Abstract
The carotid body (CB) is the major peripheral arterial chemoreceptor in mammals that mediates the acute hyperventilatory response to hypoxia. The CB grows in response to sustained hypoxia and also participates in acclimatisation to chronic hypoxaemia. Knowledge of CB physiology at the cellular level has increased considerably in recent times thanks to studies performed on lower mammals, and rodents in particular. However, the functional characteristics of human CB cells remain practically unknown. Herein, we use tissue slices or enzymatically dispersed cells to determine the characteristics of human CB cells. The adult human CB parenchyma contains clusters of chemosensitive glomus (type I) and sustentacular (type II) cells as well as nestin-positive progenitor cells. This organ also expresses high levels of the dopaminotrophic glial cell line-derived neurotrophic factor (GDNF). We found that GDNF production and the number of progenitor and glomus cells were preserved in the CBs of human subjects of advanced age. Moreover, glomus cells exhibited voltage-dependent Na+, Ca2+ and K+ currents that were qualitatively similar to those reported in lower mammals. These cells responded to hypoxia with an external Ca2+-dependent increase of cytosolic Ca2+ and quantal catecholamine secretion, as reported for other mammalian species. Interestingly, human glomus cells are also responsive to hypoglycaemia and together these two stimuli can potentiate each other's effects. The chemosensory responses of glomus cells are also preserved at an advanced age. These new data on the cellular and molecular physiology of the CB pave the way for future pathophysiological studies involving this organ in humans.
Key points
The carotid body (CB) is a key chemoreceptor organ that mediates the hyperventilatory response to hypoxia, and contributes to the process of acclimatisation to chronic hypoxaemia.
Knowledge of CB physiology at the cellular and molecular levels has advanced considerably in recent times thanks to studies on lower mammals; however, information on humans is practically absent. Here we describe the properties of human CB cells in slice preparations or after enzymatic dispersion.
Besides glomus (type I) and glia-like, sustentacular (type II) cells, adult human CBs contain nestin-positive neural progenitor cells. The human CB also expresses high levels of glial cell line-derived neurotrophic factor. These properties are maintained at an advanced age.
Human glomus cells contain a relatively high density of voltage-dependent Na+, Ca2+ and K+ channels. Membrane depolarisation with high extracellular K+ induces an increase of cytosolic [Ca2+] and quantal catecholamine release.
Human glomus cells are responsive to hypoxia and hypoglycaemia, both of which induce an increase in cytosolic [Ca2+] and transmitter release. Chemosensory responses of glomus cells are also preserved at an advanced age.
These findings on the cellular and molecular physiology of the CB provide novel perspectives for the systematic study of pathologies involving this organ in humans.
Introduction
The carotid body (CB) is a neural crest-derived bilateral arterial chemoreceptor that is mainly activated by a decrease of blood O2 tension, although it is also sensitive to increased CO2, low pH and other stimuli (see Fitzgerald & Lahiri, 1986). The CB plays a fundamental role in the body's acute hyperventilatory response to hypoxia (Teppema & Dahan, 2010) and alterations of its structure and function are implicated in several human diseases (López-Barneo et al. 2008). Moreover, as the CB is affected by anaesthetic agents, it thereby critically influences respiratory control and arousal after general anaesthesia (Fagerlund et al. 2010). The CB parenchyma is organised into clusters (glomeruli) of neuron-like, glomus (type I) cells, which have numerous secretory vesicles containing dopamine and other neurotransmitters (particularly acetylcholine and ATP) as well as several peptides. These cells are enveloped by the processes of glia-like, sustentacular (type II) cells.
Our understanding of the physiological function of the CB at the molecular and cellular levels has increased considerably during the last 25 years due to studies primarily on lower mammals (mainly rodents) (for reviews see López-Barneo et al. 1999, 2001; Prabhakar, 1999; Nurse, 2005; Peers et al. 2010). It has been shown that glomus cells, the O2-sensing elements in the CB, are excitable and contain a broad range of voltage- and ligand-gated ion channels. These cells form chemosensory synapses with afferent fibres terminating in the brainstem respiratory centre. Closure of O2-sensitive K+ channels in glomus cells during hypoxia is the signal that leads to membrane depolarisation, Ca2+ entry and transmitter release (Ureña et al. 1994; Buckler & Vaughan-Jones, 1994). Glomus cells can also depolarise and release transmitters when the extracellular glucose concentration is reduced (Pardal & López-Barneo, 2002; García-Fernandez et al. 2007; Zhang et al. 2007; Fitzgerald et al. 2009); this has lead to the proposal that the CB is a combined glucose and O2 sensor (Pardal & López-Barneo, 2002). Although the role of the CB in the regulation of plasma glucose has been the subject of some debate (Bin-Jaliah et al. 2004; Ward et al. 2007), recent systemic studies in man have yielded results compatible with CB involvement in the counter-regulatory response to hypoglycaemia (Wehrwein et al. 2010).
An intriguing property of the CB that makes it unique among other organs of the adult peripheral nervous system is that it can grow in response to exposure to sustained hypoxia (Arias-Stella & Valcarcel, 1976; McGregor et al. 1984). This is a fundamental homeostatic response that permits acclimatisation of humans and other mammals to high altitude and aids the survival of patients with cardio-respiratory diseases involving hypoxaemia (Heath et al. 1970; Arias-Stella & Valcarcel, 1976). Although a population of glomus cells can undergo mitosis (Chen et al. 2007; Pardal et al. 2007; Wang et al. 2008), it has been reported that the CB shares properties with neurogenic centres in the adult brain and that the glia-like type II cells, or a subpopulation of them, are indeed stem cells which contribute to the growth of the CB in response to chronic hypoxia (Pardal et al. 2007). CB stem cells are quiescent in normoxia but upon exposure to hypoxia they evolve into nestin-positive intermediate progenitor cells, which in turn proliferate and, eventually, differentiate into new glomus cells and other cell types (Pardal et al. 2007).
The anatomy and ultrastructure of the human CB have been studied in normal subjects and in high altitude dwellers as well as in patients with chronic lung diseases (Grimley & Glenner, 1968; Heath et al. 1970; Arias-Stella & Valcarcel, 1976; Heath, 1983). Similarly, the systemic cardio-respiratory alterations caused by bilateral CB resection (Timmers et al. 2003) or ageing (Pokorski et al. 2004; Di Giulio et al. 2012) have also been investigated in several human cohorts. However, knowledge about the cellular and molecular properties of the human CB is scant. The gene expression profile of the human CB, with a description of the molecules (ion channels, receptors and enzymes) thought to be relevant to the physiology of the CB or its response to drugs, has been described in two recent papers (Fagerlund et al. 2010; Mkrtchian et al. 2012). Nonetheless, the actual functional properties of human CB cells remain as yet unstudied and to our knowledge their responses to putative stimuli have never been investigated. Here we report the basic anatomical and physiological properties of CBs dissected from human cadavers registered with the organ donation programme at our institution. We show that the adult human CB contains chemosensory glomus cells with similar qualitative electrophysiological characteristics and responsiveness to hypoxia and hypoglycaemia compared with those described in rodents. However, these cells exhibit some differences with respect to the equivalent ones of lower mammalian species in terms of ion channel expression and density as well as the quantal size of catecholaminergic vesicles. Although the relative size of the human CB parenchyma is smaller than that of other non-primate mammalian species, CB progenitor cells and chemosensitive glomus cells appear to be preserved in human subjects of advanced age. Our research demonstrates that functional studies at the cellular and molecular levels are feasible in CBs removed from human donors, meaning that the association between CB function and human pathology can now be systematically investigated.
Methods
Ethical approval
This study was approved by the local Ethics Committee on Human Research of the University Hospital ‘Virgen del Rocío’ (HUVR) and the Animal Care and Use Committee of the University of Seville, Spain. The donation of vascular segments was included in the protocol of the organ donation programme at the HUVR. The study was performed in agreement with the standards of the 2008 revision of the Declaration of Helsinki. For comparison, some of the experiments done with human tissue were also performed with CBs from rats. Animals were sacrificed by intraperitoneal administration of a lethal dose of sodium thiopental (200 mg/kg). Experimental protocols were those described in previous papers from our laboratory (Pardal et al. 2000; Ortega-Sáenz et al. 2003).
Human CB dissection
Experiments were performed on CBs removed from human carotid artery bifurcations. We used CBs from 38 donors aged 10–76 years. Carotid bifurcations were dissected from the donor once other organs destined for transplantation, such as the liver, heart and kidneys, had been removed. When possible, the thoracic aorta was clamped to avoid perfusing the CB vascular system with high K+ Wisconsin solution, which is used to perfuse some organs. After removal, carotid artery bifurcations were kept in cold saline until the CBs were dissected in the laboratory. Each CB was cleaned of connective tissue in ice-cold saline solution and its three major diameters were measured to estimate the total CB volume. The CB was then cut into small pieces, which were used for histology, GDNF determination, slice preparation, or cell dispersion. The time of cardiac arrest and other personal and clinical data of the patients were recorded. The time that the tissue remained hypoxic until the dissection of the CB was performed was a factor that significantly affected the results of subsequent experiments. Viable cells and slices were obtained 1.5–3 h after cardiac arrest of the donor. CBs removed after longer periods in cardiac arrest seemed to yield poorer results. In some experiments the superior cervical ganglion (SCG), which is located near the carotid bifurcation, was also removed and processed.
Neurosphere assays
Dispersed CB cells were typically cultured in ultra-low binding 6-well plates (Corning Inc., Corning, NY, USA) at low density so that individual neurospheres were spatially separated from each other. Ultra-low binding 96-well plates (Corning) were used for the clonal experiments. The culture medium contained Dulbecco's modified Eagle's medium (DMEM):F-12 (Gibco BRL, Grand Island, NY, USA) with 20% serum (fetal bovine serum or human umbilical cord serum), 1% N2 supplement (Gibco), 2% B27 supplement (Gibco), 1% penicillin–streptomycin, 1%l-glutamine, 20 ng ml−1 recombinant human basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN, USA), 20 ng ml−1 recombinant human insulin-like growth factor 1 (IGF-1; R&D), and 20 ng ml−1 recombinant human epidermal growth factor (EGF; R&D). All cultures were maintained in 3% O2–5% CO2 atmosphere incubators (Thermo Electron Corp., Waltham, MA, USA) at 37°C.
Immunocytochemistry and morphological studies
After fixation in 4% paraformaldehyde, CB pieces were preserved in 30% sucrose in phosphate-buffered saline (PBS) at 4°C for 12 h. Subsequently the pieces were embedded in Tissue-Tek O.C.T. (Sakura, Zueterwoude, The Netherlands), snap-frozen by quenching in liquid nitrogen, and stored at −80°C. Sections (10 μm thick) were cut with a cryostat and stained to detect tyrosine hydroxylase (TH; rabbit polyclonal anti-TH, Novus Biologicals, 1:5000, Littleton, USA), glial fibrillary acidic protein (GFAP; polyclonal rabbit anti-GFAP, Dako, 1:500, Produktionsvej, Denmark), nestin (mouse anti-nestin human-specific monoclonal antibody, Millipore, 1:500, Darmstadt, Germany) and dopa-decarboxylase (DDC; rabbit anti-DDC affinity-purified polyclonal antibody, 1:500, Invitrogen, NY, USA). Signals were detected using fluorescent secondary antibodies (anti-rabbit IgG Alexa 568, 1:500; anti-rabbit IgG Alexa 488, and anti-mouse IgG Alexa 488, Invitrogen, NY, USA). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000). Images were acquired using a confocal or an Olympus BX-61 microscope (Olympus, Hamburg, Germany) and CB structures were measured with ImageJ software (NIH, USA). For the immunocytochemical identification of cells within the neurospheres, the latter were fixed in 4% paraformaldehyde and subjected to the same steps as tissue samples (cryoprotection, embedding and 10 μm sectioning). Sections were pre-blocked for at least 1 h at room temperature, and incubated with the different primary and secondary antibodies for 1 h at room temperature prior to DAPI counter-staining. The antibodies used for neurosphere thin sections were: rabbit anti-mouse TH (same as for tissues; see above), and mouse anti-rat nestin (1:200, Chemicon International) followed by Cy2-conjugated donkey anti-mouse secondary antibody (1:200, Jackson Immunoresearch, Philadelphia, Pa, USA) or anti-rabbit IgG Alexa 568 (1:500, Invitrogen).
RT-PCR
CB pieces and SCGs were extracted and quickly frozen in liquid nitrogen before tissue homogenisation (OMNI, Waterbury, CT, USA). Messenger RNA was extracted with TRIzol reagent (Ambion, Invitrogen) and treated with DNase (Invitrogen). Reverse transcription was performed using SuperScript II reverse transcriptase (Invitrogen) in a final volume of 20 μl. The primers used were: for GDNF, 5′-TGAAGTTATGGGATGTCGTGGCTG-3′ and 5′-ATC-CACACCTTTTAGCGGAATGC-3′ (band of 548 bp); and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Murthi et al. 2008), 5′-GCACCACCAACTGCTTAGCA-3′ and 5′-GTCTTCTGGGTGGCAGTGATG-3′ (band of 105 bp). The bands of expected size were gel purified and confirmed by sequencing (MGW Biotech, Martinsried, Germany). GAPDH was used as a housekeeping gene to normalise for mRNA.
Detection of GDNF by enzyme-linked immuno-sorbent assay (ELISA)
GDNF protein content was estimated in CB and SCG as previously described (Villadiego et al. 2005). Briefly, tissues were removed and immediately frozen in liquid nitrogen, and then homogenised in lysis buffer (137 mm NaCl, 20 mm Tris (pH 8.0), 1% NP40, 10% glycerol, 1/1000 protease inhibitor cocktail (Sigma, Madrid, Spain)) using a Polytron homogeniser (OMNI, Waterbury, CT, USA). Protein extraction and the ELISA assay were performed according to the manufacturer's instructions (GDNF Emax Immunoassay System, Promega, WI, USA), except that the anti-GDNF monoclonal and anti-hGDNF polyclonal antibodies were used at dilutions of 1:500 and 1:250, respectively. Absorbance at 450 nm was measured using a plate reader (Thermo Electron Corporation, Vantaa, Finland) and the total protein content of the samples was obtained using a Bio-Rad Protein Assay (Bio-Rad, Munich, Germany). We systematically estimated the background level of the ELISA GDNF detection kit by using lysis buffer (137 mm NaCl, 20 mm Tris (pH 8.0), 1% NP40, 10% glycerol, 1/1000 protease inhibitor cocktail (Sigma, Madrid, Spain)) supplemented with 1 μg μl−1 of bovine serum albumin (Sigma, Madrid, Spain). The estimated background level was 0.14 ± 0.03 pg (μg protein)−1 (n= 4). The values obtained from the samples of SCG (0.34 ± 0.06 pg μg−1 in human and 0.26 ± 0.11 pg μg−1 in rat) were slightly higher than the background signal. This can be explained by the cross-reactivity of the ELISA GDNF detection kit with other structurally related trophic factors, especially with members of the TGF-β superfamily (for detailed information see the technical specifications of the GDNF Emax Immunoassay system, TB221, Promega).
Amperometric recording of catecholamine secretion in CB slices
CB pieces were embedded in agarose as described previously by Pardal et al. (2000). Carotid body slices (150 μm thick) were placed in an enzyme solution (1 ml of PBS with 0.6 mg of collagenase II (Sigma, Madrid Spain), 0.3 mg of papain (Sigma), 10 U of porcine elastase (Calbiochem, Darmstadt, Germany) and 10 μl of CaCl2 from a 5 mm stock solution) and incubated at 37°C for 10 min. Afterwards, the slices were washed twice with PBS and placed in 35 mm Petri dishes with culture medium (DMEM supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 1%l-glutamine, 84 μU ml−1 insulin and 5 μm dexamethasone (Sigma)) and maintained at 37°C in a 5% CO2 incubator for 24 h before use. For the experiments, a slice was transferred to the experimental chamber (∼0.2 ml volume) placed on the stage of an upright microscope (Axioscope, Zeiss, Munich, Germany) and continuously perfused by gravity (flow 1–2 ml min−1) with a solution containing (in mm): 117 NaCl, 4.5 KCl, 23 NaHCO3, 1 MgCl2, 2.5 CaCl2, 5 glucose and 5 sucrose. The ‘normoxic’ solution was bubbled with a gas mixture of 5% CO2, 20% O2 and 75% N2 (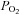 = 150 mmHg). The ‘hypoxic’ solution was bubbled with 5% CO2 and 95% N2 (
= 150 mmHg). The ‘hypoxic’ solution was bubbled with 5% CO2 and 95% N2 (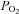 in the chamber was ∼10 mmHg). In the high K+ solutions, KCl was used to replace NaCl equimolarly. The osmolality of the solutions was ∼300 mosmol kg−1 and pH was 7.4. All the experiments were carried out at a chamber temperature of ∼36°C. Secretory events were recorded using a 12 μm polarised (+750 mV) carbon-fibre electrode positioned under visual control. Amperometric currents were recorded with an EPC-8 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfaltz, Germany), filtered at 100 Hz and digitised at 250 Hz before storage on the hard disk of a computer. Data acquisition and analysis were performed with an ITC-16 interface (Instrutech Corporation, NY, USA) and PULSE/PULSEFIT software (HEKA Electronics).
in the chamber was ∼10 mmHg). In the high K+ solutions, KCl was used to replace NaCl equimolarly. The osmolality of the solutions was ∼300 mosmol kg−1 and pH was 7.4. All the experiments were carried out at a chamber temperature of ∼36°C. Secretory events were recorded using a 12 μm polarised (+750 mV) carbon-fibre electrode positioned under visual control. Amperometric currents were recorded with an EPC-8 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfaltz, Germany), filtered at 100 Hz and digitised at 250 Hz before storage on the hard disk of a computer. Data acquisition and analysis were performed with an ITC-16 interface (Instrutech Corporation, NY, USA) and PULSE/PULSEFIT software (HEKA Electronics).
Patch clamp recordings in dispersed CB glomus cells
CB pieces were incubated at 37°C for 20 min in an enzyme solution (1 ml of PBS supplemented with 0.6 mg of collagenase II (Sigma), 0.3 mg of papain (Sigma), 10 U of porcine elastase (Calbiochem) and 10 μl of CaCl2 from a 5 mm stock solution), and cells were mechanically dispersed. Thereafter, cells were plated on glass coverslips treated with poly-l-lysine and kept in culture medium (DMEM supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 1%l-glutamine and 84 μU ml−1 insulin) at 37°C in a 5% CO2 incubator until use.
Macroscopic ionic currents were recorded using the whole-cell configuration of the patch clamp technique as described previously for our laboratory (Levitsky & López-Barneo, 2009; Ortega-Sáenz et al. 2010). Patch electrodes (1.5–2.5 MΩ) were pulled from capillary glass tubes (Kimax, Kimble Products), fire polished on a microforge MF-830 (Narishige, Japan) and coated with silicone elastomer (Sylgard 184; Dow Corning, USA) to decrease capacitance. Voltage-clamp recordings were obtained with an EPC-8 patch clamp amplifier (Heka Elektronik) using standard voltage-clamp protocols designed with PULSE software (Heka Elektronik). Unless otherwise noted, the holding potential used was −80 mV. Data were filtered at 10 kHz, digitised at a sampling interval of 20 μs with an ITC-16 A/D converter (Instrutech), and stored on a computer. Off-line data analysis was performed using custom-made software and PULSEFIT (Heka Elektronik). All experiments were conducted at room temperature (22–25°C). The standard bath solution for recording macroscopic K+ currents in dialysed glomus cells contained (in mm): 140 NaCl, 2.5 KCl, 2.5 CaCl2, 4 MgCl2, 10 glucose, 10 Hepes, pH 7.4, osmolality 300 mosmol kg−1. The pipette solution contained (in mm): 80 potassium glutamate, 60 KCl, 10 Hepes and 0.1 EGTA, pH 7.2 and osmolality 285 mosmol kg−1. Macroscopic Ca2+ and Na+ currents were recorded in dialysed glomus cells. The solutions used for recording whole-cell Na+ and Ca2+ currents were as follows. External (in mm): 140 NaCl, 9 BaCl2, 1 CaCl2, 10 Hepes and 10 glucose; pH 7.4 and osmolality 300 mosmol kg−1. Internal (in mm): 110 CsCl, 30 CsF, 10 EGTA, 10 Hepes and 4 ATP-Mg; pH 7.2 and osmolality 285 mosmol kg−1).
Cytosolic Ca2+ measurements
Glomus cells plated on coverslips were incubated in DMEM containing the acetoxymethyl ester form of Fura-2, Fura 2-AM (2 μm; TefLabs, Austin, TX, USA) and pluronic acid (Invitrogen, 1 μl of a 20% solution in DMSO added to 2 ml of DMEM) for 1 h at 37°C in a 5% CO2 incubator. For the experiments, a coverslip with attached glomus cells was placed in the recording chamber mounted on the stage of an inverted microscope (Axiovert 35, Zeiss) equipped for epifluorescence and photometry. Alternating excitation wavelengths of 340 and 380 nm were used, and background fluorescence was subtracted before obtaining the F340/F380 ratio (Ureña et al. 1994). Cytosolic [Ca2+] signals were digitised at a sampling interval of 500 ms. All the experiments were performed at room temperature.
Statistical analysis
Statistical analysis was performed using the unpaired Student's t test or, in the event of failure of the normality test, by a Mann–Whitney U test. Unless otherwise specified, data are expressed as the mean ± standard error of the mean (SEM) with the number (n) of experiments indicated. A value of P < 0.05 was considered to indicate statistical significance.
Results
Morphological and immunocytochemical features of the human carotid body
Human CBs are located at the bifurcation of the common carotid artery and appear as small round to ovoid organs in close contact with the arterial adventitia (Fig. 1A). The average estimated volume of the adult human CB in our cohort was 20.7 ± 3.8 mm3 (n= 16 carotid bodies; 11 donors); however, large differences in sizes were observed among the different individuals or even between the right and left CBs of the same subject (Fig. 1B). The average CB volume was similar in young (<50 years; 19.3 ± 4.7 mm3, n= 10 carotid bodies; 6 donors) and older (>50 years; 22.9 ± 6.6 mm3, n= 6 carotid bodies; 5 donors) donors. Although there was a trend for CBs from males (26.9 ± 5.5 mm3; n= 7 carotid bodies; 5 donors) to be larger than those of females (15.7 ± 4.7 mm3; n= 9 carotid bodies; 6 donors), this difference was not statistically significant (P= 0.14). Tissue sections were stained with selective antibodies against TH, nestin or GFAP to identify characteristic glomus (type I) and sustentacular (type II) cells, respectively. We found clusters of TH+ cells in the human CB, but as previously reported (Lazarov et al. 2009) the number of these cells was surprisingly low (∼1–2% of the cell population with DAPI-stained nuclei) in comparison with routinely observed values (>20%) in CBs from adult rodents (Ortega-Sáenz et al. 2006; Pardal et al. 2007). However, the actual number of human glomus cells was clearly higher (>10%) as demonstrated by the staining of sections with antibodies against DDC, which also showed the typical appearance of clusters of glomus cells (Fig. 1C–E). TH is a highly regulated enzyme whose expression depends on numerous factors (Kroll & Czyzyk-Krzeska, 1998). Therefore it is possible that in our cohort of subjects, most of whom had been subjected to intensive pharmacological treatments (e.g. intravenous noradrenaline administration at 0.02–0.5 μg kg−1 min−1 for several days prior to death) the enzyme was repressed. Notably, the levels of TH or DDC expression were similar among individuals of different ages (Fig. 1F and G). GFAP immunoreactivity, present on the soma of type II cells and in their long processes covering extensive areas, was high in the CB at all ages. However, the estimated number of GFAP+ cells (in comparison with the total number of DAPI+ nuclei) was only ∼1% (Fig. 1H).
Figure 1. Morphology of the human carotid body.
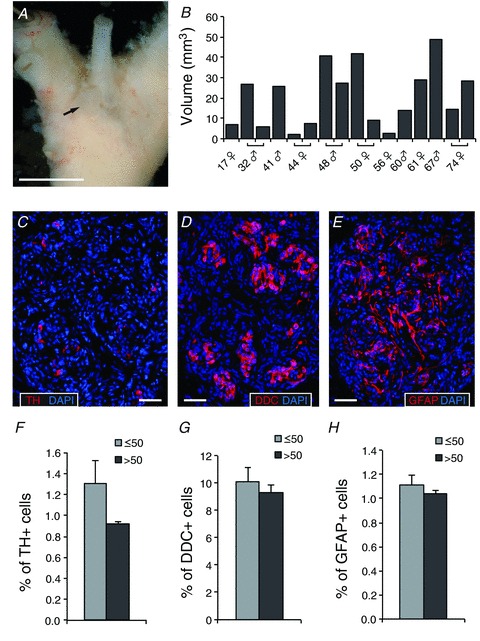
A, photograph of the human carotid artery bifurcation. Connective and adipose tissues have been removed to reveal the carotid body (indicated by a black arrow). Scale bar: 1 cm. B, volumes of human carotid bodies (in mm3) of different subjects indicating age (years), sex and location (volume of the left CB appears in the left bar of cases where both CBs have been removed from patient). Note that in some cases only one of the two CBs was dissected. C, D and E, fluorescence immunohistochemistry with antibodies against the glomus cell markers TH (C) and DDC (D), and the type II cell marker GFAP (E), in thin sections of a human carotid body. Scale bars: 50 μm. F, G and H, quantification of immunopositive cells versus total number of cells estimated in human carotid body sections. Tissues have been grouped by donor age: ≤50 years (n= 19, 15 and 24 for TH, DDC and GFAP, respectively) and >50 years (n= 10, 8 and 11, respectively). No significant changes with age were detected for any of the cell types quantified.
As in lower mammals (Pardal et al. 2007), the adult human CB is a neurogenic niche that, besides containing TH+ and GFAP+ stem cells, is also composed of nestin+ intermediate neural progenitor cells, which appear in close contact with TH+ or GFAP+ cells (Fig. 2A and B). The proximity of nestin+ cells and glomus (DDC+) cells is also illustrated at low magnification in Fig. 2C. The number of nestin+ cells represented 6.9 ± 0.6% of the cell population with DAPI-stained nuclei (n= 4 CBs from 2 donors). Indeed, dispersed CB cells were able to generate characteristic clonal colonies (neurospheres) qualitatively similar to those already reported for rat CB tissue. Human CB neurospheres had a core rich in proliferating nestin+ progenitor cells with blebs of differentiating TH+ cells budding out of the neurosphere (Fig. 2C and D). As reported previously for the rat (Pardal et al. 2007), human neurospheres were obtained in some experiments from cultures in which a single cell per well had been deposited, thus confirming their clonal origin. Nonetheless, we cannot discount the fact that in some cultures undissociated clusters of CB cells, with a neurosphere-like appearance, may have remained viable for several days. The number of human CB cultures that yielded neurospheres was small (7 out of 42 attempts), and the viability of these neurospheres was limited to a few days, thereby indicating that adaptation of this technique to human tissue requires further refinement.
Figure 2. Human carotid body stem cell niche.

A, fluorescence immunohistochemistry with antibodies against two stem cell markers, GFAP and nestin, in a thin section of the human carotid body. Scale bar: 10 μm. B, fluorescence immunohistochemistry with antibodies against TH, a glomus cell marker, and against nestin, a stem cell marker, illustrating the presence of neural progenitor cells around a CB glomerulus. Scale bar: 5 μm. C, fluorescence immunohistochemistry image at low magnification with antibodies against DDC, a glomus cell marker, and against nestin, a stem cell marker. Note the proximity between progenitor cells and glomus cells within the CB glomeruli. Scale bar: 20 μm. D, bright-field images of two typical neurospheres obtained in non-adherent cultures after growth of human carotid body stem cells. Scale bar: 100 μm. E, fluorescence immunohistochemistry with antibodies against TH and nestin in thin sections of human carotid body neurospheres, illustrating the presence of nestin+ neural progenitor cells within the core, and blebs of TH+ glomus cells on the surface of neurospheres. Scale bars: 50 μm.
GDNF production in human CB cells
In comparison with other neural crest-derived organs, the rat CB contains high levels of GDNF (Toledo-Aral et al. 2003; Leitner et al. 2005; Villadiego et al. 2005; Porzionato et al. 2008). Selective GDNF mRNA expression was also observed by PCR in the CB of young and aged adult humans (Fig. 3A). No GDNF mRNA signal was observed in the human SCG. The amount of GDNF protein, estimated by ELISA, was 10-fold higher in the human CB than in the SCG; this difference was in the range of that found between equivalent rat tissues processed in parallel using the same experimental protocol (Fig. 3B). As the SCG does not express GDNF, the amount measured by ELISA in this tissue probably represents the cross-reactivity (described by the manufacturer; see Methods) of the ELISA GDNF detection kit with other structurally related trophic factors (Villadiego et al. 2005). Interestingly, no quantitative difference was observed between GDNF protein content in CBs from young and aged donors (Fig. 3C).
Figure 3. GDNF production in human carotid body cells.
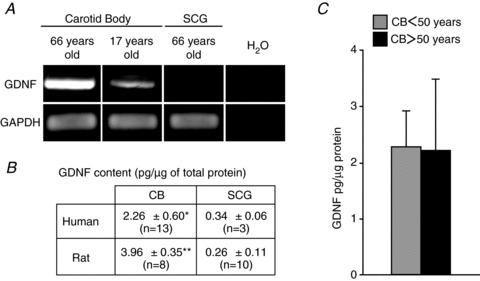
A, GDNF mRNA expression in human CB samples from 66- and 17-year-old subjects. Note the lack of expression of GDNF in the SCG tissue (66-year-old subject). GAPDH was used as housekeeping gene to normalise mRNA. B, GDNF content (in picograms per microgram of total protein) measured by ELISA in human and rat CB and SCG. C, GDNF protein levels in CBs from subjects below (n= 8) and above (n= 5) 50 years of age. *P < 0.05 and **P < 0.01 (Mann–Whitney U test).
Ionic currents in dispersed CB cells
Stable patch clamp recordings were obtained from dispersed human glomus cells, thus permitting characterisation of their main electrophysiological parameters. In the whole-cell patch clamp mode, membrane capacitance was 6.8 ± 0.4 pF (n= 40). In a parallel study performed on rat glomus cells, cell capacitance was 4.9 ± 0.3 pF (n= 52) for the same experimental conditions. Using standard external and internal solutions (high Na+/high K+), the average cell resistance of human glomus cells was 1.2 ± 0.1 GΩ (n= 14); this value clearly increased (6.2 ± 0.3 GΩ; n= 40) in experiments designed to record inward currents (where the external solution contained Ba2+ and the internal solution contained Cs+ instead of K+; see below). Under standard experimental conditions, membrane depolarisation evoked voltage-dependent inward currents of variable amplitude in all cells tested (n > 50) due to the activation of Na+ and Ca2+ channels. These currents were followed by large outward K+ currents with variable activation and inactivation kinetics, suggesting that they were generated by different subclasses of voltage-dependent K+ channels (Fig. 4A–C). Detailed characterisation of the K+ channels in human glomus cells was not attempted in this study; however, it is likely that a significant component of the outward current was mediated by Ca2+-activated K+ channels since paxilline (500 nm), a selective blocker of maxi-K+ channels (Gribkoff et al. 1996), produced a ∼30% reversible reduction of the peak K+ current at +20 mV in two cells tested (data not shown). In cells dialysed intracellularly with Cs+ to block K+ channels, we recorded inward currents that in 81% of cases (n= 42 from 15 subjects) had a clear Na+ component with characteristic fast activation and inactivation kinetics (Fig. 4D and E). The amplitude of the maximal peak Na+ current, normally recorded at +10 mV, varied from cell to cell (Fig. 4F). After complete inactivation of the Na+ current we observed small inward Ca2+ currents typically followed by large tail currents reflecting the deactivation of channels that remained open at the end of the depolarising pulse (Fig. 4D).
Figure 4. Voltage-dependent ionic currents in human glomus cells.
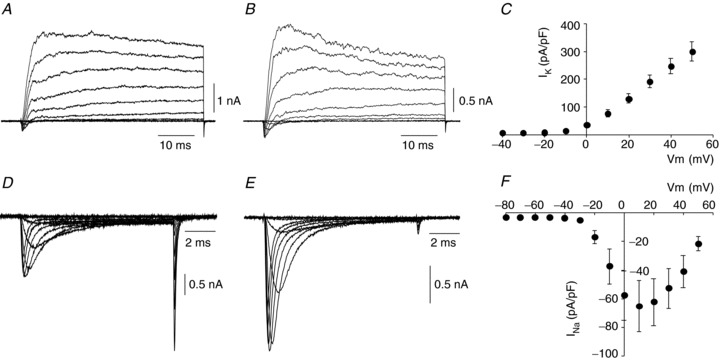
A and B, representative family of macroscopic inward and outward currents recorded in dispersed CB cells from 47- (A) and 63-year-old (B) subjects. Depolarising steps from −40 to +50 mV. Holding potential =−80 mV. C, average peak K+ current density (pA/pF) versus voltage (membrane potential, Vm) relationship obtained from human glomus cells (n= 7). Values are expressed as mean ± SEM. D and E, families of voltage-dependent Na+ and Ca2+ currents recorded in dispersed CB cells from 10- (D) and 61-year-old (E) subjects. Depolarising steps from −50 to +50 mV. Holding potential =−80 mV. Cells were dialysed with Cs+ to block outward K+ currents. Note the different proportion of Na+vs. Ca2+ currents in different cells as evidenced by the sizes of the rapidly inactivating inward currents and the tail currents at the end of the pulses. F, peak sodium current density–voltage relationship obtained in human glomus cells (n= 19). Values are expressed as mean ± SEM.
Approximately 80% of the human glomus cells analysed (n= 40) contained both fast and slowly inactivating Ca2+ channels that generated tail currents with different time courses. These currents were best fitted by a double exponential function (Fig. 5A–C) with average deactivation time constants (at −70 mV) of 1.81 ± 0.1 and 0.127 ± 0.006 ms for slow and fast deactivating channels, respectively (n= 40). The proportion of Ca2+ channels with slow or fast deactivation kinetics varied from cell to cell (Fig. 5D and E); however, few cells contained only fast (n= 7) or only slowly (n= 3) deactivating tail currents. The average peak Ca2+ current–voltage relationship demonstrated the presence of a small ‘sag’ in the negative membrane potential range (∼−20 mV) and a maximum at +20 mV (Fig. 5F). These kinetic data suggest the existence in human glomus cells of low- and high-voltage-activated Ca2+ channels (slowly and fast deactivating, respectively) as reported for other mammalian glomus cells (Ureña et al. 1989; Ortega-Sáenz et al. 2010).
Figure 5. Macroscopic Ca2+ currents recorded in human glomus cells using whole-cell patch clamp.

A, Ca2+ tail current traces generated on repolarisation to −70 mV after short (5 ms; grey) or long (50 ms; black) depolarising pulses to +20 mV. Note the reduction in the amplitude of the slow component of the tail current after the long-lasting depolarisation. C and D, single and double exponential fits (black lines) to the Ca2+ tail current recorded on repolarisation to −70 mV after short depolarising pulses. In the double exponential fit, the fast and slow time constant values are 0.102 and 1.90 ms, respectively. D and E, representative voltage-dependent Ca2+ and Na+ currents recorded during 5 ms (black) or 50 ms (grey) depolarising pulses from a holding potential of −80 mV to +20 mV. Note the large component of fast inactivating tail currents in D and the presence of a large component of slowly inactivating current in E. F, Ca2+ current density–voltage relationship obtained from human glomus cells (n= 31). Note the shoulder at negative membrane potentials (arrow) due to activation of low voltage-activated Ca2+ channels. Values are expressed as mean ± SEM.
Responses of CB cells to hypoxia, hypoglycaemia and membrane depolarisation
The responses of human glomus cells to hypoxia and hypoglycaemia, or to direct depolarisation with high extracellular K+, were studied by amperometry and microfluorimetry in undialysed cells or in tissue slices. In this work, modulation of membrane ionic currents by hypoxia in dispersed glomus cells was not studied due to the inherent difficulties associated with the human CB preparation referred to in previous sections. The responsiveness of patch clamped glomus cells to hypoxia is a labile process that is sensitive to cell dissociation protocols; this responsiveness may disappear when conditions are not absolutely optimal for a particular preparation (López-Barneo et al. 1999). On the other hand, we have previously shown that amperometry and microfluorimetry are robust and highly reproducible techniques that are less invasive than patch clamping and therefore ideal for monitoring the chemosensory responsiveness of individual glomus cells (Ureña et al. 1994; Pardal et al. 2000; García-Fernandez et al. 2007; Ortega-Sáenz et al. 2010). In human CB slices, hypoxia (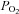 ∼10 mmHg) or high (40 mm) K+ induced a reversible secretory response (Fig. 6A) and a distribution of quantal events (Fig. 6B) similar to those evoked in glomus cells of other species (Pardal et al. 2000). However, the average quantal size in the human CB (72 ± 25 fC; n= 680) appeared to be larger than that measured in rodents (Ureña et al. 1994; Pardal et al. 2000; Pardal & López-Barneo, 2002; Fig. 6B). Of 15 cells tested, 13 responded with a secretory response to both hypoxia and high K+. Quantitative estimates of the secretion rate induced by hypoxia (∼2000 fC min−1) or high K+ (∼7000 fC min−1) yielded results (Fig. 6C and D) that are within the range of those calculated for similar responses in rats or mice with the same methodology (Ortega-Sáenz et al. 2003; Piruat et al. 2004). Although dispersed glomus cells obtained from human donors did not survive well, we were able to record hypoxia-evoked increases in cytosolic [Ca2+] in several experiments. These responses had a time course that greatly varied from cell to cell. In silent cells, hypoxia induced one or several Ca2+ oscillations that resumed after the end of the stimuli (Fig. 7A and B). In cells with spontaneous Ca2+ oscillations, hypoxia normally elicited a sustained elevation of [Ca2+] (Fig. 7C). In the two cells tested, the hypoxia-dependent increase in cytosolic Ca2+ was reversibly abolished by 0.5 mm extracellular Cd2+ (Fig. 7C), which is a blocker of Ca2+ channels in rat and rabbit glomus cells (Ureña et al. 1994). From 24 human glomus cells in which the cytosolic Ca2+ concentration increased in response to 30 mm K+, 17 also responded to hypoxia with a peak Ca2+ signal amplitude that was 14.4 ± 4% of that observed during exposure to the high K+. In parallel experiments performed in rat cells, the peak Ca2+ signal in response to hypoxia was 31 ± 1% of that seen with 30 mm K+ (n= 132).
∼10 mmHg) or high (40 mm) K+ induced a reversible secretory response (Fig. 6A) and a distribution of quantal events (Fig. 6B) similar to those evoked in glomus cells of other species (Pardal et al. 2000). However, the average quantal size in the human CB (72 ± 25 fC; n= 680) appeared to be larger than that measured in rodents (Ureña et al. 1994; Pardal et al. 2000; Pardal & López-Barneo, 2002; Fig. 6B). Of 15 cells tested, 13 responded with a secretory response to both hypoxia and high K+. Quantitative estimates of the secretion rate induced by hypoxia (∼2000 fC min−1) or high K+ (∼7000 fC min−1) yielded results (Fig. 6C and D) that are within the range of those calculated for similar responses in rats or mice with the same methodology (Ortega-Sáenz et al. 2003; Piruat et al. 2004). Although dispersed glomus cells obtained from human donors did not survive well, we were able to record hypoxia-evoked increases in cytosolic [Ca2+] in several experiments. These responses had a time course that greatly varied from cell to cell. In silent cells, hypoxia induced one or several Ca2+ oscillations that resumed after the end of the stimuli (Fig. 7A and B). In cells with spontaneous Ca2+ oscillations, hypoxia normally elicited a sustained elevation of [Ca2+] (Fig. 7C). In the two cells tested, the hypoxia-dependent increase in cytosolic Ca2+ was reversibly abolished by 0.5 mm extracellular Cd2+ (Fig. 7C), which is a blocker of Ca2+ channels in rat and rabbit glomus cells (Ureña et al. 1994). From 24 human glomus cells in which the cytosolic Ca2+ concentration increased in response to 30 mm K+, 17 also responded to hypoxia with a peak Ca2+ signal amplitude that was 14.4 ± 4% of that observed during exposure to the high K+. In parallel experiments performed in rat cells, the peak Ca2+ signal in response to hypoxia was 31 ± 1% of that seen with 30 mm K+ (n= 132).
Figure 6. Secretory response of glomus cells to hypoxia in human carotid body slices.

A, top: amperometric signal showing catecholamine release from a glomus cell exposed to low  (∼10 mmHg) and 40 mm K+. Each spike represents a single exocytotic event. Bottom: cumulative secretion signal (in picocoulombs, pC), resulting from the time integral of the amperometric recording. Slice from the CB of a 27-year-old subject. B, frequency–charge distribution of individual exocytotic events. Mean vesicle quantal size was 72.2 ± 24.9 fC (n= 1058 spikes; 6 recordings; 4 donors aged from 27 to 62 years). C and D, average secretion rate measured under basal conditions and in response to high K+ and hypoxia (basal: 120 ± 36 fC min−1; 40 mm KCl: 6821 ± 2290 fC min−1; hypoxia: 1910 ± 521 fC min−1; n = 13 recordings, 8 donors, aged from 27 to 62 years). Secretion rate is expressed in pC min−1 (mean ± SEM).
(∼10 mmHg) and 40 mm K+. Each spike represents a single exocytotic event. Bottom: cumulative secretion signal (in picocoulombs, pC), resulting from the time integral of the amperometric recording. Slice from the CB of a 27-year-old subject. B, frequency–charge distribution of individual exocytotic events. Mean vesicle quantal size was 72.2 ± 24.9 fC (n= 1058 spikes; 6 recordings; 4 donors aged from 27 to 62 years). C and D, average secretion rate measured under basal conditions and in response to high K+ and hypoxia (basal: 120 ± 36 fC min−1; 40 mm KCl: 6821 ± 2290 fC min−1; hypoxia: 1910 ± 521 fC min−1; n = 13 recordings, 8 donors, aged from 27 to 62 years). Secretion rate is expressed in pC min−1 (mean ± SEM).
Figure 7. Increase of cytosolic calcium in response to hypoxia in Fura-2-loaded glomus cells from different donors.
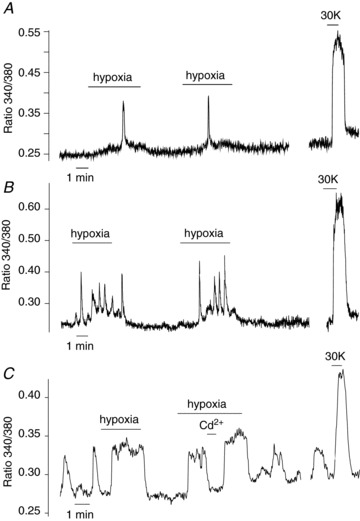
A–C, representative recordings of cytosolic [Ca2+] in dispersed glomus cells from 47- (A), 67- (B) and 27-year-old (C) donors exposed to hypoxia and high K+. Note in C that the hypoxia-induced increase of cytosolic Ca2+ was reversibly abolished by the application of extracellular Cd2+ (0.5 mm).
Under normoxic conditions (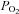 ∼150 mmHg), the removal of extracellular glucose produced membrane depolarisation and an increase in cytosolic [Ca2+] similar to that obtained in dispersed rodent CB cells subjected to the same experimental protocol (Fig. 8A and B; García-Fernandez et al. 2007; Zhang et al. 2007). The average membrane potential of dialysed glomus cells was −44 ± 3 mV (n= 10). In 3 out of 4 cells tested, removal of the glucose from the bathing medium elicited a depolarisation of 4.2 ± 0.4 mV (n= 5 exposures in 3 cells; Fig. 8A). In human CB slices, the removal of glucose from the bathing medium elicited a clear secretory response in the three experiments performed. In 2 out of the 3 cells tested, this effect was potentiated during a second exposure to low glucose in conjunction with mild hypoxia (Fig. 8C and D). The responsiveness of CB cells to hypoxia, low glucose and high K+ is further illustrated in Fig. 8E, in which recordings of cytosolic [Ca2+] changes in a Fura 2-loaded cell are presented. Note that, as for hypoxia, the removal of glucose from the medium elicited Ca2+ oscillations in this cell, and that the duration of these oscillations was reversibly increased by the simultaneous exposure of the cell to low glucose and hypoxia. High extracellular K+, applied at the end of the experiment, also produced a large increase of cytosolic [Ca2+]. From 6 human glomus cells tested, 4 responded to 0 glucose with a peak Ca2+ signal amplitude that was 15.2 ± 3% of that observed for the exposure to high K+. In parallel experiments performed on rat cells, the peak Ca2+ signal in response to hypoglycaemia was 34.5 ± 6% of that seen with 30 mm K+ (n= 13).
∼150 mmHg), the removal of extracellular glucose produced membrane depolarisation and an increase in cytosolic [Ca2+] similar to that obtained in dispersed rodent CB cells subjected to the same experimental protocol (Fig. 8A and B; García-Fernandez et al. 2007; Zhang et al. 2007). The average membrane potential of dialysed glomus cells was −44 ± 3 mV (n= 10). In 3 out of 4 cells tested, removal of the glucose from the bathing medium elicited a depolarisation of 4.2 ± 0.4 mV (n= 5 exposures in 3 cells; Fig. 8A). In human CB slices, the removal of glucose from the bathing medium elicited a clear secretory response in the three experiments performed. In 2 out of the 3 cells tested, this effect was potentiated during a second exposure to low glucose in conjunction with mild hypoxia (Fig. 8C and D). The responsiveness of CB cells to hypoxia, low glucose and high K+ is further illustrated in Fig. 8E, in which recordings of cytosolic [Ca2+] changes in a Fura 2-loaded cell are presented. Note that, as for hypoxia, the removal of glucose from the medium elicited Ca2+ oscillations in this cell, and that the duration of these oscillations was reversibly increased by the simultaneous exposure of the cell to low glucose and hypoxia. High extracellular K+, applied at the end of the experiment, also produced a large increase of cytosolic [Ca2+]. From 6 human glomus cells tested, 4 responded to 0 glucose with a peak Ca2+ signal amplitude that was 15.2 ± 3% of that observed for the exposure to high K+. In parallel experiments performed on rat cells, the peak Ca2+ signal in response to hypoglycaemia was 34.5 ± 6% of that seen with 30 mm K+ (n= 13).
Figure 8. Responses of human glomus cells to low glucose and hypoxia.
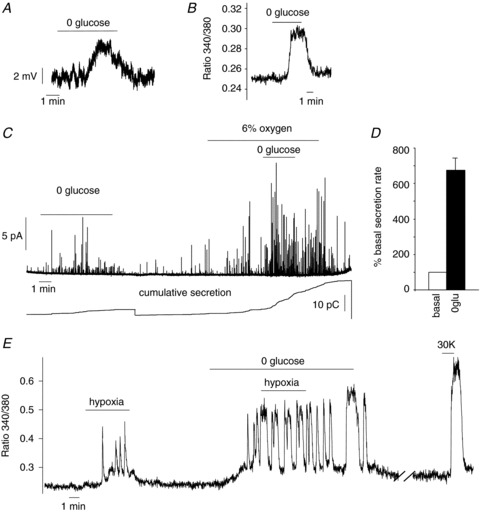
A, depolarising receptor potential recorded in a current-clamped human glomus cell in response to glucopenia. Vm=−48.5 mV. B, reversible increase of cytosolic [Ca2+] in a Fura-2-loaded glomus cell exposed to low glucose. Age of the donors was 72 (A) and 76 (B) years. C, top: secretory response to low glucose of a glomus cell in a CB slice recorded by amperometry (44-year-old subject). Potentiation of the response to low glucose induced by mild hypoxia (6% O2). Bottom: cumulative secretion signal (in picocoulombs, pC) resulting from the time integral of the amperometric recording. D, quantification of the percentage increase of secretion rate induced by hypoglycaemia (n= 3 recordings; 3 donors of 27, 42 and 44 years of age). E, representative recording of the reversible increase of cytosolic [Ca2+] in a Fura-2-loaded glomus cell in response to hypoxia, hypoglycaemia and high potassium. Note the potentiation by hypoxia of the response to low glucose. Cells were from the CB of a 67-year-old subject.
Discussion
The structure of the human CB is similar to that of lower mammals and has been studied in detail in several laboratories (see for example: Grimley & Glenner, 1968; Heath et al. 1970; Arias-Stella & Valcarcel, 1976; Di Giulio et al. 2012). In agreement with previous reports, we have found the human CB to be composed of clusters of type I (glomus) and type II (sustentacular) cells and abundant inter-parenchymal connective tissue. As shown previously (Heath et al. 1970; Arias-Stella & Valcarcel, 1976), the sizes of the CBs among our cohort of individuals, all of whom lived at sea level, were highly variable. We also observed large differences in the sizes between the right and left CBs of the same donor. The average volume of the human CB in our sample (20 mm3) was within the range of values (∼10 mm3, 12.5 mm3 and 45 mm3) estimated, respectively, by Heath et al. (1970), Shamblin et al. (1971) and Arias-Stella & Valcarcel (1976). However, the CB is surrounded by adipose and connective tissue and therefore the degree of ‘cleaning’ during the dissection of the organ can greatly influence its final apparent size. We did not detect any significant change in total volume or number of parenchymal (either type I or type II) cells between the CBs of young versus old subjects. These observations are also in agreement with previous reports comparing the histology of the CB from teenagers and elderly (>80 years) humans (Heath et al. 1970; Arias-Stella & Valcarcel, 1976). Nonetheless, several studies in rats have reported some degree of degeneration of the CB concomitant with old age (see Teppema & Dahan, 2010). In a recent report, a 10–15% decrease in the CB parenchyma was seen between infants (average age 2 years) and older subjects (average age 73 years) (Di Giulio et al. 2012). While this result was statistically significant, the difference in CB size between the groups was relatively small and further confirms the fact that human CB integrity is preserved with advancing age. These findings explain why in most studies the acute hyperventilatory response to hypoxia, which in man is completely dependent on CB activity (see Timmers et al. 2003), is unaltered by ageing (Pokorski et al. 2004; Teppema & Dahan, 2010 and references therein).
In lower mammals CB glia-like type II cells (or a subpopulation of them) are quiescent stem cells that, upon exposure to hypoxia, can differentiate into nestin+ neural progenitor cells which, in turn, give rise to new glomus cells as well as other cell types (Pardal et al. 2007). We also observed nestin+ cells within or surrounding the clusters of type I and type II cells in the human CB. Under floating culture conditions, dispersed human CB stem cells were able to form neurospheres with similar characteristics to those described previously in the rat: a central core enriched in nestin+ progenitor cells with peripheral ‘blebs’ of TH+ cells budding out of the neurosphere. As suggested previously, the CB ‘neurogenic’ stem cell niche may favour maintenance of the organ and contribute to its growth in response to chronic hypoxaemia (Pardal et al. 2007). Indeed, gene ontology groups involved in neurogenesis and axonogenesis are among those over-represented in the human CB in comparison with the adrenal gland (Mkrtchian et al. 2012). Another distinctive histochemical characteristic of rodent CBs, which was also observed in humans, is the high GDNF content, which is expressed predominantly in glomus cells (Villadiego et al. 2005). In agreement with the maintenance of the CB structure in aged subjects, and with our previous report in rodents (Villadiego et al. 2005), we found the GDNF content to be similar in young and older donors. GDNF is a well-known ‘dopaminotrophic’ factor that promotes the survival of dopaminergic neurons (see Toledo-Aral et al. 2003 and references therein). It is therefore tempting to speculate that the autocrine/paracrine action of GDNF contributes to the survival of CB cells during ageing. These observations also support the notion that GDNF-producing human glomus cells are potentially good candidates for neuroprotective cell therapy in Parkinson's disease, as suggested by previous studies in lower mammals (Espejo et al. 1998; Yu et al. 2005) and man (Minguez-Castellanos et al. 2007).
The passive electrical properties of human CB glomus cells were similar to those reported for other species under the same experimental conditions. Cell capacitance (6.8 pF) was within the range of the values estimated in the rabbit (7.3 pF; Ureña et al. 1994), mouse (2.9 pF; Ortega-Sáenz et al. 2010) and rat (4.9 pF; authors’ unpublished observations). These values correspond to unfolded spherical diameters between 9.5 and 14.5 μm, which are close to the values normally measured in dispersed glomus cells. Similar to their counterparts in rodents, human CB glomus cells express a broad range of voltage-dependent ion channels. In this way, whole-cell patch clamped cells exhibited a mixture of macroscopic voltage-dependent Na+, Ca2+ and K+ currents. Macroscopic Na+ currents with typical fast activation and inactivation kinetics were recorded in 81% of the cells studied (n= 42). Interestingly, in a parallel study performed using the same methodology on the adult rat CB, we found a much lower (26%) proportion of glomus cells with voltage-gated Na+ channels (authors’ unpublished observations). Consistent with this observation, only 10% of adult rat CB glomus cells were found to have macroscopic Na+ currents (López-López et al. 1997). The proportion of cells in the rabbit CB with macroscopic Na+ currents, although not precisely quantified, was >50% (Ureña et al. 1989). Na+ channel density measured at a membrane potential of +10 mV was also higher in human cells (∼70 pA pF−1) than in rat (61 pA pF−1) or mouse (26 pA pF−1) cells (Ortega-Sáenz et al. 2010). These differences might depend on subtle neurochemical differences in the CB cells, which are known to have highly inducible voltage-dependent Na+ channels (Stea & Nurse, 1992). All human glomus cells showed macroscopic voltage-dependent Ca2+ currents with a density (13 pA pF−1 at +20 mV) that was higher than that in rat (4.2 pA pF−1) or mouse (7.5 pA pF−1) cells (Ortega-Sáenz et al. 2010). As in lower mammals, Ca2+ currents are mediated by two main types of Ca2+ channels with either fast or slow deactivation kinetics, similar to those measured in the rabbit (Ureña et al. 1989) or mouse (Ortega-Sáenz et al. 2010) CB, and in rat chromaffin cells (Levitsky & López-Barneo, 2009).
Human glomus cells also show macroscopic K+ currents with complex kinetics indicating that, as in rodent cells (Ureña et al. 1989; López-López et al. 1997), human glomus cells have a broad variety of voltage-dependent and other classes of K+ channels. Some of these channels have been molecularly identified in rat cells (Sanchez et al. 2002) as well as in a recent study in humans (Mkrtchian et al. 2012). The macroscopic K+ current density (∼230 pA pF−1 at +30 mV) in human cells was within the range of values reported for rodents (Ortega-Sáenz et al. 2010). Our study also suggests the presence of maxi-K+ channels in human glomus cells. These channels have been shown to be important for chemotransduction in rat CB cells (Wyatt & Peers, 1995). Glomus cells of lower mammals contain several background K+ channels that contribute to a high K+ resting permeability (see Ortega-Sáenz et al. 2010 and references therein). Similar channels appear to exist in human cells, in which replacement of internal K+ with Cs+ in the patch pipette and addition of extracellular Ba2+ produced a 5-fold increase in membrane resistance.
Similar to rabbit, rat and mouse glomus cells, human glomus cells showed reversible quantal catecholamine release in response to hypoxia or high extracellular K+. The average charge of the human quantal events (∼73 fC) is somewhat higher than values estimated for rabbit (44 pC; Ureña et al. 1994) and rat (∼46 pC; Pardal & López-Barneo, 2002) quantal events. The secretion rate in rat or mouse cells exposed to hypoxia estimated in our previously published work was variable and has progressively increased over the years (between 1800 and 9900 fC min−1), which suggests that this outcome might depend on the animal species or strain as well as on methodological improvements and condition of the preparation (Ortega-Sáenz et al. 2003, 2006, 2010; Piruat et al. 2004). The secretion rate in human cells (1900 fC min−1) was within the above-mentioned range of values. Human cells exposed to hypoxia also showed a rise of cytosolic [Ca2+] that was abolished by the blockade of Ca2+ channels with Cd2+. Similar to the differences in secretion rate between human and rodent cells mentioned above, the rise of cytosolic Ca2+ induced by hypoxia in human cells was smaller than that seen in a parallel study performed on rat cells. Nonetheless, we expect that the responsiveness to hypoxia of human cells will improve as the techniques used on these cells are refined. Taken together, these data indicate that, as in other species (Buckler & Vaughan-Jones, 1994; Ureña et al. 1994; Pardal et al. 2000; Buttigieg & Nurse, 2004), human glomus cells behave as presynaptic-like elements that secrete transmitters in response to hypoxia or membrane depolarisation in an extracellular Ca2+-dependent manner.
Previous work with non-primate mammals has suggested that the CB participates in glucose homeostasis (Alvarez-Buylla & de Alvarez-Buylla, 1988; Koyama et al. 2000, 2001). Indeed, we have reported the induction of depolarisation, increase of cytosolic [Ca2+] and secretion of transmitters by glomus cells in response to hypoglycaemia due to the inhibition of K+ conductance and activation of an inward cationic current (Pardal & López-Barneo, 2002; García-Fernandez et al. 2007). The responsiveness of mammalian CB cells to hypoglycaemia was confirmed in the in vitro CB–petrosal ganglion preparation (Zhang et al. 2007) and in dispersed cat glomus cells (Fitzgerald et al. 2009). Nonetheless, other authors have failed to find any responsiveness of explanted whole CB preparations to low glucose (Bin-Jaliah et al. 2004; Conde et al. 2007) or any significant changes in cytosolic [Ca2+] in dispersed glomus cells in response to rapid glucose removal (Gallego-Martin et al. 2012). In contrast to these latter observations, we found that, as in the rat (García-Fernandez et al. 2007), glucopenia induced membrane depolarisation and increased intracellular Ca2+ levels in human CB glomus cells. Moreover, we have shown that glucose removal induces a secretory response. Both the low glucose-induced secretory response and elevation of cytosolic [Ca2+] are potentiated when the two stimuli (hypoxia and hypoglycaemia) are applied simultaneously. Therefore, our data support a role for the CB in glucose homeostasis in man. Further to this, several studies in humans have established a relationship between hypoxia and glucose tolerance (see for example Oltmanns et al. 2004). Although the subject of some debate (Ward et al. 2007), a recent study also provided convincing data supporting a role for the human CB in the counter-regulatory response to hypoglycaemia (Wehrwein et al. 2010).
In conclusion, we have shown here that human CB glomus cells have functional properties that are similar to their lower mammal counterparts, and that these properties are relatively well preserved with ageing. Human glomus cells are sensitive to hypoxia and hypoglycaemia and respond to these stimuli by elevating cytosolic [Ca2+] and catecholamine secretion. The present work should encourage the design of further studies aimed at investigating the association between CB dysfunction and human pathology.
Translational perspective
This work provides the first description to date of the functional responses of human carotid body cells. The neurochemical and physiological properties of these cells appear to be preserved at an advanced age and are qualitatively similar to those reported for cells in lower mammals. The carotid body has come under increasing medical interest in cardiorespiratory medicine due to its participation in the regulation of ventilation and of sympathetic tone. Involvement of the carotid body in the pathogenesis of several diseases can be now directly studied in humans.
Acknowledgments
We wish to express our gratitude to the members of the Coordination of Transplants Unit and the Neurosurgery Department of HUVR for their collaboration in the dissection of the vascular segments used in this study.
Glossary
Abbreviations
- CB
carotid body
- DAPI
4′,6-diamidino-2-phenylindole
- DDC
dopamine decarboxylase
- DMEM
Dulbecco's modified Eagle's medium
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GDNF
glial-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- SCG
superior cervical ganglia
- TH
tyrosine hydroxylase
Additional Information
Competing interests
The authors have no conflicts of interest to declare.
Author contributions
P.O.-S., R.P., K.L., J.V., A.B.M.-M., R.D., V.B.-H., I.A.-M. and V.S. performed the experiments and participated in the interpretation of data and the design of figures. P.O.-S., A.O., M.O., J.J.T.-A. and J.L.-B. designed and carried out the protocol for the resection and processing of carotid bodies from human donors. P.O.-S., R.P., J.J.T.-A. and J.L.-B. designed the first draft of the paper. J.L.-B. wrote the final draft of the paper and supervised the project. Experiments were performed at the Institute of Biomedicine of Seville (IBiS). All authors read and approved this manuscript.
Funding
This research was supported by the Botín Foundation and Axontherapix. Support was also received through grants from the ‘Consejería de Salud de la Junta Andalucía’ and the Spanish Ministries of Economy and Innovation, and Health (SAF, FIS, CIBERNED and TERCEL programmes).
References
- Alvarez-Buylla R, de Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Resp Physiol. 1988;72:347–359. doi: 10.1016/0034-5687(88)90093-x. [DOI] [PubMed] [Google Scholar]
- Arias-Stella J, Valcarcel J. Chief cell hyperplasia in the human carotid body at high altitudes; physiologic and pathologic significance. Hum Pathol. 1976;7:361–373. doi: 10.1016/s0046-8177(76)80052-4. [DOI] [PubMed] [Google Scholar]
- Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol. 2004;556:255–266. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1257–L1262. doi: 10.1152/ajplung.00419.2006. [DOI] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Gonzalez C. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol. 2007;585:721–730. doi: 10.1113/jphysiol.2007.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio C, Zara S, Cataldi A, Porzionato A, Pokorski M, De Caro R. Human carotid body HIF and NGB expression during human development and aging. Adv Exp Med Biol. 2012;758:265–271. doi: 10.1007/978-94-007-4584-1_36. [DOI] [PubMed] [Google Scholar]
- Espejo EF, Montoro RJ, Armengol JA, López-Barneo J. Cellular and functional recovery of Parkinsonian rats after intrastriatal transplantation of carotid body cell aggregates. Neuron. 1998;20:197–206. doi: 10.1016/s0896-6273(00)80449-3. [DOI] [PubMed] [Google Scholar]
- Fagerlund MJ, Kahlin J, Ebberyd A, Schulte G, Mkrtchian S, Eriksson LI. The human carotid body: expression of oxygen sensing and signalling genes of relevance for anaesthesia. Anesthesiology. 2010;113:1270–1279. doi: 10.1097/ALN.0b013e3181fac061. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Lahiri S. Reflex responses to chemoreceptor stimulation. In: Fishman AP, Cherniak NS, Widdicombe JS, Geiger SR, editors. Handbook of Physiology section 3, The Respiratory System, vol. II, Control of Breathing. Bethesda: American Physiological Society; 1986. [Google Scholar]
- Fitzgerald RS, Shirahata M, Chang I, Kostuk E. The impact of hypoxia and low glucose on the release of acetylcholine and ATP from the incubated cat carotid body. Brain Res. 2009;1270:39–44. doi: 10.1016/j.brainres.2009.02.078. [DOI] [PubMed] [Google Scholar]
- Gallego-Martin T, Fernandez-Martinez S, Rigual R, Obeso A, Gonzalez C. Effects of low glucose on carotid body chemoreceptor cell activity studied in cultures of intact organs and in dissociated cells. Am J Physiol Cell Physiol. 2012;302:C1128–C1140. doi: 10.1152/ajpcell.00196.2011. [DOI] [PubMed] [Google Scholar]
- García-Fernández M, Ortega-Sáenz P, Castellano A, López-Barneo J. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 2007;56:2893–2900. doi: 10.2337/db07-0122. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, Jr, Kozlowski ES, Romine JL, Trojnacki JT, McKay MC, Zhong J, Dworetzky SI. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- Grimley PM, Glenner GG. Ultrastructure of the human carotid body. A perspective on the mode of chemoreception. Circulation. 1968;37:648–665. doi: 10.1161/01.cir.37.4.648. [DOI] [PubMed] [Google Scholar]
- Heath D. The human carotid body. Thorax. 1983;38:561–564. doi: 10.1136/thx.38.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D, Edwards C, Harris P. Post-mortem size and structure of the human carotid body. Thorax. 1970;25:129–140. doi: 10.1136/thx.25.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab. 2001;281:E742–E748. doi: 10.1152/ajpendo.2001.281.4.E742. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- Kroll SL, Czyzyk-Krzeska MF. Role of H2O2 and heme-containing O2 sensors in hypoxic regulation of tyrosine hydroxylase gene expression. Am J Physiol Cell Physiol. 1998;274:C167–C174. doi: 10.1152/ajpcell.1998.274.1.C167. [DOI] [PubMed] [Google Scholar]
- Lazarov NE, Reindl S, Fisher F, Gratzl M. Histaminergic and dopaminergic traits in the human carotiod body. Res Physiol Neurobiol. 2009;165:131–136. doi: 10.1016/j.resp.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Leitner ML, Wang LH, Osborne PA, Golden JP, Milbrandt J, Johnson EM., Jr Expression and function of GDNF family ligands and receptors in the carotid body. Exp Neurol. 2005;191(Suppl. 1):S68–S79. doi: 10.1016/j.expneurol.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Levitsky KL, López-Barneo J. Developmental change of T-type Ca2+ channel expression and its role in rat chromaffin cell responsiveness to acute hypoxia. J Physiol. 2009;587:1917–1929. doi: 10.1113/jphysiol.2009.168989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI. Carotid body oxygen sensing. Eur Resp J. 2008;32:1386–1398. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Pardal R, Montoro RJ, Smani T, García-Hirschfeld J, Ureña J. K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Resp Physiol. 1999;115:215–227. doi: 10.1016/s0034-5687(99)00016-x. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Pardal R, Ortega-Sáenz P. Cellular mechanism of oxygen sensing. Ann Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- López-López JR, González C, Pérez-García MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997;499:429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor KH, Gil J, Lahiri S. A morphometric study of the carotid body in chronically hypoxic rats. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1430–1438. doi: 10.1152/jappl.1984.57.5.1430. [DOI] [PubMed] [Google Scholar]
- Minguez-Castellanos A, Escamilla-Sevilla F, Hotton GR, Toledo-Aral JJ, Ortega-Moreno A, Méndez-Ferrer S, Martin-Linares JM, Katati MJ, Mir P, Villadiego J, Meersmans M, Pérez-García M, Brooks DJ, Arjona V, López-Barneo J. Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J Neurol Neurosurg Psychiatry. 2007;78:825–831. doi: 10.1136/jnnp.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtchian S, Kåhlin J, Ebberyd A, Gonzalez C, Sanchez D, Balbir A, Kostuk EW, Shirahata M, Fagerlund MJ, Eriksson LI. The human carotid body transcriptome with focus on oxygen sensing and inflammation – a comparative analysis. J Physiol. 2012;590:3807–3819. doi: 10.1113/jphysiol.2012.231084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthi P, Fitzpatrick E, Borg AJ, Donath S, Brennecke SP, Kalionis B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta. 2008;29:798–801. doi: 10.1016/j.placenta.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Nurse CA. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci. 2005;120:1–9. doi: 10.1016/j.autneu.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL, Peters A. Hypoxia causes glucose intolerance in humans. Am J Resp Crit Care. 2004;169:1231–1237. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, López-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P, Pardal R, García-Fernandez M, López-Barneo J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol. 2003;548:789–800. doi: 10.1113/jphysiol.2003.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P, Pascual A, Gomez-Diaz R, López-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. J Gen Physiol. 2006;128:405–411. doi: 10.1085/jgp.200609591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Pardal R, Ludewig U, García-Hirschfeld J, López-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97:2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Ortega-Sáenz P, Durán R, López-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131:364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Resp Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Piruat JI, Pintado CO, Ortega-Sáenz P, Roche M, López-Barneo J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol Cell Biol. 2004;24:10933–10940. doi: 10.1128/MCB.24.24.10933-10940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorski M, Walski M, Dymecka A, Marczak M. The aging carotid body. J Physiol Pharmacol. 2004;55(Suppl. 3):107–113. [PubMed] [Google Scholar]
- Porzionato A, Macchi V, Parenti A, De Caro R. Trophic factors in the carotid body. Int Rev Cell Mol Biol. 2008;269:1–58. doi: 10.1016/S1937-6448(08)01001-0. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Resp Physiol. 1999;115:161–168. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Sanchez D, López-López JR, Pérez-García MT, Sanz-Alfayate G, Obeso A, Ganfornina MD, Gonzalez C. Molecular identification of Kvα subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid body. J Physiol. 2002;542:369–382. doi: 10.1113/jphysiol.2002.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblin WR, ReMine WH, Sheps SG, Harrison EG., Jr Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732–739. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- Stea A, Nurse CA. Whole-cell currents in two subpopulations of cultured rat petrosal neurons with different tetrodotoxin sensitivities. Neuroscience. 1992;47:727–736. doi: 10.1016/0306-4522(92)90180-a. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol. 2003;553:3–11. doi: 10.1113/jphysiol.2003.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Méndez-Ferrer S, Pardal R, Echevarría M, López-Barneo J. Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body-grafted parkinsonian rats. J Neurosci. 2003;23:141–148. doi: 10.1523/JNEUROSCI.23-01-00141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J, Fernández-Chacón R, Benot AR, Alvarez de Toledo GA, López-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci U S A. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J, López-López J, González C, López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989;93:979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadiego J, Méndez-Ferrer S, Valdés-Sanchez T, Silos-Santiago I, Fariñas I, López-Barneo J, Toledo-Aral JJ. Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation. J Neurosci. 2005;25:4091–4098. doi: 10.1523/JNEUROSCI.4312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Olson EB, Jr, Bjorling DE, Mitchell GS, Bisgard GE. Sustained hypoxia-induced proliferation of carotid body type I cells in rats. J Appl Physiol. 2008;104:803–808. doi: 10.1152/japplphysiol.00393.2007. [DOI] [PubMed] [Google Scholar]
- Ward DS, Voter WA, Karan S. The effects of hypo- and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol. 2007;582:859–869. doi: 10.1113/jphysiol.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies. J Physiol. 2010;588:4593–4601. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995;483:559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Fournier C, Hess DC, Borlongan CV. Transplantation of carotid body cells in the treatment of neurological disorders. Neurosci Biobehav Rev. 2005;28:803–810. doi: 10.1016/j.neubiorev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Zhang M, Buttigieg J, Nurse CA. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J Physiol. 2007;578:735–750. doi: 10.1113/jphysiol.2006.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]


