Abstract
Zinc (Zn) is one of the essential micronutrients required for optimum plant growth. Substantial quantity of applied inorganic zinc in soil is converted into unavailable form. Zinc solubilising bacteria are potential alternates for zinc supplement. Among 10 strains screened for Zn solubilisation, P29, P33, and B40 produced 22.0 mm clear haloes on solid medium amended with ZnCO3. Similarly, P17 and B40 showed 31.0 mm zone in ZnO incorporated medium. P29 and B40 showed significant release of Zn in broth amended with ZnCO3 (17 and 16.8 ppm) and ZnO (18 and 17 ppm), respectively. The pH of the broth was almost acidic in all the cases ranging from 3.9 to 6.1 in ZnCO3 and from 4.1 to 6.4 in ZnO added medium. Short term pot culture experiment with maize revealed that seed bacterization with P29 @ 10 g·kg−1 significantly enhanced total dry mass (12.96 g) and uptake of N (2.268%), K (2.0%), Mn (60 ppm), and Zn (278.8 ppm).
1. Introduction
Zinc is one of the imperative micronutrients required relatively in small concentrations (5–100 mg kg−1) in tissues for healthy growth and reproduction of plants. Zinc deficiency in plants leads to reduced membrane integrity and synthesis of carbohydrates, auxins, nucleotides, cytochromes, and chlorophyll and develops susceptibility to heat stress [1]. Excessive use of zinc fertilizers also poses problems to humans causing the impaired absorption of iron and copper. It is also known to repress male sexuality [2]. The solubility of Zn is highly dependent upon soil pH and moisture and hence arid and semiarid areas of Indian agroecosystems are often zinc-deficient.
In India, maize is grown in a wide range of environments, extending from extreme semiarid to subhumid and humid regions. It is grown in about 8.26 Mha with yield being 19.3 Mt (Ministry of Agriculture, Government of India). Voluminous literature indicates that Zn concentration in the grain is inherently very low, particularly when grown on Zn-deficient soils. The major reason for the widespread occurrence of Zn deficiency problems in crop plants is attributed to low solubility of Zn in soils rather than a low total amount of Zn [3]. Customary application of inorganic zinc partially caters the plant need as 96–99% of applied Zn is converted into different insoluble forms depending upon the soil types and physicochemical reactions within 7 days of application [4]. Microbes are potential alternate that could cater plant zinc requirement by solubilising the complex zinc in soil. Several genera of rhizobacteria belonging to Pseudomonas spp. and Bacillus spp. are reported to solubilise zinc. Microbes solubilise the metal forms by protons, chelated ligands, and oxidoreductive systems present on the cell surface and membranes [5–7]. These bacteria also exhibit other traits beneficial to plants, such as production of phytohormones, antibiotics, siderophores, vitamins, antifungal substances, and hydrogen cyanide [8]. In this study we reported the in vitro zinc solubilisation ability of selected strains and their ability to enhance the growth of Zea mays L.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Bacterial strains used in this study were obtained from the culture bank of Central Research Institute for Dryland Agriculture, Hyderabad, India. Five each of Pseudomonas spp. were designated as P17, P21, P29, P33, and P74 and Bacillus spp. as B40, B61, B114, B116, and B118. The cultures were originated from composite and rhizospheric soils of diverse rainfed agroecosystems of India. Pseudomonas and Bacillus spp. were maintained on King's B and nutrient agar medium at 4°C.
2.2. In Vitro Zinc Solubilization Assay
All the isolates were inoculated into liquid mineral salts medium (g·lit−1) specified by Saravanan et al. [9] containing dextrose: 10.0; (NH4)2SO4: 1.0; KCl: 0.2; K2HPO4: 0.1; MgSO4: 0.2; pH: 7.0 and insoluble Zn compound (ZnO and ZnCO3: 0.1%; Agar: 15.0 g) and autoclaved at 121°C for 20 min. Actively growing cultures of each strain were spot-inoculated (3 µL) onto the agar and plates were incubated at 28°C for 48 h. The clearing zone around colony was recorded. Quantitative study of zinc solubilization was studied in 150 mL conical flasks containing 50 mL of liquid mineral salt medium. The broth was inoculated with 10 µL of overnight grown bacterial inoculum and incubated for 72 h at 160 rpm in an incubator shaker at 28 ± 2°C. After incubation, the culture broth was centrifuged and the concentration of Zn in the supernatant was estimated in atomic absorption spectrophotometer (GBC, Australia).
2.3. Seed Bacterization
Maize seeds of cultivable variety were surface sterilized with 1% sodium hypochlorite for 5 min and washed five times with sterile distilled water. Seeds were treated with talc-based inoculum containing 108 cfu·g−1 of each strain with 0.5% carboxymethylcellulose (CMC) as adhesive.
2.4. Pot Trial
The pot culture experiment was conducted in 10 kg plastic pots (20 cm dia) filled with 9 kg sterile red soil (presterilized for three consecutive days) with six replications for each treatment. Maize cultivable variety seeds treated with bacterial inoculant were sown and the glasshouse condition was set at 28 ± 2°C and 70% humidity. Pots were watered once in two days with sterile distilled water until 60 days. The experimental setup consisted of 15 treatments namely, five treatments each of Bacillus and Pseudomonas strains as seed dresser @ 10 mg·kg−1 seed (T1 to 10), commercially available zinc solubilizing bacteria (T11), farm yard manure (FYM) @ 10 kg·acre−1 (T12), seeds primed by soaking overnight in 1.0% ZnSO4 (T13), positive control @ ZnSO4 @ 10 kg·acre−1 (T14), and uninoculated control (T15).
2.5. Plant Growth Measurement
After 60 days of sowing (DAS), plants were uprooted from the pots carefully and biometric parameters like root volume, shoot length, leaf area (measured by LI 3100, Lincoln, Nebraska, USA leaf area meter), and dry mass of plants were recorded as the indicative of plant growth.
2.6. Nutrient Analyses
Dried plants were finely ground in a mortar and pestle to amorphous powder and 100 mg was taken in 150 mL conical flask containing 10 mL nitric acid (HNO3) and perchloric acid (HClO4) in 9 : 4 ratio. The flasks were placed on a hot plate and digested at 300°C until the entire plant material turned colourless. The extract was taken in 100 mL volumetric flask and the volume was made to 100 mL with distilled water. These samples were used for estimation of sodium, potassium, and calcium by flame photometer. Phosphorus was quantified by sulphomolybdic acid method [10]. Total nitrogen content of the plants was estimated by micro-Kjeldahl method [11]. Similarly, micronutrients such as iron, copper, manganese, zinc, and magnesium were estimated by atomic absorption spectrophotometer.
2.7. Statistical Analysis
The values presented are the means of two independent experiments each with six replicates performed at different occasions. Data obtained from all the experiments were subjected to two-way analysis of variance (ANOVA). Mean values between treatments were compared with Fisher's least significant difference (LSD) test (P < 0.05).
3. Results
3.1. Zinc Solubilization Activity
All the selected strains of Pseudomonas and Bacillus used could effectively solubilize the insoluble Zn compounds used, namely, ZnCO3 and ZnO, under the assay conditions. The zone of solubilisation was comparatively high in ZnO amended medium as compared to ZnCO3. Size of the solubilisation zone ranged from 14 to 22 mm in ZnCO3 and from 17 to 33 mm in ZnO incorporated medium. Among the cultures, P29, P33, and B40 showed the highest solubilisation zone in ZnCO3 (22 mm), whereas P17 and B40 showed 31 mm zone in ZnO amended medium (Table 1). Quantitative assay for zinc solubilisation revealed that P29, P33, and B40 were able to dissolve 17, 16, and 16.8 ppm from ZnCO3, respectively, in liquid medium (Figure 1) and they were consistent with the observations on solid medium. However, P17 which was found to be the leading solubilizer on plate agar did not imitate the result in broth amended with ZnO though significant fall in pH (4.1) was noted. Instead, P29 showed the highest Zn solubilisation of 18 ppm available Zn, followed by B40 (17 ppm) (Figure 2). Across the treatments, significant reduction of pH was observed in the broth cultures amended with ZnCO3 (pH 3.9–6.1) and ZnO (pH 4.1–6.4). However there was no significant correlation between the pH and solubilisation of nutrients.
Table 1.
Zinc solubilizing ability of Pseudomonas and Bacillus isolates with insoluble zinc substrates on solid medium.
| Bacterial isolates | Solubilization zone diameter (in mm) | |
|---|---|---|
| ZnCO3 | ZnO | |
| P17 | 20 | 31 |
| P21 | 21 | 24 |
| P29 | 22 | 27 |
| P33 | 22 | 27 |
| P74 | 21 | 23 |
| B40 | 22 | 31 |
| B61 | 18 | 19 |
| B114 | 14 | 17 |
| B116 | 20 | 24 |
| B118 | 20 | 26 |
Figure 1.

Plant growth promotion of maize with zinc solubilizing Pseudomonas sp. strain-P29 (60 DAS).
Figure 2.
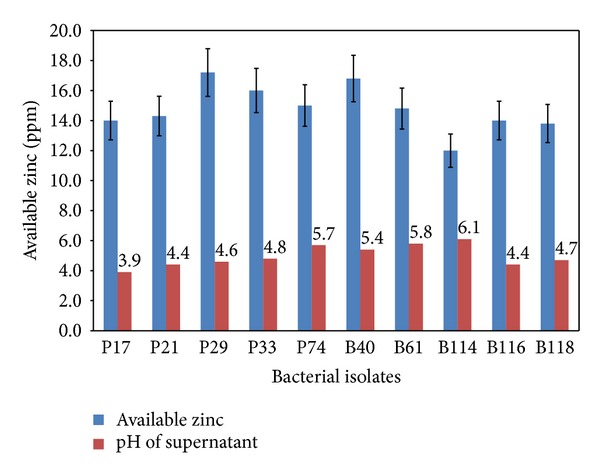
Available zinc (mg·kg−1) released by bacteria in broth medium containing zinc carbonate.
3.2. Plant Growth Promoting Activity of Bacterial Strains
Seed bacterization of maize with zinc solubilising Pseudomonas spp. and Bacillus spp. enhanced the plant growth significantly after 60 DAS (Table 2). Among all the treatments, inoculation of maize with talc-based P29 strain showed increased root volume (18.3 cm3). Other growth indicating parameters like total dry mass (TDM) and leaf area (LA) were recorded to be the highest in ZnSO4 treatment with 15.25 g and 1161.3 cm2, respectively. However, they are statistically on par with P29 treatment where 12.96 g and 1147.5 cm2 of TDM and LA were recorded, respectively (Figure 1 and Table 2).
Table 2.
Biometric growth parameters of maize seeds treated with ZSBs and inorganic source of zinc.
| Treatment | RV (cc) | SL (cm) | TDM (gm) | LA (cm2) |
|---|---|---|---|---|
| Control | 9.8j (±0.45) | 78.8h (±3.63) | 9.16h (±0.422) | 627.7i (±28.93) |
| ZnSO4 | 13.8h (±0.64) | 85.1fg (±3.92) | 15.25a (±0.703) | 1161.3a (±53.52) |
| Priming | 15.0fg (±0.69) | 96.0c (±4.42) | 12.87b (±0.593) | 861.0f (±39.68) |
| B61 | 15.0fg (±0.69) | 97.8b (±4.51) | 11.36d (±0.523) | 908.3e (±41.86) |
| B40 | 15.7de (±0.72) | 92.1d (±4.24) | 11.98c (±0.552) | 955.5d (±44.04) |
| B116 | 16.7c (±0.77) | 110.1a (±5.07) | 12.78b (±0.589) | 1113.8b (±51.33) |
| B114 | 16.2cd (±0.75) | 92.4d (±4.26) | 9.81fg (±0.452) | 901.7e (±41.56) |
| B118 | 16.3c (±0.76) | 89.0e (±4.10) | 12.08c (±0.557) | 1041.8c (±48.02) |
| P33 | 15.3e–g (±0.71) | 95.8c (±4.42) | 12.08c (±0.557) | 982.5d (±45.28) |
| P29 | 18.3b (±0.84) | 84.7fg (±3.90) | 12.96b (±0.597) | 1147.5ab (±58.02) |
| P74 | 14.8g (±0.68) | 75.5i (±3.48) | 10.13f (±0.467) | 851.7fg (±39.25) |
| P17 | 9.8j (±0.45) | 73.5i (±3.39) | 7.38i (±0.340) | 611.8i (±28.2) |
| P21 | 19.8a (±0.91) | 96.0c (±4.43) | 10.61e (±0.489) | 790.7h (±36.44) |
| ZSB | 12.8i (±0.59) | 86.3f (±3.98) | 9.67g (±0.446) | 859.7f (±39.62) |
| FYM | 15.5ef (±0.71) | 83.5g (±3.85) | 9.08h (±0.418) | 819.3gh (±37.76) |
|
| ||||
| LSD | 0.57 | 2.0 | 0.42 | 35.5 |
Values in the parentheses are standard errors and parameters were recorded at 60 DAS.
Values superscribed by the same alphabet are not significantly different according to Fisher's least significant difference test (P < 0.05).
RV: root volume; SL: shoot length; TDM: total dry mass; LA: leaf area.
3.3. Nutrient Concentrations in Plants
Significant concentration of N (2.268%) and K (2.0%) was observed in P29 treatment. The highest P (0.28%) concentration was noted in B40 treated plants as compared to other treatments (Table 3). The highest sodium (Na+) concentration in plants was recorded in Zn primed, P29, P33, and ZnSO4 treatments which were not significantly different. Significant Ca (0.434%) concentration in plant tissues was found in ZnSO4 treatment followed by P74 (0.354%) and P29 (0.341%). Na–K ratio was higher with ZSB (0.0047) and priming (0.004) treatment; P29 (0.0033) and B118 (0.0029) also recorded the highest ratio of Na–K ions.
Table 3.
Macronutrients uptake pattern of maize, seed treated with ZSBs, and inorganic source of zinc.
| Treatment | Macronutrients (%) | |||||
|---|---|---|---|---|---|---|
| Total “P” | Total “N” | K | Na | Ca | Na : K ratio | |
| Control | 0.18f (±0.160) | 1.428j (±0.160) | 1.6c (±0.074) | 0.004d (±0.00018) | 0.319d (±0.015) | 0.0025 |
| ZnSO4 | 0.15i (±0.217) | 1.883bc (±0.217) | 1.8b (±0.083) | 0.005c (±0.00023) | 0.434a (±0.020) | 0.0028 |
| Priming | 0.19e (±0.150) | 1.568fg (±0.150) | 1.5d (±0.069) | 0.006b (±0.00028) | 0.299g (±0.014) | 0.004 |
| B61 | 0.22b (±0.170) | 1.848c (±0.170) | 1.6c (±0.074) | 0.004d (±0.00018) | 0.339c (±0.016) | 0.0025 |
| B40 | 0.28a (±0.099) | 1.638de (±0.099) | 1.8b (±0.083) | 0.002f (±0.00009) | 0.197k (±0.009) | 0.0011 |
| B116 | 0.11j (±0.114) | 1.533gh (±0.114) | 1.4e (±0.065) | 0.003e (±0.00014) | 0.227j (±0.010) | 0.0021 |
| B114 | 0.16h (±0.149) | 1.883bc (±0.149) | 1.3f (±0.060) | 0.001g (±0.00005) | 0.298g (±0.014) | 0.0008 |
| B118 | 0.15i (±0.156) | 1.673d (±0.156) | 1.4e (±0.065) | 0.004d (±0.00018) | 0.311e (±0.014) | 0.0029 |
| P33 | 0.18f (±0.171) | 1.603ef (±0.171) | 1.5d (±0.069) | 0.005c (±0.00023) | 0.341c (±0.016) | 0.0033 |
| P29 | 0.20d (±0.052) | 2.268a (±0.052) | 2.0a (±0.092) | 0.005c (±0.00023) | 0.104l (±0.005) | 0.0025 |
| P74 | 0.17g (±0.177) | 1.603ef (±0.177) | 1.5d (±0.069) | 0.003e (±0.00014) | 0.354b (±0.016) | 0.002 |
| P17 | 0.21c (±0.169) | 1.498hi (±0.169) | 1.3f (±0.060) | 0.002f (±0.00009) | 0.338c (±0.016) | 0.0015 |
| P21 | 0.17g (±0.143) | 1.673d (±0.143) | 1.4e (±0.065) | 0.003e (±0.00014) | 0.285h (±0.013) | 0.0021 |
| ZSB | 0.16h (±0.152) | 1.463ij (±0.152) | 1.5d (±0.069) | 0.007a (±0.00032) | 0.303f (±0.014) | 0.0047 |
| FYM | 0.18f (±0.133) | 1.568fg (±0.133) | 1.3f (±0.060) | 0.002f (±0.00009) | 0.266i (±0.012) | 0.0015 |
|
| ||||||
| LSD | 0.015 | 0.046 | 0.042 | 0.00012 | 0.002 | |
Values in the brackets are standard errors and parameters were recorded at 60 DAS.
Values in the columns are means of two independent experiments with six replicates each time.
P29 significantly enhanced the concentration of Zn content (278.8 ppm) in plant tissue as high as 36, 32.35, and 43.11% compared to Zn priming, ZnSO4, and control treatment, respectively (Table 4). The highest Mn (60 ppm) concentration was also noted in P29 treatment which is on par with ZnSO4 treatments. Interestingly, P21 treated plants showed the highest quantity of Fe (707 ppm) which is statistically on par with B40 treatment (672.8 ppm). Mg (0.23 ppm) and Cu (4.8 ppm) were significantly found higher in inorganic ZnSO4 plants.
Table 4.
Micronutrients uptake pattern of maize, seed treated with ZSBs, and inorganic source of zinc.
| Treatment | Micronutrients (ppm) | ||||
|---|---|---|---|---|---|
| Zn | Mg | Fe | Cu | Mn | |
| Control | 158.6i (±7.3) | 0.16cd (±0.0074) | 481.4c (±22) | 2.9c (±0.13) | 34h (±1.6) |
| ZnSO4 | 188.6g (±8.7) | 0.23a (±0.0106) | 626.7b (±29) | 4.8a (±0.22) | 60a (±2.8) |
| Priming | 178.6h (±8.2) | 0.15de (±0.0069) | 488.4c (±23) | 3.0c (±0.14) | 34h (±1.6) |
| B61 | 243.4c (±11.2) | 0.17bc (±0.0078) | 672.8a (±31) | 2.9c (±0.13) | 51b (±2.4) |
| B40 | 192g (±8.8) | 0.11h (±0.0051) | 226.4f (±10) | 3.7b (±0.17) | 46c (±2.1) |
| B116 | 185.2gh (±8.5) | 0.11h (±0.0051) | 234.8f (±11) | 1.5e (±0.07) | 37fg (±1.7) |
| B114 | 235.2d (±10.8) | 0.14ef (±0.0065) | 367d (±17) | 2.1e (±0.10) | 40e (±1.8) |
| B118 | 231.8de (±10.7) | 0.15de (±0.0069) | 282.7e (±13) | 1.5e (±0.07) | 24i (±1.1) |
| P33 | 225e (±10.4) | 0.18b (±0.0083) | 381d (±18) | 0.9f (±0.04) | 38ef (±1.8) |
| P29 | 278.8a (±12.8) | 0.04i (±0.0018) | 155.2g (±7) | 3.1c (±0.14) | 60a (±2.8) |
| P74 | 230de (±10.6) | 0.16cd (±0.0074) | 495c (±23) | 1.9d (±0.09) | 44cd (±2.0) |
| P17 | 259b (±11.9) | 0.16cd (±0.0074) | 381d (±18) | 0.9f (±0.04) | 35gh (±1.6) |
| P21 | 232de (±10.7) | 0.14ef (±0.0065) | 707a (±33) | 0.5g (±0.02) | 25i (±1.2) |
| ZSB | 192g (±8.8) | 0.13fg (±0.0060) | 594b (±27) | 0.7fg (±0.03) | 43d (±2.0) |
| FYM | 212f (±9.8) | 0.13fg (±0.0060) | 359d (±17) | 0.7fg (±0.03) | 21j (±1.0) |
|
| |||||
| LSD | 7 | 0.011 | 36.2 | 0.27 | 2.5 |
Values in the brackets are standard errors and parameters were recorded at 60 DAS.
Values superscribed by the same alphabet are not significantly different according to Fisher's least significant difference test (P < 0.05).
4. Discussion
Solubilisation of zinc can be accomplished by a range of mechanisms, which include excretion of metabolites such as organic acids, proton extrusion, or production of chelating agents [12, 13]. In addition, production of inorganic acids such as sulphuric acid, nitric acid, and carbonic acid could also facilitate the solubilisation [8, 14].
It is apparent from the zinc solubilization data that the solubilization potential varied with each isolate. Organic acid production by microbial isolates has been reported to be a major mechanism of solubilization [15, 16]. This solubilization property is important in nutrient cycling. Fall in pH and acidification of medium was noted in all cases. Higher solubilization of the insoluble zinc sources was achieved in 72 h. The zinc solubilizing potential also correlated with the zinc levels that are accumulated by plant leaves. The zinc solubilization in our studies could be due to production of organic acids, like gluconic acids (especially 2-keto-gluconic acids). Zinc phosphate solubilization by a strain of Pseudomonas fluorescens was investigated by Di Simine et al. [17]. They identified that gluconic acids produced in culture medium helped in solubilization of zinc salts. In our present study also, the acidic pH shown by all the bacterial isolates gives a clue that the solubilization could be due to production of organic acids and higher the production of the same higher is the available zinc in the culture broth (Figure 3). Desai et al. [18] reported that higher availability of Zn is directly proportional to acidic pH of the culture broth. However, in some potent strains, pH did not fall drastically suggesting that in those strains other mechanisms may be active and this aspect is being accentuated.
Figure 3.
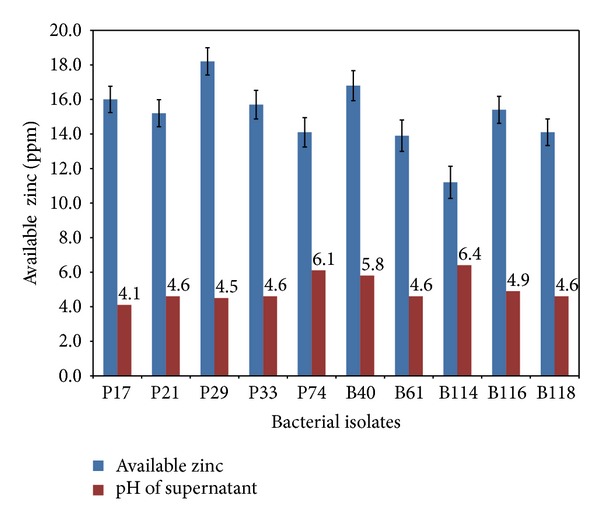
Available zinc (mg·kg−1) released by bacteria in broth medium containing zinc oxide.
The present study clearly demonstrated that inoculation with plant growth promoting rhizobacteria significantly enhanced the growth of maize in all dimensions. In this study application of ZnSO4 alone has increased the total dry mass of plants and leaf area. Increased leaf area with ZnSO4 alone was much similar to that of P29 treatment. P21 and P29 treated plants significantly enhanced the root volume and dry mass of plants, also supported by the studies carried out by Richardson [19], who showed that PGPR inoculation effectively increases surface area of roots and root weight [20]. The variation in enhancement of root volume by these strains may be due to the difference in the quantity of phytohormones produced by each strain. Auxin is a class of plant hormones of which indole-3-acetic acid is well studied, which has the capacity to enhance long term responses in plants [21]. Pseudomonas strains have increased root and shoot elongation in canola, lettuce, and tomato [22].
The plant growth promoting effects of bioinoculant PGPR strains were clearly demonstrated in many studies [23, 24]. The positive effects of PGPRs on yield and growth of maize were explained by Egamberdiyeva [25], which may be due to N2 fixation ability, P-solubilizing capacity, and phytohormones production.
From the tabulated data in Tables 2–4 it could be observed that no particular treatment with the bacterial strain or ZnSO4 or priming or FYM treatment could be able to enhance the growth of plants in all aspects. In case of dry mass and leaf area which were higher with the treatments where no bacteria were applied, much variation could not be seen on an overall comparison of treatments. This minor variation in between bacterial treatments could be due to difference in mechanisms of plant growth promotion exerted by different bacterial strains. Untreated plants showed poor growth.
In the current study, seed bacterization with zinc solubilizing plant growth promoting bacteria resulted in increased plant height (root volume and shoot height); leaf area; and dry mass. Similar increases in plant parameters were observed in different crops inoculated with Pseudomonas, Azospirillum, and Azotobacter strains [26, 27]. This improved growth by PGPR is due to making the increased availability of nutrients and decreasing metal toxicity [28].
The present study indicated that bacterial inoculation of maize with Pseudomonas and Bacillus significantly increased the nutrient content of “N” and “P” in leaves of maize (Table 3). This higher uptake of essential nutrients compared to uninoculated control plants could be justified from the fact that the unavailable forms of these nutrients were solubilized and made available in the root region by applying PGPR. Plants which are inoculated with plant growth promoting rhizobacteria usually have higher “N” content than that of uninoculated plants [29]. This fact is further strengthened by studies conducted by Murty and Ladha [30] who demonstrated that Azospirillum inoculation increased phosphate and ammonium uptake in rice plants. Even though K+ concentration was higher in other treatments than control, similar trend was not seen in case of Na+ levels (Table 3).
Results showed that all bacterial treated plants showed significant differences in Fe, Cu, Mn, and Zn content in maize leaves (Table 4) although differences between various bacterial strains were insignificant. The enhancement of macro- and micronutrient uptake by plants by inoculation with PGPR may be due to their effect on initiation and development of lateral roots [31], increased root weight, and nutrient uptake [32]. Studies by Goldstein and Liu [33] showed that phosphate and potash solubilizing bacteria may enhance mineral uptake in plants. This evidence confirmed that the percentage of various nutrients estimated in maize plants was significantly and/or relatively increased in bacterial treated plants (Table 4). On observing the critical Zn levels of treatments it can be said that the ZnSO4 treatment recorded lower Zn levels compared to other treatments. This can be explained from the fact that the presence of readily available zinc source in soil itself is not sufficient for uptake, but also the mobility of the mineral element is required which can be clearly seen in bacterial treatments there by higher presence of zinc levels in maize plants treated with PGPR. A “Z”-score statistical ranking method was followed to identify the promising bacterial isolate among all the strains and P29 strain was marked as the top strain compared to other bacteria for maize growth promotion.
5. Conclusion
Our study with PGPR and maize revealed that inoculation with beneficial rhizobacteria is an effective method for enhancing the growth of maize and maintaining the nutrient quality. Identified potential plant growth promoting rhizobacteria (P29) could be used as bioinput for improving the plant productivity as a substitute to chemical fertilizers and also to correct the nutrient deficiencies in maize for sustainable agriculture.
Acknowledgment
Financial support by Indian Council of Agricultural Research (ICAR), New Delhi, India, under a network project on Application of Microorganisms in Agriculture and Allied Sectors-Nutrient Management & PGPR (AMAAS) is gratefully acknowledged.
References
- 1.Singh B, Natesan SKA, Singh BK, Usha K. Improving zinc efficiency of cereals under zinc deficiency. Current Science. 2005;88(1):36–44. [Google Scholar]
- 2.Sharma PN, Chatterjee C, Sharma CP, Agarwala SC. Zinc deficiency and anther development in maize. Plant and Cell Physiology. 1987;28(1):11–18. [Google Scholar]
- 3.Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and Soil. 2008;302(1-2):1–17. [Google Scholar]
- 4.Saravanan VS, Subramoniam SR, Raj SA. Assessing in vitro solubilization potential of different zinc solubilizing bacterial (ZSB) isolates. Brazilian Journal of Microbiology. 2004;35(1-2):121–125. [Google Scholar]
- 5.Crane FL, Sun IL, Clark MG. Transplasma-membrane redox systems in growth and development. Biochimica et Biophysica Acta. 1985;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 6.Hughes MN, Poole RK. Metal speciation and microbial growth—the hard (and soft) facts. Journal of General Microbiology. 1991;137(4):725–734. [Google Scholar]
- 7.Wakatsuki T. Metal oxidoreduction by microbial cells. Journal of Industrial Microbiology. 1995;14(2):169–177. doi: 10.1007/BF01569900. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances. 1999;17(4-5):319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 9.Saravanan VS, Kalaiarasan P, Madhaiyan M, Thangaraju M. Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita . Letters in Applied Microbiology. 2007;44(3):235–241. doi: 10.1111/j.1472-765X.2006.02079.x. [DOI] [PubMed] [Google Scholar]
- 10.Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Department of Agriculture Circular. 1954;939:1–19. [Google Scholar]
- 11.Tandon HLS. Methods of Analysis of Soils, Plants, Water and Fertilisers. New Delhi, India: Fertiliser Develoment and Consultation Organisation; 2001. [Google Scholar]
- 12.Nahas E. Factors determining rock phosphate solubilization by microorganisms isolated from soil. World Journal of Microbiology and Biotechnology. 1996;12(6):567–572. doi: 10.1007/BF00327716. [DOI] [PubMed] [Google Scholar]
- 13.Sayer JA, Gadd GM. Solubilization and transformation of insoluble inorganic metal compounds to insoluble metal oxalates by Aspergillus niger . Mycological Research. 1997;101(6):653–661. [Google Scholar]
- 14.Seshadre S, Muthukumarasamy R, Lakshminarasimhan C, Ignaacimuthu S. Solubilization of inorganic phosphates by Azospirillum halopraeferans. Current Science. 2002;79(5):565–567. [Google Scholar]
- 15.Fasim F, Ahmed N, Parsons R, Gadd GM. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiology Letters. 2002;213(1):1–6. doi: 10.1111/j.1574-6968.2002.tb11277.x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen C, Yan W, Le Tacon F, Lapeyrie F. Genetic variability of phosphate solubilizing activity by monocaryotic and dicaryotic mycelia of the ectomycorrhizal fungus Laccaria bicolor (Maire) P.D. Orton. Plant and Soil. 1992;143(2):193–199. [Google Scholar]
- 17.Di Simine CD, Sayer JA, Gadd GM. Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biology and Fertility of Soils. 1998;28(1):87–94. [Google Scholar]
- 18.Desai S, Praveen Kumar G, Sultana U, et al. Potential microbial candidate strains for management of nutrient requirements of crops. African Journal of Microbiology Research. 2012;6(17):3924–3931. [Google Scholar]
- 19.Richardson AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Australian Journal of Plant Physiology. 2001;28(9):897–906. [Google Scholar]
- 20.Çakmakçi R, Erat M, Erdoğan Ü, Dönmez MF. The influence of plant growth-promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. Journal of Plant Nutrition and Soil Science. 2007;170(2):288–295. [Google Scholar]
- 21.Cleland R. Cell wall extension. Annual Review of Plant Physiology. 1971;22:197–222. [Google Scholar]
- 22.Glick BR, Liu C, Ghosh S, Dumbroff EB. Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Soil Biology and Biochemistry. 1997;29(8):1233–1239. [Google Scholar]
- 23.El-Hawary MI, El-Hawary Fatma I, El-Ghamry AM, El-Naggar E. Effect of application of biofertilizer on the yield and NPK uptake of some wheat genotypes as affected by the biological properties of soil. Pakistan Journal of Biological Sciences. 2002;5:1181–1185. [Google Scholar]
- 24.Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma. 2005;125(1-2):155–166. [Google Scholar]
- 25.Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Applied Soil Ecology. 2007;36(2-3):184–189. [Google Scholar]
- 26.Siddiqui IA, Shaukat SS. Zinc and glycerol enhance the production of nematicidal compounds in vitro and improve the biocontrol of Meloidogyne javanica in tomato by fluorescent pseudomonads. Letters in Applied Microbiology. 2002;35(3):212–217. doi: 10.1046/j.1472-765x.2002.01162.x. [DOI] [PubMed] [Google Scholar]
- 27.Shaukat K, Affrasayab S, Hasnain S. Growth responses of Triticwn aestivum to plant growth promoting rhizobacteria used as a bio fertilizer. Research Journal of Microbiology. 2010;5(10):1022–1030. [Google Scholar]
- 28.Burd GI, Dixon DG, Glick BR. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Canadian Journal of Microbiology. 2000;46(3):237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- 29.Puente ME, Li CY, Bashan Y. Microbial populations and activities in the rhizoplane of rock-weathering desert plants. II. Growth promotion of cactus seedlings. Plant Biology. 2004;6(5):643–650. doi: 10.1055/s-2004-821101. [DOI] [PubMed] [Google Scholar]
- 30.Murty MG, Ladha JK. Influence of Azospirillum inoculation on the mineral uptake and growth of rice under hydroponic conditions. Plant and Soil. 1988;108(2):281–285. [Google Scholar]
- 31.Rolfe BG, Djordjevic MA, Weinman JJ, et al. Root morphogenesis in legumes and cereals and the effect of bacterial inoculation on root development. Plant and Soil. 1997;194(1-2):131–144. [Google Scholar]
- 32.Canbolat MY, Bilen S, Çakmakçi R, Şahin F, Aydin A. Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biology and Fertility of Soils. 2006;42(4):350–357. [Google Scholar]
- 33.Goldstein AH, Liu ST. Molecular cloning and regulation of a mineral phosphate solubilizing gene from Erwinia herbicola . Nature Biotechnology. 1987;5(1):72–74. [Google Scholar]


