chromogen solution containing DAB. The negative control was treated with the labelling solution only. The slides were counterstained with haematoxylin, mounted and observed under a microscope (Nikon 80i) by two independent medical pathologists.
Western blotting analysis
Western blotting analysis was performed on the CGNs or astrocytes with or without treatment. Bradford assays (Bio-Rad, Hemel Hempstead, UK) were used to determine the total protein concentrations of the whole cell lysates, which were normalized to 1 μg/ml for all samples. Up to 30 μl of the protein lysates was loaded and the respective primary antibodies against IL-17 receptor (Abnova, Taipei City, Taiwan), phospho-signal transducer and activator of transcription (STAT)-3, STAT-3, phospho-extracellular-regulated kinase (ERK), ERK, phospho-p65, p65 (1:1000) and β-actin (1:1000; Sigma), and the secondary antibodies (anti-rabbit or mouse HRP-conjugated, 1:1000) were applied sequentially. Enhanced chemiluminescence (Pierce Ltd, Shanghai, China) detection was used, and the levels of total- and phosphor-proteins were quantified by densitometry with the NIH-ImageJ© software. The ratios of the mean densities of the phospho-proteins to that of the total proteins or the total β-actin were calculated and recorded as the relative expression levels of the protein activity. The antibodies and reagents were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA) if not mentioned otherwise.
ELISA assays
The protein levels of IL-17, IL-6 and IFN-γ in the supernatant of cultured cells with or without stimulation were measured using commercially available ELISA kits, including rat IL-17A/IFN-γ/IL-6 platinum ELISA (eBioscience, San Diego, CA, USA), according to the manufacturer's instructions.
Measurement of intercellular Ca2+ concentration
The intracellular Ca2+ concentration ([Ca2+]i) was determined as described previously [27,28]. Briefly, 5 × 103 primary-cultured CGNs were placed on a poly-D-lysine-coated glass cover slip. On the day of imaging, the cells were washed with normal saline solution (NSS: 137 mM NaCl, 25 mM glucose, 10 mM HEPES, 5 mM KCl, 1 mM MgCl2 and 2 mM CaCl2 at pH 7·4) and stained with 5 μM Fluo-4/AM (AM, acetoxymethyl; Invitrogen) for 30 min at 37°C. The CGNs were treated subsequently with either the recombinant rat IL-17 (100 pg/ml or 1 ng/ml), the supernatant of astrocytes stimulated by the Infl. Med. for 12 h, 10 μM of MK801 [N-methyl-D-aspartate (NMDA) receptor inhibitor; Santa Cruz] or 0·2 mM of CdCl2 (calcium channel inhibitor; Sigma) or left untreated for 30 min at 37°C before they were finally exposed to the Infl. Med. The single-cell fluorescence was captured by a laser confocal scanning microscope (Olympus, LSM, Tokyo, Japan) at an excitation of 488 nm and an emission wavelength of 530 nm for the measurement of [Ca2+]i. The fluorescent images exhibiting [Ca2+]i were recorded every 2·7 s, and the data were quantified using a specialized software Image-Master version 5.0 (Olympus).
Statistical analysis
All experiments were repeated at least three times. The data were expressed as the means ± s.e.m. Where appropriate, two group comparisons were analysed with Student's t-test. P-values less than 0·05 were considered to be statistically significant.
Results
Astrocytes protected neurones against inflammatory stimuli of high-level IL-17-containing cytokine media in vitro
For varied periods, in comparison to the other two mitogenic compounds, LPS and ConA, PMA/ionomycin-stimulated peripheral blood monocytes (PBMC) of the Wistar rats produced a series of inflammatory media that contained high levels of varied cytokines, including IL-17, IL-17F, IFN-γ and IL-6. In particular, the supernatant of 6 h-stimulated PBMC contained the highest level of IL-17 among other cytokines, including IFN-γ and IL-6, at both the mRNA and protein levels (Table 1, Fig. 1a). Such a high level of IL-17-containing cytokine milieu was used to stimulate the primary cultured neural cells to observe neural cell apoptosis and cytokine expression profiles. The media were named after the Infl. Med. (see Materials and methods). Consistent with other reports, at 6 h stimulation by PMA/ionomycin, the PBMC expressed the highest levels of multiple transcriptional factors that were relevant to different Th subsets at the mRNA level (Table 1), indicating activation of these T cells in vitro [29]. Application of the Infl. Med. (half-diluted) resulted in significant apoptosis in the primary-cultured CGNs, with cell death peaking at 12 h of incubation, yet the presence of primary-cultured astrocytes almost protected the neurones from cell apoptosis as measured by TUNEL assays (Fig. 1b).
Table 1.
Relative expression of T cell-associated molecules from PMA/ionomycin-stimulated peripheral blood mononuclear cells (PBMC) at the mRNA level
| con (Ct value) | con | 6 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|---|
| IL-17 | 40·00 | 1·00 | 167·73 ± 11·12 | 12·61 ± 2·47 | 6·54 ± 1·69 | 7·13 ± 2·14 |
| IL-17F | 33·4 | 1·00 | 6·96 ± 1·73 | 12·47 ± 1·81 | 6·59 ± 2·42 | 1·41 ± 0·17 |
| IL-6 | 31·72 | 1·00 | 17·51 ± 2·74 | 43·59 ± 5·79 | 11·79 ± 2·97 | 23·40 ± 6·44 |
| IFN-γ | 31·09 | 1·00 | 11·08 ± 2·09 | 0·53 ± 0·22 | 1·85 ± 0·15 | 0·93 ± 0·45 |
| TNF-α | 23·34 | 1·00 | 3·51 ± 1·39 | 0·91 ± 0·56 | 1·84 ± 0·11 | 1·07 ± 0·66 |
| IL-12p40 | 32·93 | 1·00 | 10·06 ± 3·56 | 5·50 ± 1·52 | 0·84 ± 0·56 | 13·53 ± 3·71 |
| IL-23p19 | 28·54 | 1·00 | 3·84 ± 1·62 | 0·93 ± 0·48 | 0·65 ± 0·24 | 0·65 ± 0·11 |
| ROR-γt | 30·92 | 1·00 | 9·99 ± 3·83 | 1·69 ± 0·35 | 1·68 ± 0·38 | 0·84 ± 0·51 |
| GATA-3 | 23·93 | 1·00 | 2·62 ± 1·15 | 0·25 ± 0·18 | 0·42 ± 0·18 | 0·11 ± 0·08 |
| T-bet | 24·22 | 1·00 | 3·10 ± 1·65 | 0·24 ± 0·19 | 0·52 ± 0·24 | 0·12 ± 0·10 |
| FoxP3 | 29·46 | 1·00 | 29·04 ± 3·05 | 4·26 ± 1·71 | 2·45 ± 1·16 | 0·63 ± 0·31 |
| TGF-β | 20·93 | 1·00 | 2·99 ± 1·22 | 2·99 ± 1·12 | 0·49 ± 0·23 | 0·50 ± 0·28 |
PBMC were activated with phorbol myristate acetate (PMA) and ionomycin for 6, 12, 24 and 48 h. The relative expression of cytokines and transcription factors was measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR). Genes expression at least a 2-fold change was recognized as being significantly altered and in bold type. IL: interleukin; IFN: interferon; TNF: tumour necrosis factor; ROR-γt: retinoic acid receptor-related orphan receptor gammat; GATA-3: GATA-binding protein 3; FoxP3: forkhead box protein 3; TGF: transforming growth factor; con: unstimulated PBMC.
Fig. 1.
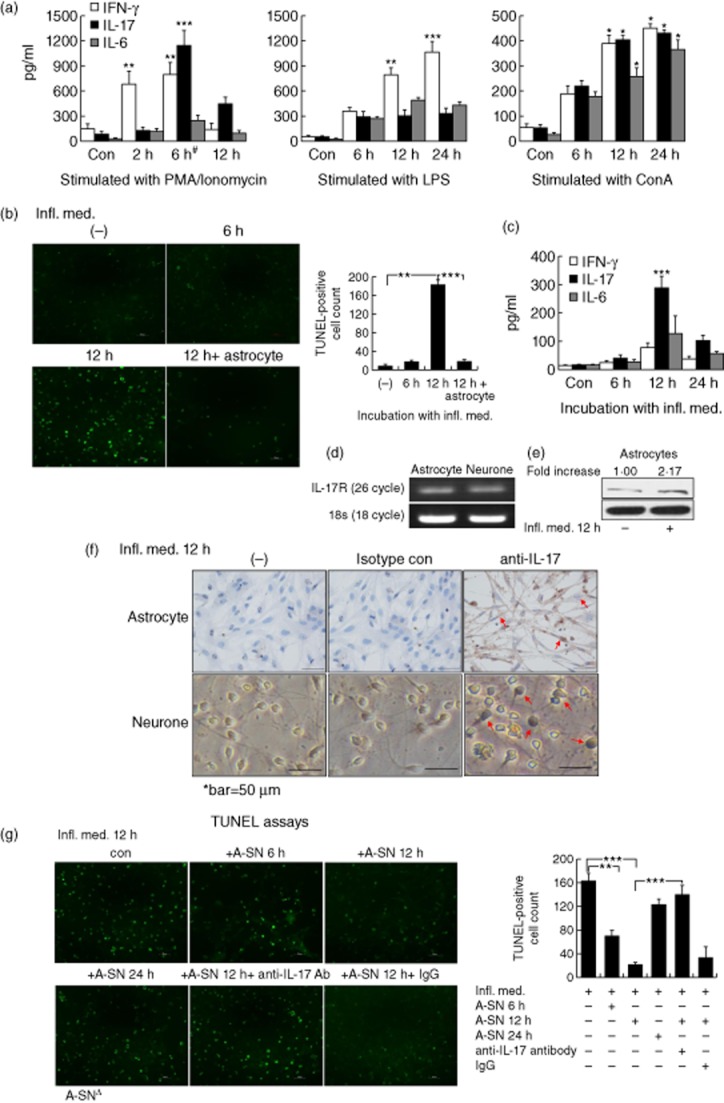
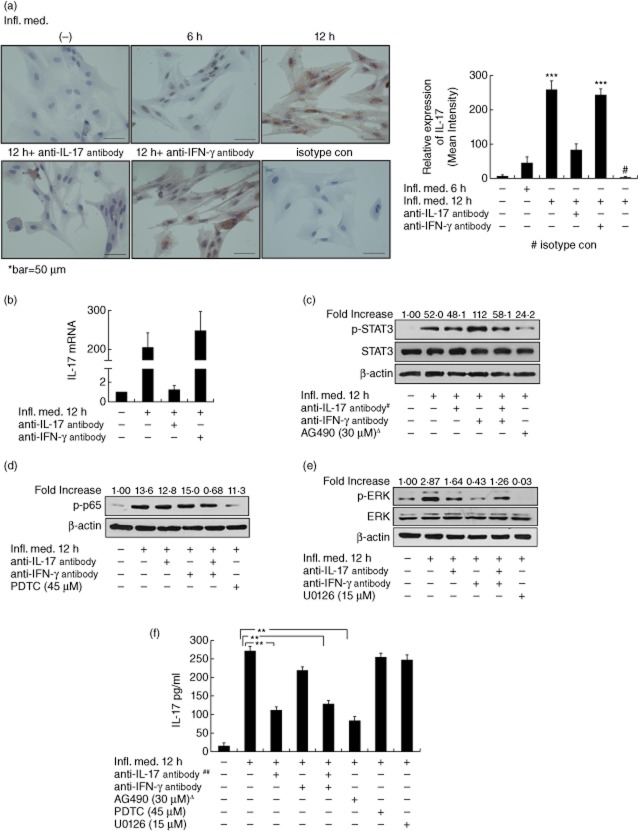
Neuronal protection effect of interleukin (IL)-17 in vitro (a) The peripheral blood mononuclear cells (PBMC) were stimulated with 50 ng/ml phorbol myristate acetate (PMA) plus 1 μg/ml ionomycin, 100 ng/ml lipopolysaccharide (LPS) or 5 μg/ml concanavalin A (ConA) for 6, 12 and 24 h. IL-17 in the supernatant were examined by enzyme-linked immunosorbent assay (ELISA). #The supernatant of PBMC stimulated by PMA/ionomycin for 6 h is referred to as ‘inflammatory media’ (Infl. Med). (b) The presence of astrocytes effectively protected primary cultured CGNs from apoptosis against stimulation with the half diluted inflammatory media by TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labelling) immunofluorescent assays. (c) The highest level of IL-17 was produced in the supernatant of the primary cultured astrocytes treated with the Infl. Med for 12 h. (d) The abundance of IL-17R expression in the CGNs (neurones) and astrocytes by reverse transcription–polymerase chain reaction (RT–PCR). (e) The inflammatory media stimulated a significant increase on the expression of IL-17R expression in the astrocytes by Western blotting. (f) IL-17-positive staining in the primary cultured astrocytes or neurones (arrows) stimulated with the inflammatory media for 12 h by immunohistochemistry with an anti-IL-17 antibody, non-immune rabbit immunoglobulin (Ig)G was used as an isotype control. (g) IL-17 secreted by astrocytes protected neurones from apoptosis against the stimulation of the Infl. Med. The primary cultured neurones were stimulated with the Infl. Med. (half diluted) for 12 h with or without the supernatant of the astrocytes that were treated by the Infl. Med. for the indicated time-periods; additional anti-IL-17 neutralizing antibodies (20 ng/ml) were added where appropriate. All data are the means ± standard error of the mean of at least three experiments. *P < 0·05; **P < 0·01; ***P < 0·001; Δ, A-SN indicates the supernatant of astrocytes stimulated by the Infl. Med.
At the mRNA level in the astrocytes, the Infl. Med. induced significant increases in the following quantities: the expression of proinflammatory cytokines, including IL-17, IFN-γ and IL-6, but not the neurotrophic factors, with the peak expressions of IL-17 and IFN-γ achieved at 12 h post-stimulation and the level of IL-17 over that of IFN-γ (Table 2). Consistently, ELISA assays showed significantly increased levels of IL-17 over other types of cytokines in the supernatant of the reactive astrocytes (Fig. 1c, Supporting information, Fig. S3). However, the direct stimulation by PMA/ionomycin on astrocytes did not induce significantly increased levels of IL-17 or other cytokine expression (Supporting information, Table S1). Moreover, whereas RT–PCR results indicated constitutive expressions of the IL-17 receptor in the primary-cultured CGNs and astrocytes without stimulation (Fig. 1d), Western assays showed a significantly increased level of the receptor in the stimulated astrocytes (Fig. 1e), and immunohistochemistry showed a significant amount of the cytokine IL-17 expression in both astrocytes and CGNs 12 h post-stimulation (Fig. 1f). Further, we found that, while the supernatant of the astrocytes incubated with the Infl. Med. for 12 h (containing the highest level of IL-17 during the incubation, Fig. 1c) attained the best protection on CGNs under inflammatory attack, neutralization of IL-17 with an anti-IL-17 antibody significantly undermined such protection (Fig. 1g). Together, these data showed that the astrocytes might mediate substantial neuronal protection against the acute inflammatory stimuli of the high-level IL-17-containing cytokine media at least partially in vitro, through their secretion of the cytokine IL-17.
Table 2.
Relative expression of T cell-associated molecules from astrocytes under the inflammatory media treatment at the mRNA level
| con (Ct value) | con | 6 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|---|
| IL-17 | 40·00 | 1·00 | 28·31 ± 4·05 | 160·53 ± 25·51 | 4·09 ± 1·47 | 10·36 ± 1·62 |
| IL-17F | 33·19 | 1·00 | 25·16 ± 4·44 | 5·05 ± 1·69 | 1·52 ± 0·30 | 1·65 ± 0·42 |
| IL-6 | 26·2 | 1·00 | 6·38 ± 1·95 | 0·96 ± 0·86 | 0·38 ± 0·19 | 0·42 ± 0·26 |
| IFN-γ | 40·00 | 1·00 | 0·66 ± 0·27 | 97·46 ± 16·24 | 4·24 ± 2·57 | 0·69 ± 0·37 |
| IL-12p40 | 32·93 | 1·00 | 0·40 ± 0·20 | 0·56 ± 0·16 | 0·53 ± 0·08 | 0·95 ± 0·33 |
| IL-23p19 | 28·1 | 1·00 | 2·50 ± 1·30 | 1·01 ± 0·53 | 0·79 ± 0·34 | 0·78 ± 0·48 |
| ROR-γt | 29·92 | 1·00 | 0·38 ± 0·05 | 0·20 ± 0·19 | 0·59 ± 0·12 | 1·58 ± 0·35 |
| GATA-3 | 30·53 | 1·00 | 3·98 ± 1·45 | 0·82 ± 0·30 | 0·38 ± 0·18 | 0·78 ± 0·38 |
| T-bet | 31·46 | 1·00 | 0·24 ± 0·05 | 5·53 ± 1·65 | 1·14 ± 0·40 | 0·62 ± 0·29 |
| FoxP3 | 32·93 | 1·00 | 0·39 ± 0·16 | 1·27 ± 0·33 | 0·68 ± 0·26 | 0·90 ± 0·31 |
| TGF-β | 21·91 | 1·00 | 0·60 ± 0·24 | 0·78 ± 0·24 | 0·56 ± 0·21 | 0·83 ± 0·36 |
| NGF | 26·93 | 1·00 | 1·19 ± 0·43 | 0·89 ± 0·32 | 0·76 ± 0·22 | 1·18 ± 0·41 |
| CNTF | 22·93 | 1·00 | 1·11 ± 0·44 | 0·68 ± 0·19 | 1·11 ± 0·51 | 1·34 ± 0·58 |
Primary astrocytes were treated with the cell-free supernatants of peripheral blood mononuclear cells (PBMC) pretreated with phorbol mysristate acetate (PMA) and ionomycin for 6 h. The relative expression of cytokines and transcription factors was measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) after 6, 12, 24 and 48 h. Genes expression at least a 2-fold change was recognized as being significantly altered and in bold type. IL: interleukin; IFN: interferon; ROR-γt: retinoic acid receptor-related orphan receptor gammat; GATA-3: GATA-binding protein 3; FoxP3: forkhead box protein 3; TGF: transforming growth factor; con: unstimulated, unstimulated astrocytes; NGF: nerve growth factor; CNTF: ciliary neurotrophic factor.
IL-17 protected neurones under the stimulation from apoptosis via inhibition of the intracellular calcium increase
We next investigated how IL-17 may achieve such a protective effect. As the disturbance of intracellular calcium homeostasis may trigger the apoptosis of neurones under stimulation [30], we measured the intracellular Ca2+ concentration ([Ca2+]i) in neurones with or without the Infl. Med. stimulation by laser confocal scanning microscopy. The data indicated that as incubation with the media significantly increased [Ca2+]i in the CGNs, the application of CdCl2, an inhibitor of the cellular L-type voltage dependent calcium channel (VDC), significantly suppressed the increase (P = 0·017 Fig. 2a). To a lesser extent, a neurone-specific N-methyl-D-aspartate receptor (NMDAR) antagonist, MK801, also significantly suppressed [Ca2+]i increase in neurones under the same stimulation (P = 0·036, Fig. 2a). Furthermore, the 30 min preincubation with the supernatant of astrocytes 12 h post-stimulated by the Infl. Med (P = 0·014), 100 pg/ml (P = 0·039) or 1 ng/ml (P = 0·016) of IL-17 all significantly prevented [Ca2+]i increases in neurones under stimulation (Fig. 2b). The intracellular calcium signalling pathways may directly activate caspase-dependent apoptotic pathways [30]. Consistently, our Western assays revealed a significantly increased expression of the cleaved-caspase-3, but decreased pro-caspase-3, in CGNs stimulated by the half-diluted Infl. Med., and almost unaffected expressions of both forms of caspase-3 in the same cells under the protection of the supernatant from reactive astrocytes (Fig. 2c,d).
Fig. 2.
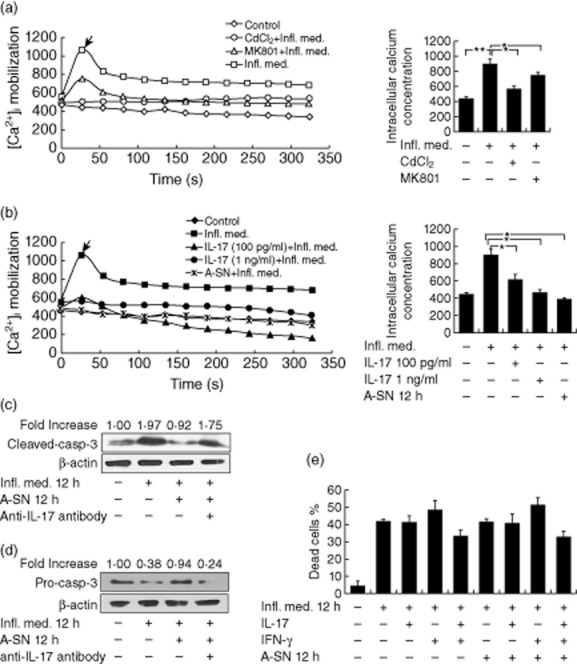
Inhibition of apoptosis and intracellular calcium increase by interleukin (IL)-17 in neurones under stimulation. (a) The laser confocal scanning microscopy indicated a significant increase of the intracellular Ca2+ concentration ([Ca2+]i) in neurones stimulated by the inflammatory media (Infl. Med). This effect was mostly prevented by an inhibitor of the L-type voltage-dependent calcium channel (VDC), CdCl2 (0·2 mM), or partially prevented by an inhibitor of the neurone-specific N-methyl-D-aspartate receptor (NMDAR), MK801 (10 μM). The intracellular Ca2+ was labelled with the fluorescent probe Fluo-4/AM. (b) Pretreatment with the supernatant of the stimulated astrocytes, or IL-17 (100 pg/ml or 1 ng/ml) for 30 min significantly prevented the [Ca2+]i increase in neurones under stimulation. (c) The cleaved-caspase 3 expression at the protein level by Western blotting in neurones with or without stimulation. (d) The pro-caspase 3 expression at the protein level by Western blotting in neurones with or without stimulation. (e) LDH assays showed necrosis in the primary cultured neurones with or without stimulation. Up to 200 pg/ml of IL-17 or interferon (IFN)-γ were applied where appropriate. All data are means ± standard error of the mean of at least three experiments. *P < 0·05; **P < 0·01.
Moreover, we found that incubation with the Infl. Med. resulted in significant neuronal necrosis along with apoptosis (Fig. 2e). However, application of the supernatant of the reactive astrocytes, IL-17, IFN-γ, or IL-17 plus IFN-γ did not significantly protect the stimulated neurones from necrosis, as measured by lactate dehydrogenase (LDH) assays (Fig. 2e). Together, these data showed that while IL-17 secreted by reactive astrocytes may largely protect neurones from apoptosis against acute proinflammatory stimuli, other mechanisms, such as other types of cytokines, contact between neurones and astrocytes or with other cells in the vicinity might protect neurones from necrosis in neuroinflammation.
The high-level IL-17-containing cytokine media stimulated acute production of IL-17 in astrocytes via the JAK/STAT signalling pathways
As we have shown, the best protective effect for neurones under the inflammatory insult was achieved by the supernatant of astrocytes 12 h post-stimulation (Fig. 1g) when the astrocytes produced the highest IL-17 level (Fig. 1c). We next asked whether the astrocytes under the condition of persistent stimulation continued to secrete high levels of protective IL-17. To answer the question, we applied a second stimulation with the Infl. Med. to the astrocytes that had just completed a 12 h post-stimulation period. We observed that the level of IL-17 secreted by the astrocytes under the second inflammatory attack quickly reached peak levels 2 h post-stimulation and then fell gradually at both the mRNA and protein levels. However, the peak levels of IL-17 in the astrocytes under the second stimulation were much lower than those under the first attack (Fig. 3a,b).
Fig. 3.
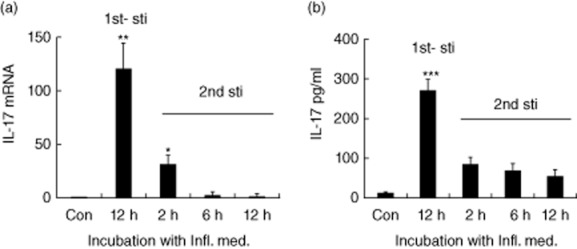
Interleukin (IL)-17 production by the astrocytes under extended time of stimulation. The dynamic expressions of IL-17 productions by the astrocytes under the primary and secondary stimulation with the inflammatory media (Infl. Med) at the mRNA levels by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) (a), or at the protein levels by enzyme-linked immunosorbent assay (ELISA) assays (b). 1st-sti: the primary stimulation with the Infl. Med.; 2nd-sti: the secondary stimulation with the same media started just after the astrocytes completed a 12 h primary stimulation. All data are means ± standard error of the mean of at least three experiments. *P < 0·05; **P < 0·01; ***P < 0·001.
The Infl. Med. for the stimulation of the primary cultured astrocytes contained high levels of proinflammatory cytokines, most prominently IL-17 and IFN-γ (Table 1 and Fig. 1a). We set out to examine whether IL-17, IFN-γ or both cytokines in the media may contribute to the production of IL-17 in reactive astrocytes. To achieve the goal, we applied the neutralizing antibodies against IL-17, IFN-γ or both cytokines, respectively, during the production of the Infl. Med. to reduce significantly the level of IL-17, IFN-γ or both in the media (Supporting information, Fig. S4). The data showed that, compared to the original and the conditional Infl. Med. with reduced level of IFN-γ, the media with less IL-17 stimulated a significantly decreased amount of IL-17 in astrocytes (Fig. 4a,b). Next, we applied up to 1 ng/ml of IL-17 to the astrocyte cultures only for 12 h; we found that IL-17 alone failed to stimulate a significant level of IL-17 in the astrocytes (data not shown). A further examination of the activation of downstream cellular signalling effectors, including STAT-3, NF-κB p65 and ERK, in stimulated astrocytes indicated that, while the Infl. Med. stimulated significant activation of multiple signalling pathways and the prominent activation of all three effector molecules compared with the non-stimulated control, the conditional Infl. Med. with lower levels of IL-17, IFN-γ or both only decreased the activation of ERK, respectively (Fig. 4c–e). Taken together, these results showed that the complex cytokine milieux, the interaction between IL-17 and IFNγ, as well as among other cytokines, and the duration of the stimulation may all contribute to the production of IL-17 by reactive astrocytes.
Fig. 4.
The role of interleukin (IL)-17 in the inflammatory media (Infl. Med). (a) IL-17-positive staining by immunochemistry with an anti-IL-17 antibody in astrocytes incubated with the Infl. Med. with different conditions. During the production of the Infl. Med. in peripheral blood mononuclear cells (PBMC) stimulated by phorbol myristate acetate (PMA)/ionomycin, neutralizing anti-IL-17, or anti-interferon (IFN)-γ antibodies were added to decrease the level of IL-17 or IFN-γ, respectively, before the media were collected to stimulate the astrocyte culture. Immunoglobulin (Ig)G isotype control antibody was applied. (b) Relative expressions of IL-17 quantitative reverse transcription–polymerase chain reaction (qRT–PCR) in astrocytes stimulated with or without the Infl. Med. Multiple downstream signalling pathway activation including signal transducer and activator of transcription-3 (STAT-3) (c), p65, a subunit of nuclear factor (NF)-κB (d), or extracellular-regulated kinase (ERK) (e) in astrocytes stimulated with different conditions. #The respective neutralizing antibodies were added during the production of the Infl. Med. to reduce the level of cytokine expression in the media. (f) IL-17 production by astrocytes stimulated with or without the the Infl. Med. with various conditions by the enzyme-linked immunosorbent assay (ELISA) assays. ΔRespective inhibitor drugs were added 2 h before, ##the antibodies 2 h after, the Infl. Med. application on the astrocytes for stimulation. All data are means ± standard error of the mean of at least three experiments. **P < 0·01.
Moreover, to examine the cellular signalling pathways downstream of IL-17 secreted by reactive astrocytes, we added neutralizing antibodies or inhibitor drugs during stimulation on astrocytes by the Infl. Med. These treatment regimens included the neutralizing anti-IFN-γ, anti-IL-17 antibodies or both, as well as the inhibitor drugs including U0126 (ERK), AG490 (JAK/STAT-3) and PDTC (p65 of NF-κB). The results showed that, whereas adding anti-IL-17 antibody during the stimulation significantly reduced the level of IL-17, treatment with AG490, an inhibitor on the JAK/STAT-3 signalling pathways, almost blocked the secretion of IL-17 by astrocytes 12 h post-stimulation by the Infl. Med. (Fig. 4f).
Systemic application of IL-17 antibody at a late stage of the monophasic EAU delayed the disease resolution and resulted in significant apoptosis in the resident neural cells
To testify the protective role of IL-17 in CNS autoimmune diseases in vivo, we established IRBP-induced autoimmune uveitis, an EAU model with Lewis rats. The disease mainly affected the retina and displayed an acute and monophasic course, including the ocular inflammation initiated 7 dpi, maximum inflammation at 14 dpi and resolution of the inflammation at 21 dpi afterwards (Fig. 5a) [24]. During the peak disease at 14 dpi, the retina underwent rigorous inflammation with intensive invasion of infiltrating immune cells, and resulted in a distorted structure of the retina (Fig. 5b–d). We [24] and others [3,31] have shown previously that the Th17-mediated autoimmune response played a dominant role in pathogenesis of the disease model. However, compared to the control at the mRNA level, analysis of the whole retina of the EAU rats indicated a significant up-regulation of multiple cytokines, including IL-17, IL-17F, IFN-γ, IL-6 and TNF-α, in addition to various transcriptional factors that were associated with different Th subsets (Table 3), indicating simultaneous involvement of these effector T cells that may include Th17, Th1, Th2 and regulatory T cells. Among these cytokines, IL-17 remained at the highest expression levels during the disease, peaking at 14 dpi (Table 3). Interestingly, these cytokines showed co-ordinated elevation of respective expression during the course of disease. Interestingly, from 21 to 35 dpi, the disease underwent a significant resolution accompanied by significantly up-regulated expressions of neurotrophic factors, such as nerve growth factor (NGF) and ciliary neurotrophic factor (CNTF) (Table 3, Fig. 5a,b); the sustained up-regulation and co-ordinated expression of multiple cytokines might imply their role in promoting the disease recovery.
Fig. 5.
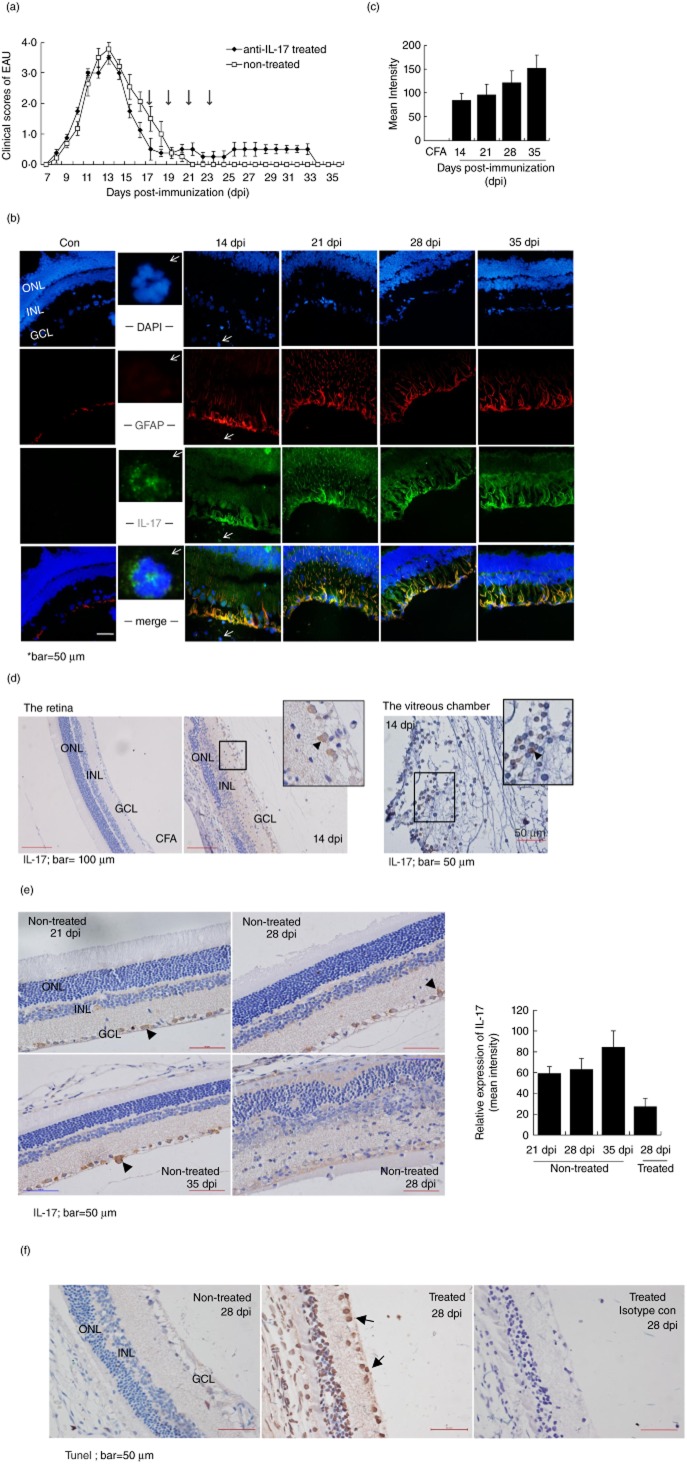
Neuroprotection effect of interleukin (IL)-17 in the late phase of an acute experimental autoimmune uveitis (EAU) model. (a) Clinical scores of the EAU in Lewis rats with or without the treatment of anti-IL-17 antibodies. The animals in the treatment group received intravenous injections of anti-IL-17-neutralizing antibody (20 μg/rat) on the 17, 19, 21 and 23 days post-immunization (dpi); the non-treated rats received phosphate-buffered saline (PBS). The mean score at each dpi is the average for five to seven animals. (b) Immunofluorescent microscopy with anti-glial fibrillary acidic protein (GFAP) (red) and anti-IL-17 antibodies (green) showed a significant number of double-positive staining cells in the EAU versus control retina from 14 to 35 dpi, indicating the expression of IL-17 in the reactive astrocytes during the development of disease. At 14 dpi, a significant single-positive staining of IL-17 on the infiltrating cells was noticed (2nd column, arrows). (c) The double-positive staining astrocytes were quantified by measuring the yellow (merged with green and red) fluorescent intensity in the total retinal area using NIS-Elements BR version 4·00·00 software [means ± standard error of the mean (s.e.m.), n = 10]; *P < 0·05. (d) Immunohistochemistry with an anti-IL-17 antibody shown at 14 dpi, the peak of disease. Intensive IL-17-positive staining was observed in the infiltrating immune cells (in the retina and the vitreous chamber) and resident neural cells (the arrows). (e) Immunohistochemistry staining with an anti-IL-17 antibody at the resolution phase of EAU with or without treatment by neutralizing anti-IL-17 antibodies. The cytoplasm staining was evident at the GCL of the neural cells (the arrows). (f) Significant neural cell apoptosis in EAU treated with an anti-IL-17-neutralizing antibody (20 μg/per animal) by TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labelling) assays. GCL: ganglion cell layer; INL; inner nuclear layer; ONL: outer nuclear layer. Data shown were means ± s.e.m. of at least three experiments.
Table 3.
Relative expression of T cell-associated factors from retina during a monophasic experimental autoimmune uveitis (EAU) with Lewis rats at the mRNA level
| con (Ct value) | con | 7 days | 14 days | 21 days | 28 days | 35 days | +anti-IL-17 antibody | |
|---|---|---|---|---|---|---|---|---|
| T cell-related | ||||||||
| IL-17 | 37·02 | 1·00 | 2·62 | 1573·6 | 15·05 | 49·24 | 80·54 | 5·36 |
| IL-17F | 35·65 | 1·00 | 0·99 | 32·22 | 0·89 | 1·49 | 1·47 | 0·22 |
| IL-6 | 32·75 | 1·00 | 1·62 | 33·01 | 3·30 | 2·86 | 6·92 | 2·52 |
| IFN-γ | 36·92 | 1·00 | 1·47 | 870·08 | 20·07 | 45·46 | 63·41 | 38·14 |
| TNF-α | 30·68 | 1·00 | 1·27 | 58·08 | 3·26 | 4·65 | 12·10 | 0·34 |
| ROR-γt | 26·37 | 1·00 | 1·10 | 6·02 | 0·70 | 0·92 | 3·09 | 0·43 |
| T-bet | 28·82 | 1·00 | 1·04 | 9·03 | 0·88 | 1·35 | 3·66 | 1·05 |
| GATA-3 | 27·13 | 1·00 | 1·17 | 5·64 | 0·14 | 0·77 | 2·46 | 0·36 |
| FoxP3 | 30·11 | 1·00 | 0·86 | 10·59 | 0·84 | 1·59 | 4·63 | 0·39 |
| Neurotrophic factor | ||||||||
| NGF | 30·35 | 1·00 | 0·96 | 1·13 | 1·00 | 3·00 | 2·91 | 0·10 |
| CNTF | 23·64 | 1·00 | 0·92 | 3·96 | 3·49 | 4·15 | 7·07 | 2·53 |
| Thy1 | 19·48 | 1·00 | 0·90 | 0·93 | 0·65 | 0·78 | 1·51 | 0·58 |
| Gap3 | 21·93 | 1·00 | 0·92 | 1·04 | 0·76 | 0·80 | 1·99 | 0·87 |
| Rho | 12·47 | 1·00 | 0·89 | 0·18 | 0·49 | 1·04 | 1·13 | 0·05 |
| Crx | 17·93 | 1·00 | 0·85 | 0·24 | 0·75 | 1·19 | 1·31 | 0·17 |
con*: tissue counterparts were obtained from the control Lewis rats. The relative expression of the genes of interest was measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR), the same method with the EAU rats, and set as 1·00. Genes expression at least a 2-fold change was recognized as being significantly altered and in bold type. IL: interleukin; IFN: interferon; TNF: tumour necrosis factor; ROR-γt: retinoic acid receptor-related orphan receptor gammat; GATA-3: GATA-binding protein 3; FoxP3: forkhead box protein 3; TGF: transforming growth factor; NGF: nerve growth factor; CNTF: ciliary neurotrophic factor.
Based on such a high level of IL-17-containing cytokine environment across the disease in vivo, immunofluorescent microscopy staining showed that the GFAP-positive retinal astrocytes became rigorously reactive starting at 14 dpi, with enlarged astrocytic processes extending to the outer nuclear layer (ONL) of the retina during the peak and resolution phases of the disease (Fig. 5b,c). Consistent with our in vitro results, most of these reactive astrocytes bore IL-17-positive staining more strongly with the development of disease (Fig. 5b). Moreover, CD11b-positive microglias were only evident at the peak of disease at 14 dpi, excluding their involvement in the late phases (Supporting information, Fig. S5). Further immunochemistry examination confirmed the high levels of IL-17 production from 14 dpi by the infiltrating cells and resident neural cells (Fig. 5b,d,e). In particular, abundant IL-17 was observed at the cytoplasm of the resident neural cells located in the retina ganglion cell (RGC, the neurones in retina) layer during the disease, especially at late stages (Fig. 5b,d,e, the retina; and Fig. 5d, the vitreous chamber of the affected eye).
To determine if IL-17 produced at the late phase of disease was protective of the resident neural cells, we applied the anti-IL-17 neutralizing antibody intravenously four times from 17 to 23 dpi (Fig. 5a). We found that, compared to non-treatment controls at 28 dpi, the treated retina showed significantly less IL-17 expression and displayed not only an unresolved inflammation and structure but also significant rates of neural cell apoptosis, leading to markedly reduced neural cell numbers in regions across the ONL, the INL and the RGC layers (Fig. 5e,f). In keeping with these data, while the expression of IL-17 was greatly reduced in the treated retina compared to that in non-treatment controls at the mRNA level, the retinal function markers, such as Thy-1, Gap43, Rho, Crx [24,32,33] and certain neurotrophic factors, such as NGF and CNTF, were also greatly decreased, indicating a significant loss of the neural cells (Table 3). Collectively, in the acute CNS autoimmunity model, we confirmed that the high-level IL-17-containing cytokine milieus in vivo may further stimulate regionally sufficient production of IL-17 by resident neural cells to maintain a protective environment during the course of the disease.
Discussion
In this study, which used an in-vitro primary-neurone and astrocyte culture system attacked by acute proinflammatory stimuli to mimic the in-vivo inflammatory environment in a Th17-dominated autoimmunity, we demonstrated that a high level of IL-17 in a complex milieu of cytokines contributed to the secretion of the same cytokine by reactive astrocytes to attain neuronal protection. These results were confirmed in a monophasic EAU model with Lewis rats, in which relatively high levels of IL-17 were maintained across the disease, and the blockade of IL-17 function with a neutralizing anti-IL-17 antibody at late phases of the disease resulted in significant neural cell apoptosis and tissue damage. The effect of the cytokine was attained through the protection of neurones from apoptosis via the activation of JAK2/STAT-3 signalling and the suppression of voltage-dependent Ca2+ influx. Furthermore, the duration or the level of IL-17 of the proinflammatory milieux, which might reflect the duration or the ratio of Th17 : Th1 in an active autoimmune response, might impact the disease outcome of CNS autoimmunity (either acute or chronic).
Recently, there have been notable debates regarding whether an autoimmune Th17 response is beneficial [34]. Our results demonstrated a virtual cycle orchestrated by IL-17 in an acute EAU model that may involve a course of protective autoimmune responses with the aim of restoring homeostasis. Based on the situation in vivo, it may be necessary for the host to initiate an intensive but controlled autoimmune response. At the peak of disease, a dominant Th17 response results in an environment that may contain multiple cytokines, including a high level of IL-17; however, the activation of Th17 cells may be paralleled with a series of protective mechanisms on local cells [24,35]. The Th17 cells and their associated cytokines were recently proved protective in the gut because application of a neutralizing IL-17 antibody [36], deletion of IL-17 [37] or IL-22 [38] all resulted in the exacerbation of disease in a colitis model [34]. In our results, whereas a high-level IL-17-containing environment was deleterious to neurones, the presence of astrocytes may largely protect neurones under stimulation from apoptosis, partially through secretion of IL-17. Interestingly, IL-6 and TGF-β, which are responsible for the induction of Th17 cells, are pleiotrophic cytokines and are often implicated in neuroprotective processes [39,40]. IL-17 was shown recently to have properties similar to those of neurotrophic factors [16,17]. Together, these findings highlight significance of Th17 and related cytokines in the homeostasis of CNS diseases or autoimmunity.
The precise composition of a high-level IL-17-containing environment and its effect on disease outcome is unclear. It has been shown that Th17 can co-secrete IFN-γ, IL-10, IL-4 or co-express forkhead box protein 3 (FoxP3); thus, multiple subsets of Th17 cells with functional specializations may co-exist [41]. Several studies indicate that the balance and interplay of the Th1/Th17 response, as measured by IFN-γ/IL-17 ratios, may determine the course of the autoimmune disease [42]. An acute or monophasic EAU is more Th17-prone, whereas the chronic disease is dependent upon Th1 cells [43]. Kaufmann et al. [44] recently showed that the maintenance of a high level of IL-17 in a mixed cytokine milieu might be vital to promote disease resolution in acute rather than chronic disease. These researchers compared the in-vivo dynamics between a monophasic and a relapsing–remitting EAU, in which the monophasic disease displayed a single course, whereas the relapsing model presented a relapsing–remitting course [44]. Their data showed that the acute EAU presented rising numbers of cells expressing IFN-γ and IL-17 (Th1/Th17) and cells expressing IL-10 or FoxP3; in contrast, during relapsing disease an increase of the intraocular IFN-γ+ cells and a concomitant decrease of the IL-17+ cells is detected, while the IL-10+ populations remained stable [44].
Notably, other cellular sources can also produce IL-17 and act to amplify the cytokine production by Th17 cells [11–13,45]. Overall, the maintenance of a high level of IL-17 in vivo may contribute to immune balance and disease resolution [24]. In our results, when inflammation was subdued, the resident neural cells may still produce IL-17, resulting in a sustained level of the cytokine secretion during the disease resolution, whereas systemic administration of a neutralizing anti-IL-17 antibody resulted in significant neural cell apoptosis and tissue damage. Furthermore, the parallel production of relatively high levels of other cytokines, such as IFN-γ, TNF-α and IL-6, at the same phase of the disease suggested that IL-17 may act in synergy with these cytokines to achieve their special functions. This effect would be consistent with the notably features of IL-17 action in vivo [6]. We showed that IL-17 alone was insufficient to stimulate astrocytes to produce IL-17 in vitro. Moreover, whereas IL-17 over-expression is linked to active disease [10], a low frequency of IL-17-producing cells are observed with late-stage chronic inflammation [8]. Consistent with these clinical data, we showed that prolonged stimulation with a high-level IL-17-containing cytokine medium stimulates significantly lowers levels of IL-17 by reactive astrocytes for a shorter duration, leading to less protection on the neurones under attack in vitro. Collectively, these data reflect the complex immune-regulation mechanisms that may be critical for the outcomes of an autoimmune disease-mediated neuroinflammation.
Previous reports showed that neurotrophic IL-6 prevents cultured CGNs from glutamate- or NMDA-induced [Ca2+]i overload and neuronal apoptosis, thereby attaining a neuroprotective effect [46]. A recent study demonstrated that IL-17 inhibits voltage-dependent Ca2+ influx in neurones from the superior mesenteric ganglion [16]. These data are consistent with ours, which showed that IL-17 or the supernatant from reactive astrocytes may protect neurones from an [Ca2+]i increase and apoptosis induced by the Infl. Med. Furthermore, a recent significant paper shows that Th17 cells interact directly with neurone cell bodies, forming immune-neuronal synapses during EAE and resulting in neuronal dysfunction via increased [Ca2+]i in neurones and exacerbation of disease [47]. Following similar mechanisms in neurones under the stimulation of proinflammatory stimuli, we showed that the increased [Ca2+]i was reduced significantly by treatment with an NMDA-receptor antagonist; such a result suggests the importance of glutamate in such processes [47]. Moreover, recent studies show that the astrocyte-specific deletion of STAT-3, rather than that of NF-κB, resulted in impaired reactive astrogliosis and increased inflammation and tissue damage in various models of CNS disease or injury [48]. This evidence implies that STAT-3 signalling may be essential to neuroprotective effects in reactive astrocytes. We also demonstrate that JAK2/STAT-3 signalling was required for a neuroprotective effect achieved by IL-17 in reactive astrocytes in vitro. Taken together, these data imply that Th17 and related effector molecules may play a part in a broader spectrum of neurological processes.
In conclusion, our study demonstrates for the first time the neuroprotective effects of IL-17 that is involved in both an active immune response and astrogliosis in neuroinflammation. These results provide novel insights into the nature of autoimmune responses in CNS injuries and diseases and may have direct implications for immune regulation-based treatment modalities in autoimmunity-mediated neuroinflammation.
Acknowledgments
This work was supported by the Foundation of the Heilongjiang Provincial Education Department (12521180), the Foundation of the Heilongjiang Provincial Health Office (2011-208), the Foundation of the ‘Spring Sunshine Program’ (Z2010003) and the Foundation of the HarBin Science and Technology Bureau (RC2011XK003008).
Disclosure
None of the authors has any potential financial conflict of interest related to this manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website:
Fig. S1. Purity of the primary neural cell cultures. (a) Purity of the primary cultured astrocytes and cerebellar granule neurones (CGNs) were confirmed by immunofluorescent microscopy with the anti-glial fibrillary acidic protein (GFAP) antibody or a neurone-specific class III beta-tubulin antibody (Tuj1), respectively. (b) Detection of the microglias with anti-CD11b antibodies in the primary astrocyte culture by immunofluorescent microscopy. Similar results were obtained from three independent experiments.
Fig. S2. Selection of the dilution factor for the inflammatory media (Infl. Med.) that applied to the primary CGN cultures. The half-diluted Infl. Med. induced increased neuronal apoptosis over time and allowed further experiments, whereas a full Infl. Med. induced neuronal cell death quickly and thoroughly within 6 h of incubation. Similar results were obtained from three independent experiments.
Fig. S3. Reduction of the level of interleukin (IL)-17 in the inflammatory media (Infl. Med.) over time at a 37°C incubator. The peripheral blood mononuclear cells (PBMC) were stimulated with 50 ng/ml phorbol myristate acetate (PMA) plus 1 μg/ml ionomycin for 6 h, the supernatant (as the Infl. Med.) were collected and left in the 37°C incubator for 2, 4 and 6 h; the levels of IL-17 of these supernatants were then examined by enzyme-linked immunosorbent assay (ELISA). All data are means ± standard error of the mean of at least three experiments. **P < 0·01; ***P < 0·001.
Fig. S4. The reduced level of interleukin (IL)-17 in the inflammatory media (Infl. Med.) by the neutralizing anti-IL-17 antibody. The peripheral blood mononuclear cells (PBMC) were stimulated with 50 ng/ml phorbol myristate acetate (PMA) plus 1 μg/ml ionomycin for 6 h; the supernatant was collected as the phorbol myristate acetate (PMA) (Infl. Med.). The respective neutralizing antibodies against IL-17 or interferon (IFN)-γ were applied at 4 h of stimulation, resulting in the conditional Infl. Med. with either reduced levels of IL-17 or IFN-γ, compared to the original inflammatory media. The level of IL-17 was measured by enzyme-linked immunosorbent assay (ELISA). All data are means ± standard error of the mean of at least three experiments. *** P < 0·001.
Fig. S5. The involvement of microglias during the development of experimental autoimmune uveitis (EAU) in rats. Immunofluorescent microscopy was used to detect microglias with anti-CD11b antibodies on the retina of EAU rats across the disease. The positive-staining was shown only at the peak of disease at 14 dpi. Similar results were obtained from three independent experiments.
Table S1. Relative expression of T cell-associated molecules from phorbol myristate acetate (PMA)/ionomycin-stimulated astrocytes at the mRNA level by real-time reverse transcription–polymerase chain reaction (qRT-PCR).
References
- 1.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl. 1):S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markiewicz I, Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp (Warsaw) 2006;66:343–358. doi: 10.55782/ane-2006-1623. [DOI] [PubMed] [Google Scholar]
- 3.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Angkasekwinai P, Dong C, Tang H. Structure and function of interleukin-17 family cytokines. Protein Cell. 2011;2:26–40. doi: 10.1007/s13238-011-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang SC, Long AJ, Bennett F, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 6.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 8.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 9.Horai R, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011;31:733–744. doi: 10.1089/jir.2011.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becher B, Segal BM. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. 2011;23:707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends Immunol. 2013;34:151–154. doi: 10.1016/j.it.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteiro M, Almeida CF, Agua-Doce A, Graca L. Induced IL-17-producing invariant NKT cells require activation in presence of TGF-beta and IL-1beta. J Immunol. 2013;190:805–811. doi: 10.4049/jimmunol.1201010. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184:4898–4906. doi: 10.4049/jimmunol.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chisholm SP, Cervi AL, Nagpal S, Lomax AE. Interleukin-17A increases neurite outgrowth from adult postganglionic sympathetic neurons. J Neurosci. 2012;32:1146–1155. doi: 10.1523/JNEUROSCI.5343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hymowitz SG, Filvaroff EH, Yin JP, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke Y, Liu K, Huang GQ, et al. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009;182:3183–3190. doi: 10.4049/jimmunol.0802487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 20.John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 21.D'Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983;96:920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilimoria PM, Bonni A. Cultures of cerebellar granule neurons. CSH Protoc. 2008;2008:pdb prot5107. doi: 10.1101/pdb.prot5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia X, Hu M, Wang C, et al. Coordinated gene expression of Th17- and Treg-associated molecules correlated with resolution of the monophasic experimental autoimmune uveitis. Mol Vis. 2011;17:1493–1507. [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004;102:395–419. doi: 10.1385/1-59259-805-6:395. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Gong Q, Zhong S, et al. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia–reperfusion injury. Nephrol Dial Transplant. 2011;26:469–478. doi: 10.1093/ndt/gfq466. [DOI] [PubMed] [Google Scholar]
- 27.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 28.Duncan RS, Hwang SY, Koulen P. Differential inositol 1,4,5-trisphosphate receptor signaling in a neuronal cell line. Int J Biochem Cell Biol. 2007;39:1852–1862. doi: 10.1016/j.biocel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Flori L, Lecardonnel J, et al. Transcriptome analysis of porcine PBMCs after in vitro stimulation by LPS or PMA/ionomycin using an expression array targeting the pig immune response. BMC Genomics. 2010;11:292. doi: 10.1186/1471-2164-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annunziato L, Amoroso S, Pannaccione A, et al. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett. 2003;139:125–133. doi: 10.1016/s0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R, Qian J, Guo J, Yuan YF, Xue K. Suppression of experimental autoimmune uveoretinitis by anti-IL-17 antibody. Curr Eye Res. 2009;34:297–303. doi: 10.1080/02713680902741696. [DOI] [PubMed] [Google Scholar]
- 32.Carr AJ, Vugler AA, Yu L, et al. The expression of retinal cell markers in human retinal pigment epithelial cells and their augmentation by the synthetic retinoid fenretinide. Mol Vis. 2011;17:1701–1715. [PMC free article] [PubMed] [Google Scholar]
- 33.Barber AC, Hippert C, Duran Y, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013;110:354–359. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLOS ONE. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobolyi A, Vincze C, Pal G, Lovas G. The neuroprotective functions of transforming growth factor beta proteins. Int J Mol Sci. 2012;13:8219–8258. doi: 10.3390/ijms13078219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng YP, Qiu YH, Lu JH, Wang JJ. Interleukin-6 protects cultured cerebellar granule neurons against glutamate-induced neurotoxicity. Neurosci Lett. 2005;374:192–196. doi: 10.1016/j.neulet.2004.10.069. [DOI] [PubMed] [Google Scholar]
- 41.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev. 2012;248:205–215. doi: 10.1111/j.1600-065X.2012.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Toerne C, Sieg C, Kaufmann U, Diedrichs-Mohring M, Nelson PJ, Wildner G. Effector T cells driving monophasic vs. relapsing/remitting experimental autoimmune uveitis show unique pathway signatures. Mol Immunol. 2010;48:272–280. doi: 10.1016/j.molimm.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann U, Diedrichs-Mohring M, Wildner G. Dynamics of intraocular IFN-gamma, IL-17 and IL-10-producing cell populations during relapsing and monophasic rat experimental autoimmune uveitis. PLOS ONE. 2012;7:e49008. doi: 10.1371/journal.pone.0049008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price AE, Reinhardt RL, Liang HE, Locksley RM. Marking and quantifying IL-17A-producing cells in vivo. PLOS ONE. 2012;7:e39750. doi: 10.1371/journal.pone.0039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang XX, Jiang XL, Han XH, Peng YP, Qiu YH. Neuroprotection of interleukin-6 against NMDA-induced neurotoxicity is mediated by JAK/STAT3, MAPK/ERK, and PI3K/AKT signaling pathways. Cell Mol Neurobiol. 2013;33:241–251. doi: 10.1007/s10571-012-9891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siffrin V, Radbruch H, Glumm R, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Allaman I, Belanger M, Magistretti PJ. Astrocyte–neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.