Abstract
Antioxidants are an important group of medicinal preventive compounds as well as being food additives inhibiting detrimental changes of easily oxidizable nutrients. The present investigation has been carried out to evaluate the antioxidant properties of different solvent extracts of Agriophyllum pungens seeds by various in vitro systems. The anti-oxidative activities of these samples were determined using four methods: 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, ferric-reducing antioxidant potential (FRAP), and hydroxyl (OH) radical scavenging activities. Additionally, total flavonoids and phenolic contents (TPC) were also determined. Yield of extracts varied widely among solvents and was the highest for water extract (5.642% based on dry weight basis), while ethyl acetate extract exhibited the highest total phenolic content (0.149 mg/mL), total flavonoid content (0.111 mg/mL), and antioxidant activities (P<0.05). The ABTS radical scavenging activity of A. pungens seeds occurred in the following order: ascorbic acid (92.9157%)>BHA (90.1503%)>α-tocopherol (87.7527%)>APEA (83.9887%) >APWR (75.5633%); the antioxidant activity of the extracts might be attributed to the presence of these phenolics. This suggests that A. pungens seed extract is a potential source of natural antioxidants, which could be added to dietary supplements to help prevent oxidative stress.
Keywords: Agriophyllum pungens, Mongolian Sand-rice, antioxidant activity, phenols, flavonoid
INTRODUCTION
Reactive oxygen species (ROS) and free radicals, such as superoxide anion, hydrogen peroxide, and hydroxyl radical, are constantly formed in the human body by normal metabolic action. Their action is opposed by a balanced system of antioxidant defenses, including antioxidant compounds and enzymes. Upsetting this balance causes oxidative stress, which can lead to cell injury and death (1). Recently, much attention has been given to naturally occurring antioxidants, which may play an important role in inhibiting both free radicals and oxidative chain reactions within tissues and membranes (2). Moreover, ROS are a predominant cause of qualitative decay of foods, which leads to rancidity, toxicity, and destruction of biomolecules important in physiologic metabolism (3). Therefore, the evaluation of antioxidant activities of extracts and fractions is considered an important step prior to the isolation of antioxidant phytochemicals they contain (4). Many phenolic compounds, particularly flavonoids, exhibit a wide range of biological effects, including antibacterial, antiviral, anti-inflammatory, anti-allergic, anti-thrombotic, and vasodilatory actions (5). The health promoting effect of antioxidants from plants is thought to arise mainly from their protective effects by counteracting reactive oxygen species, which are believed to play a significant role in the etiology and pathogenesis of various chronic diseases, premature ageing, and the oxidative deterioration of cosmetics, foods, and pharmaceutical preparations (6). In plants, antioxidants prevent lipid peroxidation and oxidative modification of low-density lipoproteins (7). To find new natural sources of active compounds, we studied the antioxidant potential of various extracts from Agriopyllum pungens seeds.
Agriophyllum pungens (Vahl) Link ex A. Diterich is a native Mongolian medicinal plant belonging to the Chen-opdiaceae family, which is widely distributed in Middle Khalkha, Khovd, East-Mongolia, Depression of Great Lakes, Valley of Lakes, Eastern Gobi, Trans-Altai Gobi, Dzungarian Basin semi-desert and Alashan Gobi. In Mongolia, this plant commonly known as “Tsulihir, and Sand-rice”, is widely used in local foods. The seeds are an ingredient in most traditional milk products and mainly used to prepare flour and tea material. This special-tasting flour is mixed with milk and eaten for breakfast. Traditionally, the seeds have been used in the treatments of cough, and kidney and liver diseases. The seeds also showed preventive effects on hepatitis, hyperlipidemia, and fatty liver in vivo. A. pungens seeds contain high levels of crude protein with essential amino acids (lysine, threonine, and methionine), fatty acids, and polysaccharides (8). However, information on the anti-oxidant activity of A. pungens seeds is scanty.
Based on the highly acclaimed properties of this plant, the present investigation was carried out to evaluate the antioxidant properties of various extracts of A. pungens seeds by different in vitro models.
MATERIALS AND METHODS
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) diammonium salt, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), sodium bicarbonate, 2-deoxy-D-ribose, trichloroacetic acid (TCA), Folin & Ciocalteu’s phenol reagent, naringin, L-ascorbic acid, α-tocopherol, and butylated hydroxyanisole (BHA) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Tannic acid was obtained from Yakuri Pure Chemicals Co., Ltd. (Kyoto, Japan). All organic solvents and chemicals used in this study were of analytical grade.
Sample preparation
Agriophyllum pungens seed were collected from fresh plants in the East Gobi located in the Southeastern regions of Mongolia from September to October in 2012. The plant specimens were authenticated and deposited in the Botany Herbarium of the Department of Biology, Ulaanbaatar University of Mongolia. The plants were cleaned and air-dried under shade. The dried samples were stored at −70°C until used.
Preparation of extract
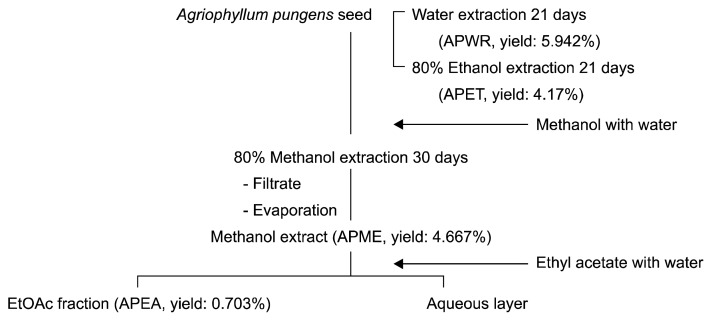
The dried and powdered Agriophyllum pungens (AP) seeds (3 kg) were exhaustively extracted with 9 L of 80% methanol for 30 days at room temperature. Finally, the material was macerated using 80% ethanol and water (1,000 mL) with constant stirring at room temperature for 21 days. The residual materials were re-extracted by the same procedure (3 times). The extracts were filtered over filter paper (No. 3, Whatman International Ltd., Kent, England), and the filtrate was evaporated by a rotary evaporator (N11; Yamato Co., Tokyo, Japan) under reduced pressure at a temperature lower than 40°C. The residue was dissolved in distilled water (200 mL) and then extracted with ethyl acetate (1 L) using a separating funnel. The ethyl acetate layer was collected and evaporated to dryness in a vacuum to yield the ethyl acetate (21.1 g) fractions. The dried samples were dissolved in respective solvents and filtered with a 0.45 μm membrane filter (Millipore, Billerica, MA, USA). The dried extract obtained with each solvent was weighed. The percentage yield was calculated in terms of the air-dried plant material. The extract obtained was used for the assessment of various antioxidant assays and for further analyses. The positive controls (α-tocopherol, BHA, butylated hydroxyanisole, ascorbic acid) and each solvent extract (APME, APEA, APWR, APET) were dissolved in methanol to the concentration of 100 mg/mL as stocks. All samples were placed in a glass bottle and stored at 4°C until further analyses. The extraction procedure is shown in Fig. 1.
Fig. 1.
Procedure to obtain different solvent extracts (APME, APEA, APET, and APWR) of Agriophyllum pungens seed.
Extract recovery percentage
The amount of crude extract recovered after successive extraction was weighed and the percentage of yield was calculated by the following formula,
Determination of total phenolics
Total phenolic contents in each extract were determined with Folin-Ciocalteu reagent according to the method of Singleton (9). The plant sample was dissolved using 20 mM phosphate buffered saline (PBS, pH 7.4), to a final concentration of 100 mg/mL. An aliquot (0.33 mL) of the extract was added to a test tube containing 2.5 mL of distilled water, vortexed, and then mixed with 0.16 mL of Folin-Ciocalteu reagent. After 5 min, 0.3 mL of 10% sodium bicarbonate solution was added. The mixture was allowed to stand 30 min at room temperature in darkness, and the absorbance was measured at 760 nm (DU650, Beckman Coulter, Brea, CA, USA). A standard curve was prepared to express the results as tannic acid equivalents, i.e. the quantity of tannic acid (mg/mL).
Determination of total flavoniod contents
According to the method of Davis (10), total flavonoid contents were determined by preparing 0.4 mL of extracts in 4.0 mL of 90% diethylene glycol and 40 mL of 1 N NaOH. The mixture was shaken thoroughly, and allowed to stand for 1 h at room temperature. The absorbance of the solution was measured at 420 nm. Total flavonoid contents were determined using a standard curve obtained from various concentrations of naringin.
DPPH radical scavenging activity
The free radical scavenging activity was carried out according to the Blois method (11) with slight modifications. Methanol solution (200 μL) of sample at various concentrations (0.1~10 mg/mL) was added to 0.1 mM methanolic solution of DPPH (4 mL) and shaken vigorously. The reaction mixture was allowed to stand for 30 min at room temperature in the dark. The absorbance of the mixture was determined using a spectrophotometer (DU650, Beckman Coulter) at 517 nm. The antioxidant activity of plant extracts was expressed as IC50, which was defined as the concentration of extract required to inhibit the formation of DPPH radicals by 50%. This activity is given as percent DPPH scavenging and calculated as: [(control absorbance–extract absorbance)/(control absorbance)]×100. BHA, α-tocopherol and ascorbic acid were used as references.
Ferric-reducing antioxidant power (FRAP) assay
The antioxidant capacity of samples was determined according to the Benzie and Strain method (12) with some modifications. The method is based on the reduction of the Fe3+-TPTZ complex to the ferrous form at low pH. The fresh working solution (FRAP reagent) was prepared by mixing 25 mL of 0.3 M acetate buffer, 2.5 mL of TPTZ solution, and 2.5 mL of FeCl3•6H2O solution, and then warmed (preheated) at 37°C before used. A properly diluted sample (0.1 mL) was added to 4.0 mL of FRAP reagent to form a mixture. The mixture was incubated at 37°C for 10 min in the dark, and the absorbance was measured at 593 nm against a blank that was prepared using distilled water. The results, obtained from triplicate analyses, were expressed as aqueous solution of ferrous sulfate (FeSO4•7H2O), and derived from a calibration curve of the standards (20~1,000 μM). The FRAP values of BHA, α-tocopherol and ascorbic acid were obtained by the same procedure.
ABTS radical scavenging activity assay
This assay was based on the ability of different substances to scavenge the 2,2′-azino-bis(3-ethylbenzothia-zoline-6-sulfonic acid) diammonium salts radical cation (ABTS•+) according to the methods of Re et al. (13) with some modifications. The ABTS•+ stock solution was diluted with ethanol (about 1:88 v/v) to an absorbance of 0.70±0.02 at 734 nm (Beckman Coulter DU650) for measurement, and equilibrated at 30°C. Aliquots (0.1 mL) of the samples were mixed with 2.9 mL of diluted ABTS•+ solution. After incubation at room temperature for 20 min, the absorbance at 734 nm was measured. Appropriate solvent blanks were run in each assay. BHA, α-tocopherol and ascorbic acid were employed as positive controls. A lower absorbance of the reaction mixture indicates higher ABTS radical scavenging activity. The activity is given as percentage ABTS radical scavenging, which is calculated with the equation:
Hydroxyl radical scavenging activity
Hydroxyl radical (OH•) scavenging assay was carried out by measuring the competition between deoxyribose and the extract for hydroxyl radicals generated from the Fe3+/ascorbate/EDTA/H2O2 system (14). Hydroxyl radicals were generated by direct addition of iron (II) salts to the reaction mixture (15). The reaction mixture was prepared with 0.2 mL of 10 mM FeSO4•7H2O containg 10 mM ethylenediaminetetraacetic acid (EDTA), 10 mM 2-deoxy-D-ribose solution (0.2 mL), sample solution (0.15 mL) or 0.1 M phosphate buffer (1.0 mL pH 7.4). The reaction was initiated by addition of 10 mM H2O2 (0.2 mL), followed by incubation at 37°C for 4 h. After incubation, 1 mL of 2.8% trichloroacetic acid (TCA) and 1.0% thiobarbituric acid (TBA) were added, and the mixture was heated in boiling water for 10 min. Finally, the reaction mixture was cooled in ice water and centrifuged at 800×g for 10 min. The absorbance of the supernatant was measured at 532 nm. Each assay was performed in triplicate. Hydroxyl radical scavenging activity was expressed as 50% inhibitory concentration (IC50), which is the concentration to reduce the absorbance of treated samples by 50% with reference to the control. α-tocopherol, ascorbic acid, and BHA were used as the positive controls.
Statistical analysis
The results are expressed as the mean±standard deviation (SD). All statistical analyses were performed using the SPSS 12.0 (SPSS Inc., Chicago, IL, USA) software. The differences among groups were evaluated by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests. A level of P<0.05 was used as the criterion for statistical significance.
RESULTS AND DISCUSSION
Recovery percent, total phenolics, and flavonoids
The extraction yields were 4.667, 0.703, 5.942, and 4.170 in 80% methanol, ethyl acetate, water and 80% ethanol extracts, respectively (Table 1). The highest extraction yield was recorded for the water seed extract of AP (APWR), whereas the lowest was for the ethyl acetate extract. However, no significant difference was observed between 80% methanol and ethanol extracts. Different solvent systems have been used for extraction of poly-phenols from plant material (16), but the reason for choosing these solvents has not been justified. Generally known, the yield of chemical extraction depends on the type of solvents with varying polarities and pH, extraction time, and temperature as well as on the chemical compositions and physical characteristics of the sample (17). Phenolic compounds are secondary metabolic products that occur throughout the plant kingdom. They contain the phenolic hydroxyl group, which has an anti-oxidative effect via interactions with the phenol ring and its resonance stabilization effect (18). Since plant-derived phenolic compounds have shown various physiological activities, levels of phenolic and flavonoid contents from A. pungens seed were first assessed (Table 1).
Table 1.
Extraction yield and total phenolic and flavonoid contents of different solvent extracts from Agriophyllum pungens seeds
| Sample1) | Yield (%)2) | Total phenol (Tannic mg/mL)3) | Total flavonoid (Naringin mg/mL)4) |
|---|---|---|---|
| APME | 4.6672) | 0.119±0.001308b5) | 0.073±0.00819ab6) |
| APEA | 0.703 | 0.149±0.01557a | 0.111±0.05808a |
| APWR | 5.642 | 0.138±0.01803ab | 0.098±0.01752a |
| APET | 4.170 | 0.060±0.01411c | 0.036±0.00265b |
Extracts from AP: APME, 80% methanol; APEA, ethyl acetate; APWR, water; and APET, 80% ethanol.
Based on weight of aerial parts extracted, Yield (%)=(extract weight/dry weight)×100.
Tannic acid was used as a standard for measuring total phenolic contents.
Naringin was used as a standard for measuring total flavonoid contents.
Measurements were done in triplicate and values represent mean±SD.
Mean values followed by different superscripts in a column are significantly different by Duncan’s multiple range test at P<0.05.
The ethyl acetate extract of AP contained significantly (P<0.05) higher amounts of total phenolics (0.149 mg/mL) than methanol (0.119 mg/mL), water (0.138 mg/mL) and ethanol (0.060 mg/mL) extracts. Previously, Zhou et al. (19) reported the presence of chemical constituents (p-(acetylamino)phenol, ayamenin A, irisone, and salicin) of the water and ethyl acetate extracts of Agriophyllum squarrosum. Gómez-Caravaca et al. (20) reported on the phenolic composition of Chenopodium species. Mirando et al. (21) noted the presence of phenolics in quinoa seeds. Our results are also similar to Gennari et al. (22) in that Beta bulgaris seeds had higher amounts of phenolic compounds when compared to other plants within the family Chenopodiaceae.
Flavonoids are also potent antioxidants, free radical scavengers, and metal chelators and they inhibit lipid peroxidation. This activity is believed to be mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (23). The total flavonoid contents in methanol, ethyl acetate, water and ethanol extracts were 0.073, 0.111, 0.098, and 0.036 mg/mL, respectively (Table 1). In a recent study by Li et al. (24), flavones (quercetin) and coumarins were reported in butanol extracts and ethyl acetate extracts of the aerial parts of Agriophyllum squarrosum. The flavonol conjugates quercetin and kaempferol oligomeric glycosides have previously been identified in the quinoa seeds (25). Awaad et al. (26) found that the ethanol, diethyl ether, chloroform, ethyl acetate, and n-butanol extracts of the Chenopodiaceous plant (Artiplex lentiformis) had 985 and 895 μmol Trolox equivalent/g and flavonoid contents, respectively. Similarly, Ranilla et al. (27) and Kumar et al. (28) had also reported antioxidant activity and flavonoid content in quinoa (Chenopoium quinoa Willd), Kaniwa (Chenopodium pallidicaule Aelen) and Chenopodium album. Plants containing flavonoids have been reported to possess strong antioxidant activities (29). Moreover, flavonoids are known to possess a wide range of therapeutic uses due to their antimutagenic and anticarcinogenic properties and the potential to decrease cardiovascular complications (30). Since the phenolic compounds are considered to be major contributors to the antioxidant capacities of plants, the relatively large phenolic and flavonoid contents in the ethyl acetate extract and water extract was suggestive for further research on isolating antioxidant compounds. This study supports our prediction that Agriophyllum pungens seeds are a good source of natural anti-oxidants such as phenolic and flavonoid compounds.
DPPH radical scavenging property
Free radicals produced in the body are partly associated with the etiology of cancers and other chronic diseases. Therefore, determining the radical scavenging effect of antioxidants in this seeds is important. Antioxidant activities of the A. pungens seed extracts by the different solvents were analyzed by measuring DPPH radical scavenging activities. DPPH• assay is widely used for evaluating antioxidant activities in a short time compared with other methods. The effect of antioxidants on DPPH radical scavenging was thought to be due to their hydrogen donating ability (31). The results on DPPH radical scavenging activities of the methanol (APME), ethyl acetate (APEA), water (APWR), and ethanol (APET) extracts and the positive control reference standards BHA, α-tocopherol, and ascorbic acid are shown in Table 2. According to the results, as the IC50 value decreases, the antioxidant activity increases. The IC50 value of each extract was compared with α-tocopherol (IC50, 0.575 mg/mL), BHA (IC50, 0.570 mg/mL) and ascorbic acid (IC50, 0.553 mg/mL). DPPH radical scavenging activity of APEA was found to be higher than that of APET. Our findings are partially comparable with previous studies by Gorinstein et al. (32) and Nsimba et al. (2) in which some quinoa seeds of various extracts showed high anti-oxidative activities. Gennari et al. (22) had recently reported that ethyl acetate seed extract of Swiss chard (B. vulgaris) has high antioxidant activity. Similar to our result, Wang et al. (33) reported that Agtiopllyllum squarrosum also showed high antioxidant activity. According to a well-established thought, antioxidant activities of various vegetables, fruits, and natural plants are attributed to the contents of phenolic compounds (34). The radical-scavenging activity of polyphenols depends on the molecular structure and the substitution pattern of the hydroxyl groups, the availability of phenolic hydrogens, and the possibility of stabilization of the resulting phenoxyl radicals via hydrogen donation or by expanded electron delocalization (35). This radical scavenging ability of extracts could be related to the nature of phenolics, thus contributing to their election transfer/hydrogen donating system. These results indicate that A. pungens seeds have significant effects on scavenging free radicals.
Table 2.
DPPH radical scavenging activity of different solvent extracts from Agriophyllum pungens seeds
| Sample extract1) | IC50: mg/mL2) |
|---|---|
| APME | 4.833±0.398923)b |
| APEA | 2.6487±0.47171c |
| APWR | 2.9982±0.06918c |
| APET | 8.5083±0.71914a |
| α-Tocopherol4) | 0.575±0.02879d |
| BHA4) | 0.570±0.03568d |
| Ascorbic acid4) | 0.553±0.04709d |
Same extracts as described in Table 1.
IC50 (mg/mL), concentration for scavenging 50% of DPPH radicals.
Measurements were done in triplicate, and values represent mean±SD.
α-Tocopherol, BHA (butylated hydroxyanisole) and ascorbic acid were used as positive controls.
Mean values followed by different superscripts in a column are significantly different by Duncan’s multiple range test at P<0.05.
Determination of reducing power
FRAP assay is commonly used to study the antioxidant capacity of plant materials. Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The ferrous state of iron can stimulate lipid peroxidation by the Fenton reaction and also accelerate peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals, which can themselves abstract hydrogen and perpetuate the chain reaction of lipid peroxidation. The simple and reliable FRAP assay measures the reducing potential of an antioxidant reacting with a ferric-TPTZ (Fe(III)-TPTZ) complex and producing a colored ferrous-TPTZ (Fe(II)-TPTZ) complex by a reductant at a low pH (about 3.6). The ferric-TPTZ complex can be monitored at 593 nm. A higher absorbance power indicates a higher ferric reducing power (36). In the present study, the FRAP values for APME, APEA, APWR and APET are shown in Table 3. The APEA (0.1921 mmol Fe(II)/mg extract) registered significantly higher (P<0.05) ferric reducing antioxidant activity than the APET (0.0753 mmol Fe(II)/mg extract), indicating that APEA possesses higher reducing capacity than APET. These observations showed that the reducing characteristics of the plants can act as antioxidants, reducing agents, hydrogen donators and oxygen quenchers (37). Similar to our result, Wang et al. (33) reported that Agtiopllyllum squarrosum showed the strongest anti-oxidant activity through the FRAP assay. Paško et al. (38) and Alvarez-Jubete et al. (39) also observed the highest antioxidant capacity in wild plants, especially in the Chenopodiaceae family, using the FRAP assay. According to Oktay et al. (40), a highly positive relationship between total phenolics and antioxidant activity appears to be the trend in many plant species. The presence of abundant total phenolics in several medicinal plants resulted in high a FRAP value. The reductive ability of the samples assessed in this study suggests that ethyl acetate and water extracts were able to donate an electron. Hence, the samples should be able to donate electrons to free radicals in actual biological or food systems, making the radicals stable.
Table 3.
Ferric-reducing activities of different solvent extracts from Agriophyllum pungens seeds
| Sample extract1) | mmol Fe(II)/mg extract2) |
|---|---|
| APME | 0.096±0.00900c3) |
| APEA | 0.1921±0.00301a |
| APWR | 0.1292±0.00857b |
| APET | 0.0753±0.00057d |
| α-Tocopherol4) | 1.137±0.02200a |
| BHA4) | 1.164±0.04078a |
| Ascorbic acid4) | 1.137±0.1506a |
Same extracts as described in Table 1.
Concentration of substance having ferric-TPTZ reducing ability expressed as μmol Fe(II) equivalents.
Measurements were done in triplicate and values represent mean±SD.
α-Tocopherol, BHA (butylated hydroxyanisole) and ascorbic acid were used as positive controls.
Mean values followed by different superscripts in a column are significantly different by Duncan’s multiple range test at P<0.05.
Antioxidant activity by the ABTS•+ assay
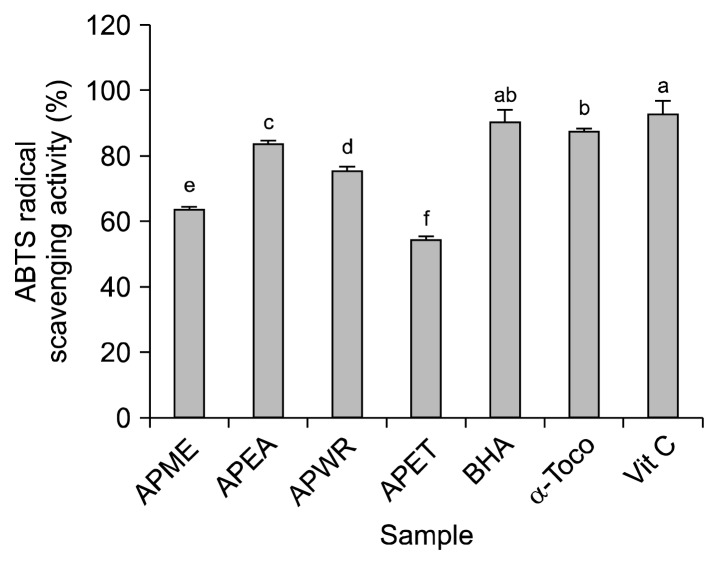
Another stable free radical cation, ABTS, was used to evaluate the antioxidant activity of the methanol, ethyl acetate, water and ethanol extracts from Agriophyllum pungens. The ABTS systems have been commonly used to measure the total antioxidative status of various biological specimens by measuring radical scavenging through electron donation (41). The ABTS radical scavenging effect of different solvent extracts from A. pungens exhibited a similar trend to the DPPH radical scavenging activity. The results were expressed as μmol Trolox equivalents/g extract. A steady increase was observed in the percentage inhibition of the ABTS radicals by the APME, APEA, APWR and APET (Fig. 2). The APEA exhibited higher radical scavenging activity than the APME when reacted with the ABTS radicals. As seen in Fig. 2, the scavenging effect on ABTS radical by dilution concentrations (2 mg/mL) of APME, APEA, APWR and APET were found as: 63.7083%, 83.9887%, 75.5633%, and 54.5510%, respectively. Quinoa seed extracts have already been reported to exhibit strong ABTS•+ scavenging activity (42). Hagerman et al. (15) have reported that the high molecular weight phenolics (tannins) have a greater ability to quench free radicals (ABTS•+) and their effectiveness depends on the molecular weight, the number of aromatic rings and nature of the hydroxyl group substitution than the specific functional groups. The position of hydroxyl groups might be more important for mediating potent total antioxidant activity (43). These results indicated that the APEA contains high amounts of radical scavengers and may be useful as a natural source of antioxidants.
Fig. 2.
ABTS radical scavenging activity of different solvent extracts from Agriophyllum pungens seed: APME, 80% methanol; APEA, ethyl acetate; APWR, water; and APET, 80% ethanol. Data were presented as mean±SD (n=3). Means sharing different letters (a–f) are significantly different (P<0.05). Same extracts as created in Fig 1. BHA (butylated hydroxyanisole), α-tocopherol and ascorbic acid at the concentration of 0.5 mg/mL were used as positive controls.
Hydroxyl radical scavenging activity
Hydroxyl radicals can easily cross cell membranes, react readily with most biomolecules including carbohydrates, proteins, lipids, and DNA in cells, and cause tissue damage or cell death. Thus, removing OH• is very important for the protection of living systems (44). Hydroxyl radical scavenging activity was estimated by generating hydroxyl radicals using ascorbic acid-ion EDTA. The scavenging abilities of APME, APEA, APWR and APET of Agriophyllum pungens are shown in Table 4. The APEA and APWR significantly decreased hydroxyl radical scavenging activity, and IC50 values were 5.8680 and 8.2978 mg/mL, respectively (P<0.05). The scavenging ability of the APEA and APWR might be due to the active hydrogen donor ability of hydroxyl substitution. Hydrogen peroxide can be toxic to cells when active. Therefore, removing H2O2 and O2•− is very important for antioxidant defense in cell or food systems.
Table 4.
Hydroxyl radical scavenging activity of different solvent extracts from Agriophyllum pungens seeds
| Sample extract1) | IC50: mg/mL2) |
|---|---|
| APME | 9.426±3.044093)ab |
| APEA | 5.8680±1.41030c |
| APWR | 8.2978±2.18027bc |
| APET | 11.5813±2.32485a |
| α-Tocopherol4) | 0.3930±0.10930d |
| BHA4) | 0.4820±0.18949d |
| Ascorbic acid4) | 0.4050±0.14147d |
Same extracts as described in Table 1.
IC50 (mg/mL), concentration for scavenging 50% of hydroxyl radicals.
Measurements were done in triplicate and values represent the mean±SD.
α-Tocopherol, BHA (butylated hydroxyanisole) and ascorbic acid were used as positive controls.
Mean values followed by different superscripts in a column are significantly different by Duncan’s multiple range test at P<0.05.
In conclusion, ethyl acetate extract of Agriophyllum pungens seeds possessed good antioxidant activities. Although further research is necessary, our study shows that a supplementation with this plant extract could at least help in preventing or decreasing the damages caused by oxidative stress. Based on these data, additional studies are needed to characterize the bioactive compounds responsible for the antioxidative activities in Agriophyllum pungens. Therefore, the study suggests that the whole plant might be a potential source of natural antioxidants.
ACKNOWLEDGMENTS
This Research was financially supported by the Women Scientists Project (Grant 2011-0013606) of Korean Research Foundation.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. J Nephrol. 2010;15:S29–S36. [PubMed] [Google Scholar]
- 2.Nsimba RY, Kikuzaki H, Konishi Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008;106:760–766. [Google Scholar]
- 3.Valko M, Izakovic M, Rhodes CJ, Telser J. Role of oxygen raidicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 4.Elif Korcan S, Aksoy O, Erdoğmuş SF, Ciğerci İH, Konuk M. Evalution of antibacterial, antioxidant and DNA protective capacity of Chenopodium album’s ethanolic leaf extracts. Chemosphere. 2013;90:374–379. doi: 10.1016/j.chemosphere.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Cook NC, Samman S. Flavonoids–Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- 6.Kaur C, Kapoor HC. Antioxidants in fruits and vegetables–the millennium’s health. Int J Food Sci Tech. 2001;36:703–725. [Google Scholar]
- 7.Servili M, Selvaggini R, Esposto S, Taticchi A, Montedoro G, Morozzi G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J Chromatography A. 2004;1054:113–127. [PubMed] [Google Scholar]
- 8.Ligaa U, Davaasuren B, Ninjil N. Medicinal plants of Mongolia used in Western and Eastern medicine. JKC Printing; Ulaanbaatar, Mongolia: 2006. pp. 478–479. [Google Scholar]
- 9.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 10.Chae SK, Kang GS, Ma SJ, Bang KM, Oh MW, Oh SH. Standard Food Analysis. Jigu-moonwha Sa; Seoul, Korea: 2002. pp. 381–382. [Google Scholar]
- 11.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 12.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–79. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 13.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 14.Kunchandy E, Rao MNA. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–240. [Google Scholar]
- 15.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 16.Pinelo M, Rubilar M, Sineiro J, Nunez MJ. Extraction of antioxidant phenolics from almound hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster) Food Chem. 2004;85:267–273. [Google Scholar]
- 17.Xu BJ, Chang SK. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72:159–166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 18.Shahidi F, Wansundara PK. Phenolic antioxidant. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 19.Zhou YH, Zhan KX, Gong B, Zhang L, Wang C, Li YS. Chemical constituents of the whole plants of Agriophyllum squarrosum (L.) Moq. (II) J Shenyang Pharmaceutical University. 2012;29:753–757. [Google Scholar]
- 20.Gómez-Caravaca AM, Iafelice G, Lavini A, Pulvento C, Caboni MF, Marconi E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J Agric Food Chem. 2012;60:4620–4627. doi: 10.1021/jf3002125. [DOI] [PubMed] [Google Scholar]
- 21.Miranda M, Vega-Galvez A, Lopez J, Parada G, Sanders M, Aranda M, Uribe E, Scala KD. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.) Ind Crop Prod. 2010;32:258–263. [Google Scholar]
- 22.Gennari L, Felletti M, Blasa M, Angelino D, Celeghini C, Corallini A, Ninfali P. Total extract of Beta vulgaris var. cicla seeds versus its purified phenolic components: antioxidant activities and antiproliferative effects against colon cancer cells. Phytochem Anal. 2011;22:272–279. doi: 10.1002/pca.1276. [DOI] [PubMed] [Google Scholar]
- 23.Osawa T. Novel antioxidants for utilization in food and biological system. In: Uritani I, Garcia VV, Mendoza EM, editors. Postharvest Biochemistry of Plant Food Materials in the Tropics. Japan Scientist Societies Press; Tokyo, Japan: 1994. pp. 241–251. [Google Scholar]
- 24.Li BY, Zhan KX, Zhou YH, Guo YG, Hui YG, Li YS. Isolation and identification of flavonoids and coumarins from the aerial parts of Agriophyllum squarrosum (L) Moq. J Shenyang Pharmaceutical University. 2012;29:923–926. [Google Scholar]
- 25.Dini I, Tenore GC, Dini A. Phenolic constituents of Kancolla seeds. Food Chem. 2004;84:163–168. [Google Scholar]
- 26.Awaad AS, Maitland DJ, Donia Ael R, Alqasoumi SI, Soliman GA. Novel flavonoids with antioxidant activity from a Chenopodiaceous plant. Pharm Biol. 2012;50:99–104. doi: 10.3109/13880209.2011.591806. [DOI] [PubMed] [Google Scholar]
- 27.Ranilla LG, Apostolidis E, Genovese MI, Lajolo FM, Shetty K. Evalution of indigenous grains from the Peruvian Andean region for antidiabetes and antihypertension potential using in vitro methods. J Med Food. 2009;12:704–713. doi: 10.1089/jmf.2008.0122. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Kumar D. Antioxidant and free radical scavenging activities of edible weeds. AJFAND. 2009;9:1174–1190. [Google Scholar]
- 29.Radami S, Gupta MK, Suresh B. Antioxidant activity of the ethanolic extract of Stiga orobanchiodes. J Ethnopharmacol. 2003;85:227–230. doi: 10.1016/s0378-8741(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 30.Chang CH, Lin HY, Liu YC. Comparisons on the antioxidant properties of fresh, freeze-dried, and hot air dried tomatoes. J Food Eng. 2006;77:478–485. [Google Scholar]
- 31.Soares JR, Dins TC, Cunha AP, Almeida LM. Anti-oxidants activity of some extracts of Thymus zygis. Free Radic Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 32.Gorinstein S, Lojek A, Cíž M, Pawelzik E, Delgado-Licon E, Medina OJ, Moreno M, Salas IA, Goshev I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int J Food Sci Tech. 2008;43:629–637. [Google Scholar]
- 33.Wang YZ, Ping ZK, Song Y. Study on the extracting conditions and antioxidant activity of chlorogenic acid in Agtiopllyllum squarrosum. Food Ferment Ind. 2007;33:131–134. [Google Scholar]
- 34.Wong PYY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Pertrosselium crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–515. [Google Scholar]
- 35.Benković V, Orsolić N, Knezević AH, Ramić S, Dikić D, Basić I, Kopjar N. Evaluation of the radioprotective effects of propolis and flavonoids in gamma-irradiated mice: the alkaline comet assay study. Biol Pharm Bull. 2008;31:167–172. doi: 10.1248/bpb.31.167. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B. Reactive oxygen species in living systems. Sourse biochemistry and role in human disease. Am J Med. 1991;91:14–21. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 37.NCCLS. Performance standard for antimicrobial susceptibility testing. 12th ed. National Committee for Clinical Laboratory Standard; Wayne, PA, USA: 2002. pp. M100–512. [Google Scholar]
- 38.Paško P, Bartoñ H, Zagrodzki P, Gorinstein S, Fołta M, Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009;115:994–998. [Google Scholar]
- 39.Alvarez-Jubete L, Wijngaard H, Arendt EK, Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. [Google Scholar]
- 40.Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vivo antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci Technol. 2003;36:263–271. [Google Scholar]
- 41.Rufián-Henares JA, Morales FJ. Functional properties of melanoidins: In vitro antioxidant, antimicrobial, and antihypertensive activities. Food Res Int. 2007;40:995–1002. [Google Scholar]
- 42.Gorinstein S, Vargas OJM, Jaramillo NO, Salas IA, Ayala ALM, Arancibia-Avila P, Toledo F, Katrich E, Trakhtenberg S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur Food Res Technol. 2007;225:321–328. [Google Scholar]
- 43.Okawa M, Kinjo J, Nohara T, Ono M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol Pharm Bull. 2001;24:1202–1205. doi: 10.1248/bpb.24.1202. [DOI] [PubMed] [Google Scholar]
- 44.Sun Z, Zhang L, Zhang B, Niu T. Structural characterisation and antioxidant properties of polysaccharides from the fruiting bodies of Russula virescens. Food Chem. 2010;118:675–680. [Google Scholar]