Abstract
Previous strength training with or without the use of anabolic steroids facilitates subsequent re-acquisition of muscle mass even after long intervening periods of inactivity. Based on in vivo and ex vivo microscopy we here propose a cellular memory mechanism residing in the muscle cells. Female mice were treated with testosterone propionate for 14 days, inducing a 66% increase in the number of myonuclei and a 77% increase in fibre cross-sectional area. Three weeks after removing the drug, fibre size was decreased to the same level as in sham treated animals, but the number of nuclei remained elevated for at least 3 months (>10% of the mouse lifespan). At this time, when the myonuclei-rich muscles were exposed to overload-exercise for 6 days, the fibre cross-sectional area increased by 31% while control muscles did not grow significantly. We suggest that the lasting, elevated number of myonuclei constitutes a cellular memory facilitating subsequent muscle overload hypertrophy. Our findings might have consequences for the exclusion time of doping offenders. Since the ability to generate new myonuclei is impaired in the elderly our data also invites speculation that it might be beneficial to perform strength training when young in order to benefit in senescence.
Key points
Training studio folklore suggests that previous strength training, with or without the use of anabolic steroids facilitates re-acquisition of muscle mass even after long intervening periods of inactivity. This ‘muscle memory’ has previously been attributed to motor learning, but our data suggest the existence of a cellular memory residing in the muscle fibres themselves.
Muscle fibres have multiple nuclei, and the number of nuclei increases when muscle mass increases.
When mice were briefly treated with steroids the muscle mass and number of nuclei increased. The drug was subsequently withdrawn for 3 months and the muscle mass returned to normal, but the excess cell nuclei persisted. When such muscles were subjected to overload they grew by 30% over 6 days while controls grew insignificantly.
Our data suggest that previous strength training might be beneficial later in life, and that a brief exposure to anabolic steroids might have long lasting performance-enhancing effects.
Introduction
Together with neurons, muscle fibres constitute one of the two major post-mitotic tissues of the body. They are by volume the largest cells in mammals (Bruusgaard et al. 2003), and represent one of very few syncytia in vertebrates. The permanent fibres can contain hundreds of nuclei (Bruusgaard et al. 2003), each surrounded by its own synthetic machinery synthesizing protein for a local domain in its immediate vicinity (Hall & Ralston, 1989; Pavlath et al. 1989). During overload hypertrophy the increase in fibre volume is preceded by the incorporation of new myonuclei from interstitial muscle stem cells (satellite cells; Schiaffino et al. 1976; Bruusgaard et al. 2010; Wang & Rudnicki, 2012). Recent evidence suggest that these ‘extra’ nuclei are permanent or at least long lasting even under subsequent conditions where muscle mass is lost (Wada et al. 2002; Aravamudan et al. 2006; Bruusgaard & Gundersen, 2008; Gundersen & Bruusgaard, 2008; Bruusgaard et al. 2010, 2012; van der Meer et al. 2011). Thus, while muscle hypertrophy is completely reversible when it comes to muscle mass, a previous hypertrophic condition seems to convey a lasting imprint on muscle fibres in the form of an elevated number of myonuclei.
We here report that episodic treatment with steroids induced larger fibres with more myonuclei. These extra myonuclei were not lost even months after the muscle size had returned to normal upon drug removal. When such muscles were subjected to overload exercise, however, they grew much faster than controls.
Methods
Animal experiments
Female NMRI mice weighing 25–30 g were used. The animal experiments were approved by the Norwegian Animal Research Authority and were conducted in accordance with the Norwegian Animal Welfare Act of 20th December 1974. The Norwegian Animal Research Authority provided governance to ensure that facilities and experiments were in accordance with the Act, National Regulations of January 15th, 1996, and the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes of March 18th, 1986.
Inhalation gas anaesthesia with 2% isoflurane in air was used for all non-terminal experiments. For terminal experiments, intraperitoneal injections of 5 μl (g body wt)−1 of Equithesin (Ullevål Sykehus, Norway; 42.5 mg ml−1 chloral hydrate and 9.7 mg ml−1 pentobarbitone) were used. All imaging and surgery was performed under deep anaesthesia. The depth of anaesthesia was checked regularly by pinching the metatarsus region of the limb, and additional doses were given if necessary. Animals were killed by neck dislocation while deeply anaesthetized.
In order to overload the extensor digitorum longus muscle (EDL), approximately two-thirds of the distal end of the tibialis anterior muscle was excised. Overload of the soleus muscles was performed by excising similar parts of both gastrocnemius and plantaris.
Pellets containing testosterone propionate or sham pellets (A-211 Innovative Research of America, 2.5 mg, release time 21 days) were implanted subcutaneously by making a 5 mm long incision in the skin of the neck.
Analysis of testosterone levels in blood
In six mice, blood samples were drawn before implantation of the pellet, then 1 and 14 days after implantation, and finally 7 days after removal of the pellet (see inset in Fig. 2A). The analyses were performed at the approved (NS-EN ISO/IEC 17025) Hormone Laboratory at Oslo University Hospital, using a radioimmunoassay (RIA) kit from Orion Diagnostica (Espoo, Finland) after ethyl ether extraction.
Figure 2. The effect of prior administration of anabolic steroids on the number of nuclei myonuclei per fibre found on cross-sections (CS; A) and fibre cross-sectional area (CSA; B).
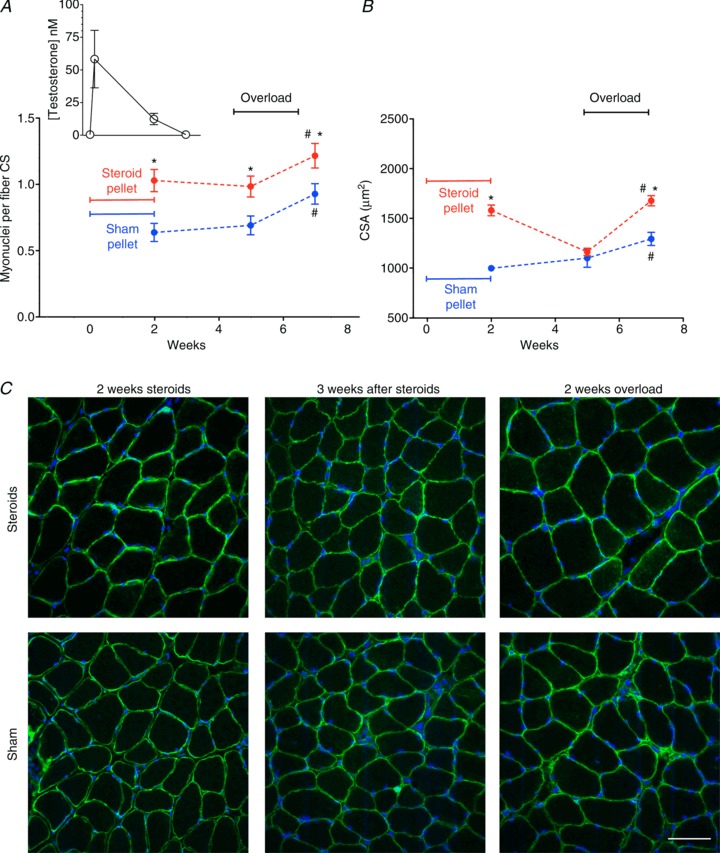
Inset in A shows blood testosterone concentration on same time axis as in main figure. *Statistically significantly different from sham (P < 0.05); #statistically significantly different from overload (P < 0.05). Each data point represents 300 fibres from 6 muscles. C, representative micrographs of cryosections stained with Hoechst dye 33342 (blue) to label DNA, and antibodies against dystrophin (green) after treatment with anabolic steroid or sham pellets for 2 weeks, 3 weeks after pellet removal, and followed by functional overload for 2 weeks. Scale bar is 50 μm.
In vivo myonuclear imaging
For in vivo labelling of myonuclei, single fibres in the EDL were injected with a solution containing a 5′-TRITC-labelled random 17-mer oligonucleotide with a phosphorothioated backbone (Yorkshire Biosciences Ltd, Heslington, United Kingdom) dissolved in an injection buffer (10 mm NaCl, 10 mm Tris, pH 7.5, 0.1 mm EDTA and 100 mm potassium gluconate). The oligonucleotides are taken up into the nuclei inside the injected fibres apparently by active transport, and serve solely as an intravital nuclear dye in our experiments. The methods have been described in detail previously (Utvik et al. 1999; Bruusgaard et al. 2003, 2010). In the present study surface fibres were removed to expose fibres in the interior of the muscles. This allowed us to inject fibres that are more representative of the muscle as a whole compared to just the surface fibres that we have injected previously. Thus, both surface and deeper fibres are included in the material. The oligonucleotides contained the randomly selected sequence TAGTCCTAAGTGGACGC, and a BLAST analysis confirmed that the sequence was not represented in the mouse genome either in the sense or antisense direction.
In vivo imaging was performed essentially as described previously (Balice-Gordon & Lichtman, 1994; Utvik et al. 1999; Bruusgaard et al. 2003, 2010). Fibre segments of 250–1000 μm were analysed by acquiring images in different focal planes 5 μm apart on an Olympus BX-50WI compound microscope with a ×20 0.3 NA long working distance water immersion objective. All images were acquired with an Andor iXion+ camera, controlled by Andor SOLIS software. By importing the images to a Macintosh computer running Adobe Photoshop and NIH ImageJ software, a stack was generated and used to count all the nuclei in the segment. The counting of nuclei was performed by evaluating all of the images in each stack. Illustration images (Fig. 3C) are composites of several focal planes in order to show all the myonuclei in each fibre segment.
Figure 3. In vivo imaging demonstrated that prior administration of anabolic steroids increased the number of myonuclei per mm fibre (A and B) and enhanced a subsequent hypertrophy response to overload in EDL muscles (A and C).
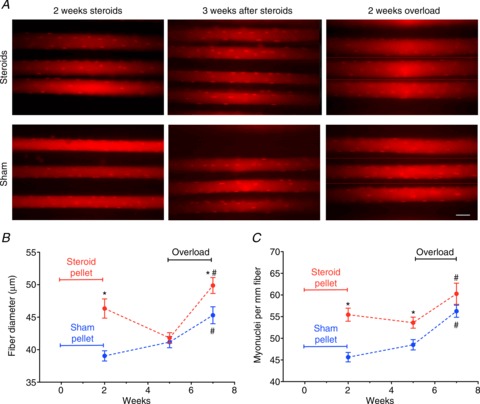
*Statistically significantly different from sham (P < 0.001); #statistically significantly different from overload (P < 0.001). Each data point represents 33–50 fibres from 6–8 muscles. C, representative in vivo images of muscles each showing three oligo-injected muscle fibres labelling myonuclei and the outline of single fibres immediately after treatment with anabolic steroid or sham pellets for 2 weeks, 3 weeks after pellet removal, and followed by functional overload for 2 weeks. In A hairlines indicate boundaries when different focal planes are depicted. Scale bar is 50 μm.
Immunohistochemistry
Muscles were excised and embedded in OCT Tissue-Tek before being frozen slightly stretched in melting isopentane and stored at −80°C followed by cryosectioning at 10 μm. The sections were subsequently blocked in 1% bovine serum albumin, and stained with anti-dystrophin monoclonal antibodies against Dystrophin (Abcam, clone MANDYS8 ab7163). Secondary antibody was goat anti-mouse TRITC conjugated IgG (Sigma T7782). Both antibodies were used at a dilution of 1:200. Nuclei were finally co-stained using Hoechst dye 33342 (Invitrogen; 0.1 μg ml−1 in PBS). As discussed previously (Gundersen & Bruusgaard, 2008; Bruusgaard et al. 2012), to ensure that only the nuclei inside muscle fibres were included in the analysis, myonuclei were defined as nuclei with their geometrical centre inside the inner rim of the dystrophin ring.
Histological analysis of myosin heavy chain fibre type was performed as described previously (Lunde et al. 2011) using the following panel of monoclonal antibodies: BA-D5 (I), SC-71 (IIa), 6H1 (IIX) and BF-F3 (IIb). All of these were grown in-house from hybridoma stocks obtained from ATCC/LGC Standards. In order to estimate fibre type composition, a grid was placed over the whole muscle section, and fibre type was determined for each fibre that was located at grid intersections.
Statistics
Differences were tested by analysis of variation (ANOVA) and Bonferroni's correction for multiple comparisons. Values are given as means ± SEM.
Results
We have investigated the effects of anabolic steroids and overload exercise on muscle cells in vivo. Adult female mice had pellets releasing testosterone propionate implanted subcutaneously for 14 days. Some of the animals in both groups also had synergist muscles ablated in order to overload either the oxidative/slow soleus or the glycolytic/fast extensor digitorum longus (EDL) muscle during the drug treatment.
In the soleus, steroid treatment alone increased the number of myonuclei by 66% compared to treatment with sham pellets whereas overload alone increased the number of myonuclei by 51% (Fig. 1A). When steroids and overload were combined, the increase was 92% (Fig. 1A). Qualitatively similar, but less dramatic effects were observed in the EDL (Fig. 1B). Steroid use has also been shown to increase the number of myonuclei in humans (Eriksson et al. 2005).
Figure 1. Effect of anabolic steroids and functional overload on cross-sectional area (CSA) and the number of myonuclei per fibre found per fibre on cross-sections (CS).
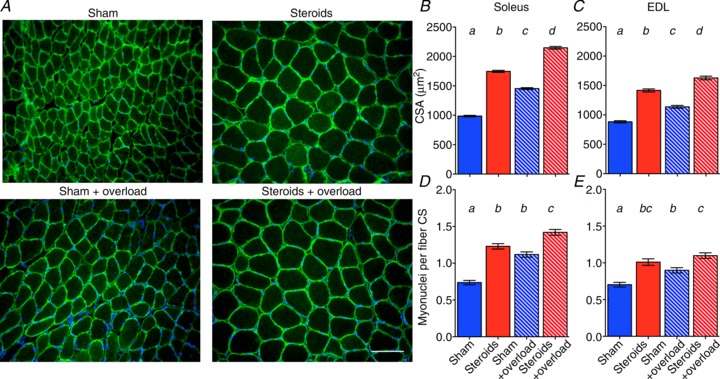
A, representative micrographs of cryosections stained with Hoechst dye 33342 (blue) to label DNA, and antibodies against dystrophin (green) from soleus muscles after 2 weeks of sham or steroid treatment and with or without simultaneous functional overload. Scale bar is 100 μm. CSA, and number of myonuclei per fibre cross-section (CS) from soleus (B and D), and EDL (C and E). Bars show means ± SEM. Letters a–d above columns indicate statistical significant differences (P < 0.01); columns designated with the same letter were not statistically different. Each column represents 317–896 fibres from 6 muscles.
While steroids had no effects on muscle fibre type (Supplemental Fig. S1), changes in fibre size roughly mirrored those of the myonuclei (Fig. 1C and E). In the soleus, steroid treatment alone increased fibre cross-sectional area (CSA) by 77% compared to treatment with sham pellets, while overload alone only induced a 48% hypertrophy. When steroids and overload were combined, a hypertrophy of 118% was observed, and thus the effect of the two treatments was roughly additive. As for the myonuclei, qualitatively similar, but less dramatic effects were observed in the EDL (Fig. 1D). These findings in mice were similar to the effects of resistance exercise and testosterone treatment in humans (Bhasin et al. 1996).
We next asked if steroids could have long lasting effects on the number of myonuclei, and if the extra nuclei could aid in building muscle mass after the drug was withdrawn and the size had reverted to normal. The variation in the blood testosterone concentration during the pellet implantation and after its removal is shown in Fig. 2A (inset), and we determined that the testosterone levels were below the detection limit (<0.05 nm) less than a week after pellet removal. When soleus muscles were investigated 3 weeks after pellet removal, the number of myonuclei was not significantly reduced and remained on average 42% higher than in the sham group (Fig. 2A). The CSAs were, however, completely reversed and the fibre size of steroid and sham treated muscles was indistinguishable (Fig. 2B and C). When overload was introduced at this time and for a subsequent period of 14 days, the steroid group displayed a hypertrophy of 44%, while in the sham treated mice the cells grew by only 17% (Fig. 2B and C).
Even though the EDL responded less strongly to steroids than the soleus (Fig. 1), it was important to investigate EDL further. The soleus is an oxidative postural muscle, and is probably less relevant for strength performances. Moreover, EDL is more suitable for in vivo imaging, allowing myonuclei to be observed directly in single fibres in situ in the living animal. This was desirable since conventional ex vivo histological techniques can introduce artefacts confounding the interpretations in this type of experiments (Gundersen & Bruusgaard, 2008; Bruusgaard et al. 2012). The in vivo single fibre observations in EDL supported the ex vivo analysis of the soleus. Thus, 2 weeks of steroid treatment lead to an increase in the number of myonuclei from 46 to 56 nuclei per millimetre fibre length at sarcomere lengths of 3 μm, i.e. up 20% (Fig. 3A and C). Fibre diameters were increased by 18%, and assuming cylindrical fibres this corresponds to an increase in CSA of 39% (Fig. 3B and C). Three weeks after pellet removal fibre diameters in the steroid and sham groups were indistinguishable (Fig. 3B and C) while the number of nuclei had not changed significantly in any of the groups (Fig. 3A and C). When overload was introduced at this time, the increases in fibre diameters indicated that during 14 days CSA grew by 42% in the steroid group and by 21% in the sham group (Fig. 3B and C).
In order to demonstrate a longer term memory, we also investigated the effects of the episodic steroid treatment 3 months after pellet removal. NMRI mice in captivity live for about 2 years (Lhrke et al. 1984), so 3 months constitutes ≈12% of their lifespan, and would correspond to approximately a decade in humans. At this time, while there was no difference in CSA or fibre type (Supplemental Fig. S2), the number of nuclei was 28% higher in the steroid group than in the sham group (Fig. 4A). When overload was introduced, CSA increased by 31% in the steroid group during the first 6 days, while in the sham group it increased by only 6% (non significant; Fig. 4B and C). The two groups afterwards grew in parallel, but the CSA was still 20% higher in the steroid group after 14 days of overload (Fig. 4B).
Figure 4. Long-term effects of a short period of anabolic steroid administration on myonuclei (A) and fibre growth in response to overload in EDL muscles (B and C).
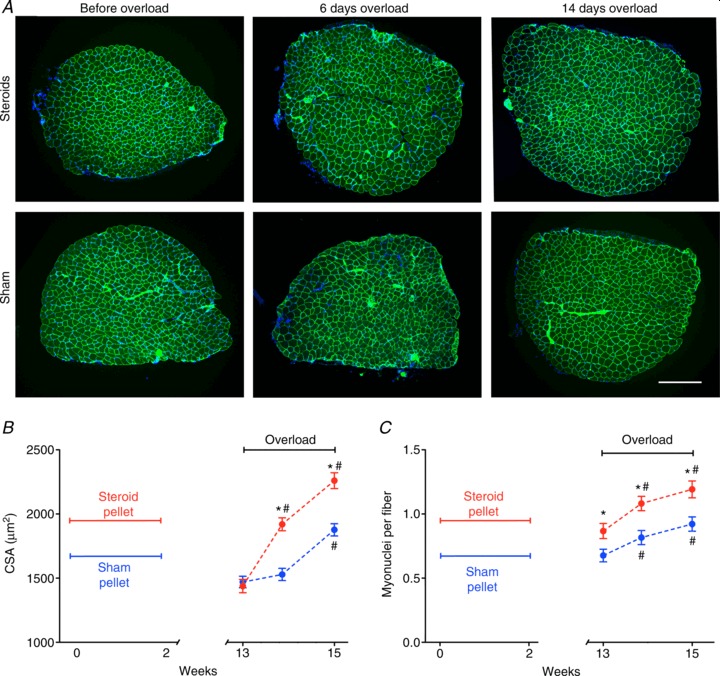
*Statistically significantly different from sham (P < 0.001); #statistically significantly different from overload (P < 0.001). Each data point represents 669–856 fibres from 6 muscles. C, representative micrographs of cryosections stained with Hoechst dye 33342 (blue) to label DNA, and antibodies against dystrophin (green) immediately after treatment with anabolic steroids or sham pellets for 2 weeks and muscles that 3 months later were functionally overloaded for 6 and 14 days. Scale bar is 300 μm.
Discussion
We here provide evidence for a new epigenetic mechanism in the form of an elevated number of nuclei within the muscle syncytium aiding restoration of muscle mass in muscles that have previously been large. Such mechanisms might have been evolutionary important since it would allow, for example, individuals performing seasonal strength-demanding tasks to regain strength quickly without having to maintain a large permanent muscle mass. In modern society it has been commonly observed, that previous strength exercise seems to make it easier to regain muscle mass later in life even after long intervening periods of inactivity and mass loss, and this phenomenon has been dubbed ‘muscle memory’ (Staron et al. 1991; Taaffe & Marcus, 1997). There has previously been no known mechanism for a cellular memory in muscle, and long lasting effects of previous training was solely attributed to motor learning in the CNS (Rutherford & Jones, 1986).
Mechanisms for muscle hypertrophy and memory
The ability of muscle cells to maintain large cytoplasmic volumes spanning vast intracellular distances without a well developed intracellular transport system seems to be related to having a high number of nuclei optimally distributed throughout the cell (Bruusgaard et al. 2003; Metzger et al. 2012; Qaisar et al. 2012). A dislocation of nuclei is commonly observed in many myopathies, and, for example, in Emery–Dreifuss syndrome the muscular dystrophy is frequently linked to mutations in nuclear envelope genes (Puckelwartz & McNally, 2011). During overload, recruitment of new nuclei from satellite cells precedes the increase in cytoplasmic volume (Bruusgaard et al. 2010), but previous literature concluded that myonuclei were lost during subsequent atrophy, suggesting a complete reversibility in cytoarchitecture. These findings were, however, based mostly on conventional histology that might not have provided a clear separation between myonuclei and the other nuclei in the tissue (e.g. satellite cells). Recently (and in this paper), in vivo and ex vivo imaging of single fibres has, however, revealed that no myonuclei are lost and that the ‘extra’ nuclei remain in the cells during atrophy (Wada et al. 2002; Aravamudan et al. 2006; Bruusgaard & Gundersen, 2008; Gundersen & Bruusgaard, 2008; Bruusgaard et al. 2010, 2012; Jackson et al. 2012a). This represents a cytoarchitectural hysteresis that allowed us to hypothesize that the elevated number of nuclei could be a mechanism for a muscle memory related to muscle mass. The present data indicate the existence of such a cellular memory that is beneficial and residing in the muscle cells themselves.
In normal animals the addition of new nuclei seems to accompany hypertrophic growth under a variety of conditions (Enesco & Puddy, 1964; Moss, 1968; Cheek et al. 1971; Schiaffino et al. 1976; Seiden, 1976; Cabric & James, 1983; Cabric et al. 1987; Giddings & Gonyea, 1992; Winchester & Gonyea, 1992; Allen et al. 1995; McCall et al. 1998; Kadi et al. 1999; Roy et al. 1999; Bruusgaard et al. 2010; Wang & Rudnicki, 2012). It has been speculated that addition of nuclei is related mainly to conditions likely to induce muscle damage, such as after elimination of synergistic muscles, or after heavy exercise with eccentric contractions (Schiaffino et al. 2013), implying that it is connected more to muscle repair than hypertrophic growth of pre-existing fibres. Addition of myonuclei, however, seems also to accompany hypertrophy related to treatment with hormones such as testosterone (the present data and Kadi, 2008) or IGF-1 (Barton-Davis et al. 1999), which is less likely to produce damage.
In overload hypertrophy studies, it has been shown both by 3H-thymidine labelling (Aloisi et al. 1973) and in vivo imaging (Bruusgaard et al. 2010), that new myonuclei are incorporated before any apparent change in CSA, suggesting a causal role for myonuclei in contributing to hypertrophy. We are, however, not suggesting that the number of myonuclei is the only determinant of muscle fibre size; for example, we show here that in spite of a larger number of myonuclei induced by steroid treatment the muscle fibres shrink, and are no larger than control fibres with fewer nuclei when the steroids are withdrawn. Moreover, there is no addition of nuclei during regrowth back to normal fibre size after hindlimb suspension (Bruusgaard et al. 2012; Jackson et al. 2012b), leading us to suggest that the number of nuclei might represent the largest size the fibre has had in its history rather than its current size (Bruusgaard et al. 2012). Moreover, with similar in vivo imaging methods to those used in the present paper we have previously found that the number of nuclei in fibres of the same size is higher in slow than in fast fibres (Bruusgaard et al. 2003). This might be related to the higher turnover and higher demands on protein synthesis in slow muscles (Goldberg, 1967; Kelly et al. 1984). Generally there seems to be a stronger correlation between number of nuclei and fibre size under conditions of relatively high protein turnover (Bruusgaard et al. 2006). We suggest that fibre size is determined by: (i) proteolysis, (ii) protein synthesis per nucleus and (iii) number of nuclei.
Several gene-manipulated mice have displayed hypertrophy without a corresponding increase in the number of nuclei, such as mice overexpressing the proteins Akt (Blaauw et al. 2009), JunB (Raffaello et al. 2010), or Ski (Bruusgaard et al. 2005), or mice harbouring loss-of-function mutations in the myostatin gene (Amthor et al. 2009). The Ski mice have a reduced content of contractile material (Bruusgaard et al. 2005) and the myostatin mice display reduced specific force (Amthor et al. 2007; Mendias et al. 2011), abnormalities that could be related to the low number of nuclei. In contrast, the Akt mice seemed to have normal specific force after 3 weeks (Blaauw et al. 2009). Similarly, specific force appeared normal after 2 weeks of overload hypertrophy in mice where 90% of satellite cells were ablated using an inducible Pax7–diphtheria toxin A transgene (McCarthy et al. 2011).
For the latter models more research is required to determine if hypertrophy without the normal addition of myonuclei is sustainable over time, is more costly, or leads to suboptimal functional properties (other than short term specific force). Alternatively, hypertrophy without the addition of nuclei might represent mechanistic redundancy, which is quite common in biological systems. Although we cannot extrapolate to other experimental models or exclude the possibility that testosterone has other long lasting effects on muscle than to increase the number of nuclei, we suggest that muscles with more nuclei seem better adapted to a rapid regrowth, and thus are bearers of a muscle memory.
Muscle memory in sport and public health
Hypertrophy induced by overload is greatly attenuated in older animals (Carson et al. 1995; Alway et al. 2002), and in humans more than 50% of the individuals currently fill the clinical criteria for frailty at ages >80 years (Matthews et al. 2011), creating major health problems in the ageing western population (Dutta & Hadley, 1995; Hughes & Schiaffino, 1999). Hard resistance training in the elderly has, however, proven to have benefits even after years of detraining, in particular if the individuals maintain a regime of moderate-intensity training (Smith et al. 2003). The ability to generate new myonuclei is, however, impaired with ageing (Schultz & Lipton, 1982), possibly due to reduced notch signalling (Conboy et al. 2003; Conboy & Rando, 2005). Our data suggests the existence of a long-term muscle memory related to myonuclei. Investigations are needed to establish whether a similar nuclei-related muscle memory related to strength exercise in humans exists; and if it does, public health advice for strength training in younger individuals should be considered, since it might aid in maintaining muscle mass more easily in senescence.
Our data demonstrate that in least in mice, an episode of testosterone use may recruit a long lasting pool of excess myonuclei, and a persistent increased ability to regain muscle mass by resistance exercise in the absence of further steroid exposure. Thus, the benefits of even episodic drug abuse might be long lasting, if not permanent, in athletes. Our data suggest that the World Anti-Doping Code calling for only 2 years of ineligibility after a conviction for steroid use (WADA, 2009) should be reconsidered.
Acknowledgments
We are grateful to Siobhan L. Anton for comments on the manuscript.
Glossary
Abbreviations
- CSA
cross-sectional area
- EDL
extensor digitorum longus
Additional Information
Competing interests
None.
Author contributions
Conception and design of experiments: I.M.E., J.C.B. and K.G. Collection and analysis of data I.M.E., J.C.B. and E.E. Drafting the article K.G. and revising it critically for important intellectual content: all authors.
Funding
This work was supported by grants from the Norwegian Research Council, Anti Doping Norway.
Supplementary material
Supplemental Fig. S1
Supplemental Fig. S2
References
- Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol. 1995;78:1969–1976. doi: 10.1152/jappl.1995.78.5.1969. [DOI] [PubMed] [Google Scholar]
- Aloisi M, Mussini I, Schiaffino S. Basic Research in Myology. Amsterdam: Excerpta Medica; 1973. Activation of muscle nuclei in denervation and hypertrophy; pp. 338–345. [Google Scholar]
- Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol. 2002;283:C66–C76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci U S A. 2009;106:7479–7484. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC. Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol. 2006;100:1617–1622. doi: 10.1152/japplphysiol.01277.2005. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Eng J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009;23:3896–3905. doi: 10.1096/fj.09-131870. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Brack AS, Hughes SM, Gundersen K. Muscle hypertrophy induced by the Ski protein: cyto-architecture and ultrastructure. Acta Physiol Scand. 2005;185:141–149. doi: 10.1111/j.1365-201X.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J App Physiol. 2012;113:290–296. doi: 10.1152/japplphysiol.00436.2012. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest. 2008;118:1450–1457. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A. 2010;107:15111–15116. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Liestol K, Ekmark M, Kollstad K, Gundersen K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J Physiol. 2003;551:467–478. doi: 10.1113/jphysiol.2003.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Liestol K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J Appl Physiol. 2006;100:2024–2030. doi: 10.1152/japplphysiol.00913.2005. [DOI] [PubMed] [Google Scholar]
- Cabric M, Appell HJ, Resic A. Effects of electrical stimulation of different frequencies on the myonuclei and fibre size in human muscle. Int J Sports Med. 1987;8:323–326. doi: 10.1055/s-2008-1025677. [DOI] [PubMed] [Google Scholar]
- Cabric M, James NT. Morphometric analyses on the muscles of exercise trained and untrained dogs. Am J Anat. 1983;166:359–368. doi: 10.1002/aja.1001660309. [DOI] [PubMed] [Google Scholar]
- Carson JA, Yamaguchi M, Alway SE. Hypertrophy and proliferation of skeletal muscle fibers from aged quail. J Appl Physiol. 1995;78:293–299. doi: 10.1152/jappl.1995.78.1.293. [DOI] [PubMed] [Google Scholar]
- Cheek DB, Holt AB, Hill DE, Talbert JL. Skeletal muscle cell mass and growth: the concept of the deoxyribonucleic acid unit. Pediat Res. 1971;5:312–328. [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):1–4. doi: 10.1093/gerona/50a.special_issue.1. [DOI] [PubMed] [Google Scholar]
- Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat. 1964;114 doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Kadi F, Malm C, Thornell LE. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol. 2005;124:167–175. doi: 10.1007/s00418-005-0029-5. [DOI] [PubMed] [Google Scholar]
- Giddings CJ, Gonyea WJ. Morphological observations supporting muscle fibre hyperplasia following weight-lifting exercise in cats. Anat Rec. 1992;233:178–195. doi: 10.1002/ar.1092330203. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein synthesis in tonic and phasic skeletal muscles. Nature. 1967;216:1219–1220. doi: 10.1038/2161219a0. [DOI] [PubMed] [Google Scholar]
- Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost. J Physiol. 2008;586:2675–2681. doi: 10.1113/jphysiol.2008.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell. 1989;59:771–772. doi: 10.1016/0092-8674(89)90597-7. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Schiaffino S. Control of muscle fibre size: a crucial factor in ageing. Acta Physiol Scand. 1999;167:307–312. doi: 10.1046/j.1365-201x.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Jackson J, Mula J, Kirby T, Fry C, Lee J, Ubele M, Campbell K, McCarthy J, Peterson C, Dupont Versteegden E. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012a;303:C854–C861. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol. 2012b;303:C854–C861. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol. 2008;154:522–528. doi: 10.1038/bjp.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol. 1999;111:189–195. doi: 10.1007/s004180050348. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Lewis SE, Anderson P, Goldspink DF. Pre- and postnatal growth and protein turnover in four muscles of the rat. Muscle Nerve. 1984;7:235–242. doi: 10.1002/mus.880070309. [DOI] [PubMed] [Google Scholar]
- Lhrke H, Hesse B, Goerttler K. Spontaneous tumors and lifespan of female NMRI mice of the outbred stock Sut:NMRT during a lifetime study. J Cancer Res Clin Onc. 1984;108:192–196. doi: 10.1007/BF00402466. [DOI] [PubMed] [Google Scholar]
- Lunde I, Anton S, Bruusgaard J, Rana Z, Ellefsen S, Gundersen K. Hypoxia inducible factor 1 links fast-patterned muscle activity and fast muscle phenotype in rats. J Physiol. 2011;589:1443–1454. doi: 10.1113/jphysiol.2010.202762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall GE, Allen DL, Linderman JK, Grindeland RE, Roy RR, Mukku VR, Edgerton VR. Maintenance of myonuclear domain size in rat soleus after overload and growth hormone/IGF-I treatment. J Appl Physiol. 1998;84:1407–1412. doi: 10.1152/jappl.1998.84.4.1407. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fibre hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GD, Huang CL, Sun L, Zaidi M. Translational musculoskeletal science: is sarcopenia the next clinical target after osteoporosis. Ann N Y Acad Sci. 2011;1237:95–105. doi: 10.1111/j.1749-6632.2011.06236.x. [DOI] [PubMed] [Google Scholar]
- Mendias CL, Kayupov E, Bradley JR, Brooks SV, Claflin DR. Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation. J Appl Physiol. 2011;111:185–191. doi: 10.1152/japplphysiol.00126.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger T, Gache V, Xu M, Cadot B, Folker E. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature. 2012;484:120. doi: 10.1038/nature10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP. The relationship between the dimensions of the fibers and the number of nuclei during normal growth of skeletal muscle in the domestic fowl. Am J Anat. 1968;122:555–564. doi: 10.1002/aja.1001220308. [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature. 1989;337:570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Puckelwartz M, McNally EM. Emery-Drefuss muscular dystrophy. Handb Clin Neurol. 2011;101:156–166. doi: 10.1016/B978-0-08-045031-5.00012-8. [DOI] [PubMed] [Google Scholar]
- Qaisar R, Renaud G, Morine K, Barton E, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fibre level. FASEB J. 2012;26:1077–1085. doi: 10.1096/fj.11-192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, Milan G, Masiero E, Carnio S, Lee D, Lanfranchi G, Goldberg AL, Sandri M. JunB transcription factor maintains skeletal muscle mass and promotes hypertrophy. J Cell Biol. 2010;191:101–113. doi: 10.1083/jcb.201001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy RR, Monke SR, Allen DL, Edgerton VR. Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. J Appl Physiol. 1999;87:634–642. doi: 10.1152/jappl.1999.87.2.634. [DOI] [PubMed] [Google Scholar]
- Rutherford OM, Jones DA. The role of learning and coordination in strength training. Eur J Appl Physiol Occup Physiol. 1986;55:100–105. doi: 10.1007/BF00422902. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Bormioli SP, Aloisi M. The fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Archiv B, Cell pathology. 1976;21:113–118. doi: 10.1007/BF02899148. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- Schultz E, Lipton BH. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev. 1982;20:377–383. doi: 10.1016/0047-6374(82)90105-1. [DOI] [PubMed] [Google Scholar]
- Seiden D. Quantitative analysis of muscle cell changes in compensatory hypertrophy and work-induced hypertrophy. Am J Anat. 1976;145:459–465. doi: 10.1002/aja.1001450405. [DOI] [PubMed] [Google Scholar]
- Smith K, Winegard K, Hicks AL, McCartney N. Two years of resistance training in older men and women: the effects of three years of detraining on the retention of dynamic strength. Can J Appl Physiol. 2003;28:462–474. doi: 10.1139/h03-034. [DOI] [PubMed] [Google Scholar]
- Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol. 1991;70:631–640. doi: 10.1152/jappl.1991.70.2.631. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Marcus R. Dynamic muscle strength alterations to detraining and retraining in elderly men. Clin Physiol. 1997;17:311–324. doi: 10.1111/j.1365-2281.1997.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Utvik JK, Njå A, Gundersen K. DNA injection into single cells of intact mice. Hum Gene Ther. 1999;10 doi: 10.1089/10430349950019075. [DOI] [PubMed] [Google Scholar]
- van der Meer SFT, Jaspers R, Jones D, Degens H. Time-course of changes in the myonuclear domain during denervation in young-adult and old rat gastrocnemius muscle. Muscle Nerve. 2011;43:212–222. doi: 10.1002/mus.21822. [DOI] [PubMed] [Google Scholar]
- WADA. Montreal, Canada: World Anti-Doping Agency; 2009. World anti-doping code. [Google Scholar]
- Wada KI, Takahashi H, Katsuta S, Soya H. No decrease in myonuclear number after long-term denervation in mature mice. Am J Physiol Cell Physiol. 2002;283:C484–C488. doi: 10.1152/ajpcell.00025.2002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rudnicki M. Satellite cells, the engines of muscle repair. Nature Rev Mol Cell Biol. 2012;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- Winchester PK, Gonyea WJ. A quantitative study of satellite cells and myonuclei in stretched avian slow tonic muscle. Anat Rec. 1992;232:369–377. doi: 10.1002/ar.1092320306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


