Abstract
A specific antibody to rat collagen proline hydroxylase has been used to measure the amount of “enzyme” protein in cultured mouse fibroblasts (L-929 cells) during normal growth, and under other conditions that cause an increase in enzyme activity. Collagen proline hydroxylase activity per mg of cell protein increased 24-fold as the cells progressed through the logarithmic to the stationary phase of growth, while the cellular concentration of immunologically reactive protein changed only slightly. Similar results were obtained with cells in early log phase in which enzyme activity was stimulated severalfold by cell concentration or lactate treatment without a corresponding change in cellular antigen. It has also been shown that the enzymatically inactive antigen in these fibroblasts competes effectively for antibody-binding sites with partially purified enzyme. It is concluded that early-log-phase fibroblasts contain an inactive form of collagen proline hydroxylase which may be a precursor of the functional enzyme.
Keywords: activation, cell concentration, lactate
Full text
PDF
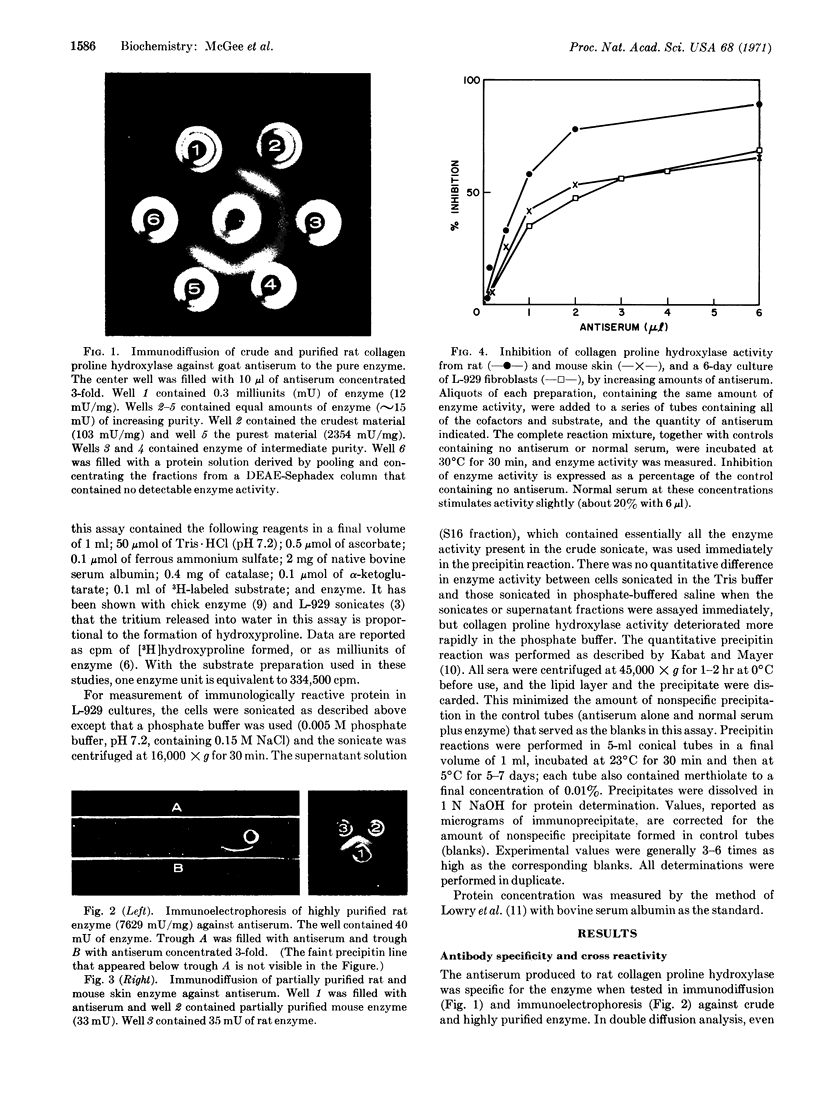
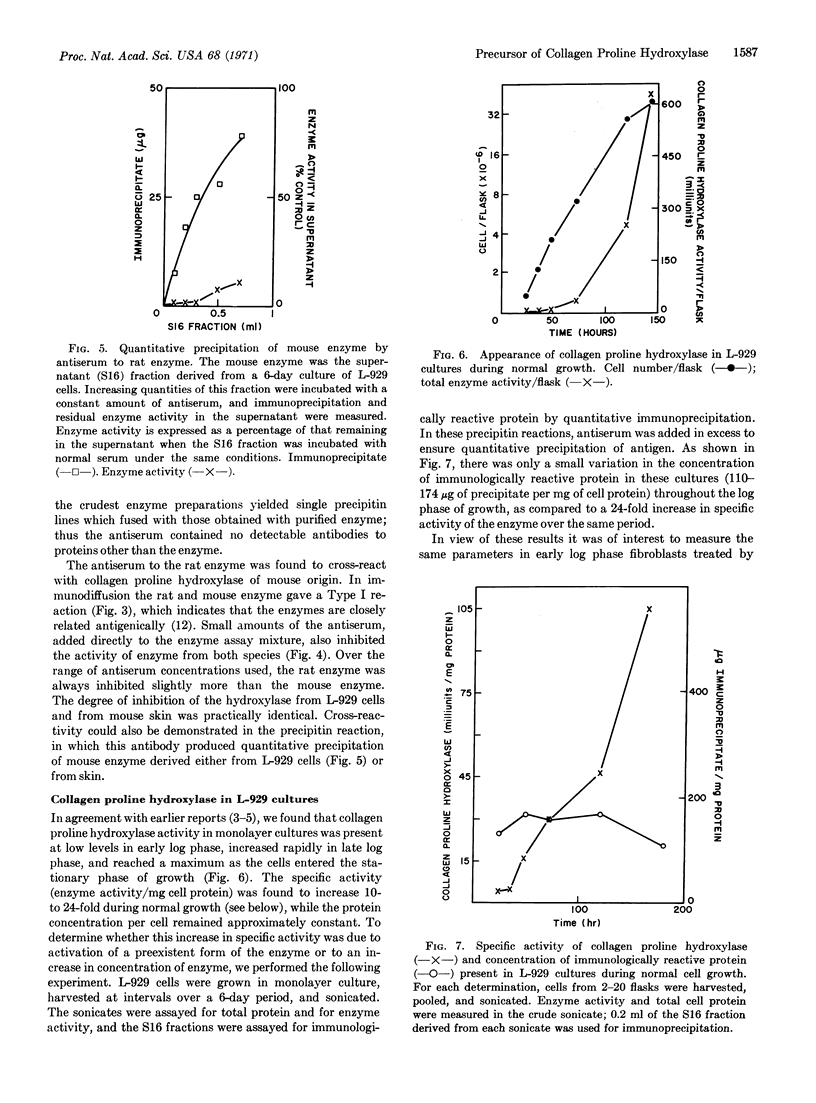
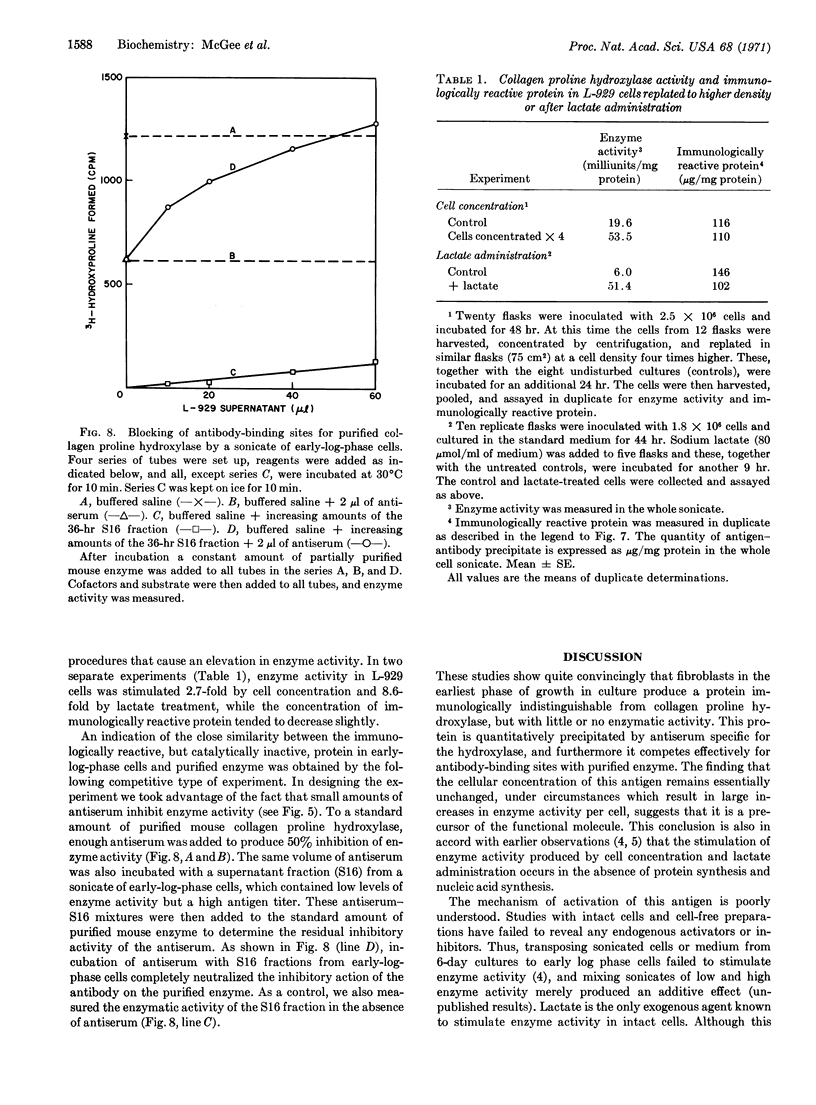

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Comstock J. P., Gribble T. J., Udenfriend S. Further study on the activation of collagen proline hydroxylase in cultures of L-929 fibroblasts. Arch Biochem Biophys. 1970 Mar;137(1):115–121. doi: 10.1016/0003-9861(70)90417-0. [DOI] [PubMed] [Google Scholar]
- Comstock J. P., Udenfriend S. Effect of lactate on collagen proline hydroxylase activity in cultured L-929 fibroblasts. Proc Natl Acad Sci U S A. 1970 Jun;66(2):552–557. doi: 10.1073/pnas.66.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. COLLAGEN AND CELL PROTEIN SYNTHESIS BY AN ESTABLISHED MAMMALIAN FIBROBLAST LINE. Nature. 1964 Oct 24;204:347–349. doi: 10.1038/204347a0. [DOI] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. KINETICS OF COLLAGEN SYNTHESIS BY ESTABLISHED MAMMALIAN CELL LINES. Nature. 1963 Dec 14;200:1097–1098. doi: 10.1038/2001097a0. [DOI] [PubMed] [Google Scholar]
- Gribble T. J., Comstock J. P., Udenfriend S. Collagen chain formation and peptidyl proline hydroxylation in monolayer tissue cultures of L-929 fibroblasts. Arch Biochem Biophys. 1969 Jan;129(1):308–316. doi: 10.1016/0003-9861(69)90180-5. [DOI] [PubMed] [Google Scholar]
- Hartman B. K., Udenfriend S. A method for immediate visualization of proteins in acrylamide gels and its use for preparation of antibodies to enzymes. Anal Biochem. 1969 Sep;30(3):391–394. doi: 10.1016/0003-2697(69)90132-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Purification and properties of collagen proline hydroxylase from newborn rat skin. Arch Biochem Biophys. 1970 Aug;139(2):329–339. doi: 10.1016/0003-9861(70)90485-6. [DOI] [PubMed] [Google Scholar]