Abstract
Artemisia princeps Pampanini (AP) has been used as a traditional medicine in Korea, China and Japan and reported to exhibit various beneficial biological effects including anti-inflammatory, antioxidant, anti-atherogenic and lipid lowering activities; however, its antiplatelet and anticoagulant properties have not been studied. In the present study, we evaluated the effects of an ethanol extract of Artemisia princeps Pampanini (EAP) and its major flavonoids, eupatilin and jaceosidin, on platelet aggregation and coagulation. To determine the antiplatelet activity, arachidonic acid (AA)-, collagen- and ADP (adenosine diphosphate)-induced platelet aggregation were examined along with serotonin and thromboxane A2 (TXA2) generation in vitro. The anticoagulant activity was determined by monitoring the activated partial thromboplastin time (aPTT) and prothrombin time (PT) in vitro. The data showed that EAP and its major flavonoids, eupatilin and jaceosidin, significantly reduced AA-induced platelet aggregation and the generation of serotonin and TXA2, although no significant change in platelet aggregation induced by collagen and ADP was observed. Moreover, EAP significantly prolonged the PT and aPTT. The PT and/or aPTT were significantly increased in the presence of eupatilin and jaceosidin. Thus, these results suggest that EAP may have the potential to prevent or improve thrombosis by inhibiting platelet activation and blood coagulation.
Keywords: Artemisia princeps Pampanini, eupatilin, jaceosidin, anticoagulation, antiplatelet
INTRODUCTION
Platelets are essential for primary hemostasis and repair of the endothelium (1,2), but they also play a role in the development of cardiovascular diseases such as atherosclerosis and thrombotic events (3). When a blood vessel is damaged by injury or pathological alterations, such as in atherosclerosis, platelets adhere to the site of vascular injury, which triggers the subsequent platelet activation followed by platelet aggregation (4). During platelet aggregation, additional platelets are recruited from the circulation to the site of injury, causing the formation of an occlusive platelet thrombus (4). The recruitment of additional platelets is mediated by a variety of stimuli that are produced or released from activated platelets, including adenosine diphosphate/adenosine triphosphate (ADP/ATP), thromboxane A2 (TXA2) and serotonin, which amplify and sustain the initial platelet response, recruit circulating platelets and thereby, promote thrombus growth and stability (5).
Blood coagulation is also an important part of hemostasis, which refers to secondary hemostasis. Platelets stimulate the blood coagulation cascade at the site of vascular injury, which leads to the generation of a fibrin-containing clot to stop the bleeding and repair the damaged vessel (6,7). The coagulation cascade is initiated by two pathways, known as the intrinsic pathway and extrinsic pathway (8). The intrinsic pathway is initiated by substances within the damaged blood vessel, whereas the extrinsic pathway is activated when blood is exposed to tissue factors from the surface of extravascular cells. Regardless of which pathway is activated, the end result of both is the generation of factor Xa, which then catalyzes the conversion of prothrombin, an inactive form of thrombin, to thrombin (9,10). Thrombin, in turn, converts fibrinogen to fibrin, which promotes platelet aggregation at the site of vascular injury and causes vascular atherothrombotic disorders (8–10). Accordingly, the inhibition of platelet activation and coagulation is considered a strategy for the treatment of thrombotic diseases. However, many drugs, which are chemically or biologically synthesized, with antiplatelet and anticoagulant functions have a variety of adverse effects including hemorrhaging, neutropenia, agranulocytosis, thrombotic thrombocytopenia and gastrointestinal upset (11–13). Thus, in recent years, much attention has been given to the development of herbal medicines for treating cardiovascular diseases, due to their safety and eco-friendly image (14,15).
Artemisia princeps Pampanini (AP) is an herbaceous plant that is widely used as Korean, Chinese, and Japanese traditional medicine for the treatment of multiple disorders including colic, diarrhea, and irregular uterine bleeding (16). The ethanol extract of AP (EAP) exhibited anti-atherosclerotic and anti-inflammatory activities in LDL receptor knockout mice (17). Jaceosidin isolated from AP methanolic extracts inhibited LDL oxidation, reactive oxygen species generation and inflammation in vitro(18). Our previous experiments have also found that EAP and its main flavonoids, eupatilin and jaceosidin, reduced the plasma lipids levels as well as fasting blood glucose level in animals and subjects with type 2 diabetes (19–21). However, little is known about the antiplatelet and anticoagulation effects of EAP. Thus, we investigated whether EAP and its major flavonoids, eupatilin and jaceosidin, could modulate platelet activation and blood coagulation in vitro.
MATERIALS AND METHODS
Materials
A. princeps Pampanini was harvested in Ganghwa County and the leaves were collected from air-dried whole plants. The leaves were extracted with 70% ethanol, and concentrated under reduced pressure. The concentrated solution was freeze-dried to powder. After freezing at −40°C, the frozen matrix was dried from 0°C~30°C in a programmable freeze dryer (PVTFD 100R, Ilshin Lab., Gyeonggi, Korea). The yield of the ethanol extract of A. princeps Pampanini was 17.6%, producing 4.7 kg of dried ethanol extract from 26.67 kg of powdered leaves.
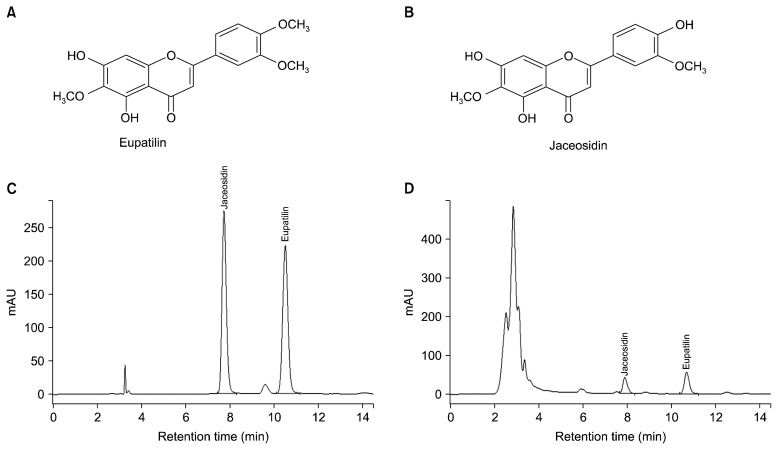
The quantities of the two major flavonoids, eupatilin and jaceosidin, present in the freeze-dried ethanol extract of the leaves were assayed by high-performance liquid chromatography on an Agilent 1100 series system (Agilent Technologies, Santa Clara, CA, USA) using a Venusil XBP C18 column (4.6×250 mm, film thickness 5 μm; Bonna-Agela Technologies, Wilmington, DE, USA). The mobile phase using isocratic elution was a solvent mixture of 65% methanol containing 0.1% trifluoroacetic acid. The flow rate was 1.0 mL/minute with an injection volume of 10 mL, and ultraviolet detection was performed at 330 nm. According to these measurements, 1 g of freeze-dried ethanol extract from the leaves contained 9.7 mg of eupatilin and 6.6 mg of jaceosidin. As shown in the HPLC chromatograms of mixed standard compounds and extract in Fig. 1, the quantification of the two major flavonoids was determined by the calibration curve. Eupatilin and Jaceosidin were purchased from NPC BioTech. (Daejon, Korea). EAP, eupatilin and jaceosidin were dissolved in dimethyl sulfoxide (DMSO) for the in vitro test.
Fig. 1.
Isolation of natural compounds jaceosidin and eupatilin. The chemical structures of eupatilin (A) and jaceosidin (B). Analytical HPLC chromatograph of mixed standard compound (C) and EAP (D).
Arachidonic acid, ADP and collagen were purchased from Chrono-log Corporation (Chicago, IL, USA). aPTT and PT reagents were purchased from Fisher Diagnostics (Middletown, VA, USA).
Preparation of human platelets
Whole blood was collected from 6 healthy male volunteers (20~35 years old) into 3.2% sodium citrate Vacutainer (Becton-Dikinson, Franklin Lakes, NJ, USA) and heparin Vacutainer (Becton-Dikinson) blood collection tubes, separately. The citrated whole blood was centrifuged for 10 min at 1,000×g. The supernatant fraction platelet-rich plasma (PRP) was used for the aggregation study. One-half of the PRP fraction was further centrifuged for 5 min at 2,000×g at room temperature and its supernatant was obtained as platelet-poor plasma (PPP). A small aliquot of fresh PPP was used to measure AA-, ADP- and collagen-induced platelet aggregation. The rest of the PRP fraction was stored at −80°C until the anticoagulant activity and serotonin concentration were measured. The heparin-treated blood was centrifuged for 10 min at 1,000×g, and the resultant plasma was then stored at −80°C until the TXA2 concentration was measured. All experiments were conducted at least four times. Experiments were performed according to the guidelines of the Ethics Committee of Kyungpook National University.
In vitro platelet aggregation assay
Platelet aggregation was determined by the turbidimetry method using an aggregometer (Chrono-Log Co., Havertown, PA, USA). The PRP (250 μL) was pre-incubated with various concentrations of EAP, eupatilin and jaceosidin for 10 min at 37°C, and platelet aggregation was stimulated by adding collagen (2 μg/mL), ADP (5 μM) and arachidonic acid (AA, 0.5 mM). The platelet aggregation was monitored for 7 min using an aggregometer with constant stirring at 1,000 rpm and expressed as the percent change in light transmission. Light transmission of PRP represented 0% and PPP represented 100%. This measurement for platelet aggregation was performed in less than 2 h.
Measurement of serotonin generation
The serotonin concentration was measured using a commercial serotonin ELISA kit (MyBioSource, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, after preincubation of PRP with EAP, eupatilin or jaceosidin for 20 min at 37°C, PRP was acylated by the acylation reagent, and the acylated plasma (25 μL) and serotonin antiserum (100 μL) were incubated for 30 min at room temperature. After washing the plates, a conjugate (anti-rabbit IgG conjugated with peroxidase) (100 μL) was added into the plate and further incubated for 15 min at room temperature. Thereafter, substrate (tetramethylbenzimide, TMB) (100 μL) for ELISA detection and stop solution (0.25 M H2SO4) were added and the absorbance was monitored at 450 nm for 5 min.
Measurement of TXB2 generation
The generation of TXA2 in platelets was measured by determining the TXB2 concentration, because TXA2 is unstable and is quickly converted to TXB2, using a commercial TXB2 ELISA kit (MyBioSource). After a suspension of PRP was preincubated with various concentrations of EAP, eupatilin and jaceosidin for 20 min at 37°C, the plasma in the plates was incubated for 2 h at 37°C. Antibodies (Biotin-antibody) (100 μL) were added to the mixture and incubated for 1 hour at 37°C. Then, horseradish peroxidase (100 μL) was added and further incubated for 1 hour at 37°C. Thereafter, the substrate (tetramethylbenzimide, TMB) (100 μL) was added to the mixture. H2SO4 was added to stop TXA2 generation and the stable metabolite of TXA2 was determined according to the manufacturer’s instructions.
In vitro anticoagulation assay
The activated partial thromboplastin time (aPTT) and prothrombin time (PT) were determined using a Thrombotimer (Behnk Elektronik, Norderstedt, Germany) according to the manufacturer’s instructions. In brief, PRP (90 μL) was incubated with various concentrations of EAP, eupatilin or jaceosidin (10 μL) for 3 min at 37°C and then aPTT reagent (100 μL) was added to the mixture and incubated for 3 min at 37°C. Thereafter, 20 μM CaCl2 (100 μL) was added and the clotting time was recorded. For the PT assay, PRP (90 μL) was incubated with various concentrations of EAP, eupatilin or jaceosidin (10 μL) for 3 min at 37°C. PT reagent was then added and the clotting time was recorded. PBS (phosphate buffered saline) was used as a control.
Statistical analysis
All data were expressed as the means±standard error of mean (SEM) of at least four different experiments. SPSS (version 18, SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis. Significant differences between groups were analyzed with the Student’s t-test. Differences were considered to be statistically significant when P<0.05.
RESULTS
Effects of EAP and its major flavonoids, eupatilin and jaceosidin, on antiplatelet aggregation in vitro
We evaluated the antiplatelet activity of EAP and its major flavonoids against AA (0.5 mM), ADP (5 μM) and collagen (2 μg/mL)-stimulated platelet aggregation (Table 1). The AA-induced platelet aggregation was significantly reduced by EAP (1 mg/mL), eupatilin (10 μM) and jaceosidin (10 μM). However, ADP- and collagen-induced platelet aggregation was not altered by EAP and its major flavonoids.
Table 1.
Effects of EAP and its major flavonoids on AA-, ADP- and collagen-induced platelet aggregation
| Samples1) | Agonist-induced platelet aggregation (%)2) | ||
|---|---|---|---|
|
| |||
| AA | ADP | Collagen | |
| Control | 86.5±5.80 | 81.5±0.50 | 87.0±1.73 |
| EAP | 1.0±0.00*** | 74.5±6.50 | 85.7±2.08 |
| Eupatilin | 0.7±0.58*** | 78.0±12.00 | 83.0±1.00 |
| Jaceosidin | 1.0±0.00*** | 85.5±0.50 | 83.7±0.58 |
Data represent the mean±SEM.
P<0.001 vs. control, DMSO was used as the control.
EAP, ethanol extract of Artemisia princeps Pampanini, 1.0 mg/mL; eupatilin, 10 μM; jaceosidin, 10 μM.
Concentration of agonists: AA, 0.5 mM; ADP, 5 μM; collagen, 10 μg/mL. Normal range of agonist-induced platelet aggregation (%): AA, 74~99; ADP, 69~88, collagen, 70~94.
AA, arachidonic acid; ADP, adenosine diphosphate.
Effects of EAP and its major flavonoids, eupatilin and jaceosidin, on serotonin generation
The serotonin concentration was measured to investigate whether EAP and its major flavonoids could attenuate serotonin generation. As shown in Fig. 2, EAP at concentrations of 1 and 2 mg/mL significantly and dose-dependently attenuated serotonin generation. Moreover, both eupatilin and jaceosidin significantly inhibited serotonin generation at or greater than 0.5 μM in a dose-dependent manner.
Fig. 2.
Effect of EAP and its major flavonoids on serotonin generation. Plasma was preincubated with various concentrations of EAP and its major flavonoids for 20 min at 37°C. The serotonin content was determined using an ELISA kit. (A) EAP (B) major flavonoids of EAP, eupatilin and jaceosidin. Data are the mean±SEM. **P<0.01 vs. concentration of 0 μM. EAP, ethanol extract of Artemisia princeps Pampanini.
Effects of EAP and its major flavonoids, eupatilin and jaceosidin, on TXB2 generation
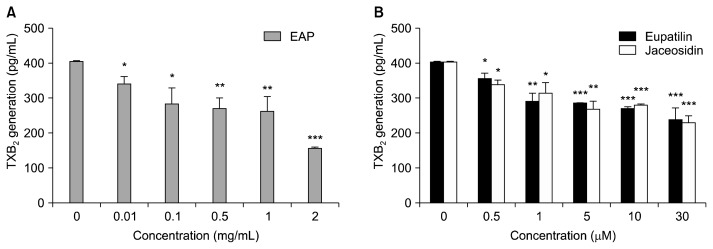
EAP and its major flavonoids were tested to determine whether they could inhibit the generation of TXB2. EAP significantly inhibited TXB2 generation at concentrations of 0.01 and 2 mg/mL significantly and dose-dependently (Fig. 3). Jaceosidin and eupatilin, the major bioflavonoids of EAP, also significantly inhibited TXB2 generation at concentrations higher than 0.5 μM.
Fig. 3.
Effect of EAP and its major flavonoids on TXB2 generation. Plasma was preincubated with various concentrations of EAP and its major flavonoids for 20 min at 37°C. TXB2 content was determined using a TXB2 ELISA kit. (A) EAP (B) major flavonoids of EAP, eupatilin and jaceosidin. Data are the mean±SEM. *P<0.05, **P<0.01, ***P<0.001 vs. concentration of 0 μM. EAP, ethanol extract of Artemisia princeps Pampanini; TXB2, thromboxane B2.
Effects of EAP and its major flavonoids, eupatilin and jaceosidin, on anticoagulant activity
To determine whether EAP and its major flavonoids have anticoagulant properties, PT and aPTT were evaluated using human plasma. EAP significantly prolonged PT and aPTT at or greater than 0.1 mg/mL in a dose-dependent manner (Table 2). Among the major flavonoids of EAP, eupatilin significantly and dose-dependently prolonged aPTT and PT (Table 3). However, jaceosidin only significantly prolonged PT at concentrations of 0.5, 1 and 30 μM.
Table 2.
Effects of EAP on anticoagulant activity based on PT and aPTT
| Sample | Concentration | PT (s) | aPTT (s) |
|---|---|---|---|
| PBS1) | 11.2±0.00 | 26.3±1.6 | |
| EAP | 0.1 mg/mL | 13.4±0.06*** | 36.2±2.46** |
| 0.25 mg/mL | 13.4±0.35*** | 36.5±2.13** | |
| 0.5 mg/mL | 13.9±0.00*** | 37.9±1.63** | |
| 1 mg/mL | 14.2±0.60*** | 38.4±1.03*** | |
| 2 mg/mL | 14.8±0.00*** | 40.5±1.74** | |
| Heparin2) | 20 nM | 43.9±1.80*** | >200*** |
Data represent the mean±SEM.
P<0.01,
P<0.001 vs. control.
Phosphate buffered saline (PBS) was used as the control.
Heparin was used as the positive control.
EAP, ethanol extract of Artemisia princeps Pampanini; PT, prothrombin time; aPTT, activated partial thromboplastin time.
Table 3.
Effects of the major flavonoids of EAP on anticoagulant activity based on PT and aPTT
| Sample | Concentration | PT (s) | aPTT (s) |
|---|---|---|---|
| PBS1) | 12.7±0.05 | 30.7±0.90 | |
| Jaceosidin | 0.5 μM | 13.0±0.20* | 31.5±0.17 |
| 1 μM | 13.4±0.37** | 31.0±0.72 | |
| 5 μM | 13.0±0.34 | 29.3±1.24 | |
| 10 μM | 12.8±0.34 | 30.1±0.58 | |
| 30 μM | 13.2±0.26* | 29.9±0.15 | |
| Eupatilin | 0.5 μM | 13.3±0.46** | 34.2±0.17** |
| 1 μM | 13.5±0.26** | 34.4±0.05** | |
| 5 μM | 13.6±0.32* | 36.4±0.11** | |
| 10 μM | 13.6±0.29*** | 37.6±0.15** | |
| 30 μM | 13.7±0.23*** | 38.8±0.10** | |
| Heparin2) | 20 nM | 43.9±1.80*** | >200*** |
Data represent the mean±SEM.
P<0.05,
P<0.01,
P<0.001 vs. control.
Phosphate buffered saline (PBS) was used as the control.
Heparin was used as the positive control.
PT, prothrombin time; aPTT, activated partial thromboplastin time.
DISCUSSION
In recent years, some herbal medicines have been considered as potential novel antiplatelet and anticoagulant agents, and much attention has been focused on the identification of plant-based materials with antiplatelet and anticoagulant activities. In the present study, the antiplatelet and anticoagulant properties of EAP and its major flavonoids were examined. We first demonstrated that EAP and its major flavonoids, eupatilin and jaceosidin, can inhibit platelet activation and coagulation in vitro.
Abnormal platelet activation and aggregation play an important role in the development of thrombosis (1,2). After vascular injury, platelet adhesion occurs at the site of vascular injury, which triggers platelet activation and subsequent thrombus formation via aggregation (4). The process of platelet activation and aggregation can be enhanced and amplified by the cooperative actions of multiple factors, including AA, ADP, collagen, serotonin and TXA2. Among them, TXA2 and serotonin are released from activated platelets promoting the activation and recruitment of additional platelets. Thus, the inhibition of TXA2 and serotonin generation can lead to anti-thrombosis (22,23). In the present study, we found that EAP and its major flavonoids, eupatilin and jaceosidin, significantly attenuated the generation of TXB2, a stable form of TXA2, and serotonin in a dose-dependent manner. In addition, eupatilin and jaceosidin, as well as EAP, markedly inhibited AA-induced platelet aggregation; however, they exhibited a relatively weak inhibitory effect on collagen and ADP-induced platelet aggregation, suggesting their selective inhibitory activity for the AA-induced platelet activation pathways. AA is a precursor of TXA2, prostaglandin and other eicosanoids. When exogenously applied to platelets, AA is metabolized to TXA2 by cyclooxygenase (COX) (24). Aspirin, a well-known antiplatelet agent, is reported to inhibit platelet TXA2 generation and AA-induced platelet aggregation by inhibiting COX (25). Several vegetables, such as spinach, garlic bolt, blanched garlic and Chinese leek, also inhibit COX and human platelet aggregation induced by AA in vitro(26). A number of polyphenols, including eupatilin and jaceosidin, are known inhibitors of COX-1 or COX-2 (27,28). Thus, these data suggest that the inhibition of TXA2 generation and AA-induced platelet aggregation in presence of EAP could be mediated through the suppression of platelet COX activity, although we did not determine this activity. Also plausible is that eupatilin and jaceosidin are major bioactive components, which may contribute to the antiplatelet aggregation activity of EAP.
Blood coagulation and platelets are mutually dependent in their interactive processes of hemostasis and thrombosis. Many coagulation factors appear to bind to platelets and induce platelet activation during initiation of coagulation (29). The concerted actions of coagulation factors on the platelet surface cause thrombin formation, so that a stable fibrin clot can be formed (30,31). Accordingly, assessment of coagulation activation as well as platelet activation and aggregation is a strategy to examine thrombotic risk and blood circulation (29). The aPTT and PT are by far the most common screening tests for coagulation abnormalities. Prolonged aPTT suggests inhibition of the intrinsic coagulation pathway, whereas prolonged PT suggests inhibition of the extrinsic coagulation pathway (32). We found that EAP as well as eupatilin prolonged the coagulation parameters such as PT and aPTT in vitro. Jaceosidin also significantly prolonged PT but did not affect aPTT. These results possibly indicate that eupatilin with or without jaceosidin may be responsible for the anticoagulant activity of EAP.
In conclusion, this study showed that EAP inhibited the intrinsic and extrinsic pathways of blood coagulation, AA-induced platelet aggregation and the generation of TXA2 and serotonin in vitro. Eupatilin and jaceosidin, flavonoids present in EAP, also exhibited antiplatelet and anticoagulant effects, indicating that these flavonoids, especially eupatilin, may be responsible for the antiplatelet and anticoagulant property of EAP. These results are also supported by the quantitative HPLC analysis of eupatilin and jaceosidin. As referred in materials, 1.0 mg/mL of EAP contains 9.7 μg/mL of eupatilin and 6.6 μg/mL of jaceosidin and the concentrations are converted into 28 μM and 20 μM in 1 g of EAP, respectively. Because the concentrations of tested samples are similar (0.5 μM~30 μM), EAP could possibly have synergistic effects compared to single compounds.
Together, our findings suggest that EAP may be useful in the prevention or improvement of vascular diseases, although further studies are required to elucidate the in vivo effects.
ACKNOWLEDGMENTS
This work was supported by High Value added Food Technology Development Program (No. 111125-3) from the Ministry for Food, Agriculture, Forestry and Fisheries and Kyungpook National University Research Fund (2012), Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Yu HY, Park SW, Chung IM, Jung YS. Anti-platelet effects of yuzu extract and its component. Food Chem Toxicol. 2011;49:3018–3024. doi: 10.1016/j.fct.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thrombo Haemost. 2009;102:248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 3.Cho HJ, Kittaka R, Abdou AM, Kim M, Kim HS, Lee DH, Park HJ. Inhibitory effects of oligopeptides from hen egg white on both human platelet aggregation and blood coagulation. Arch Pharm Res. 2009;32:945–953. doi: 10.1007/s12272-009-1618-y. [DOI] [PubMed] [Google Scholar]
- 4.Konkle BA. Bleeding and thrombosis. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. McGraw-Hill Professional; New-York, NY, USA: 2008. pp. 363–369. [Google Scholar]
- 5.Son DJ, Cho MR, Jin YR, Kim SY, Park YH, Lee SH, Akiba S, Sato T, Yun YP. Antiplatelet effect of green tea catechins: a possible mechanism through arachidonic acid path way. J PLEFA. 2003;71:25–31. doi: 10.1016/j.plefa.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Gopinath SCB. Anti-coagulant aptamers. Thromb Res. 2008;122:838–847. doi: 10.1016/j.thromres.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka KA, Key NS, Levy JH. Blood coagulation: Hemostasis and thrombin regulation. Anesth Analg. 2009;108:1433–1446. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 8.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinath SCB, Shikamoto Y, Mizuno H, Kumar PK. A potent anti-coagulant RNA aptamer inhibits blood coagulation by specifically blocking the extrinsic clotting pathway. Thromb Haemost. 2006;95:767–771. [PubMed] [Google Scholar]
- 10.Gopinath SC, Shikamoto Y, Mizuno H, Kumar PK. Snake-venom-derived Factor IX-binding protein specifically blocks the gamma-carboxyglutamic acid-rich-domain-mediated membrane binding of human Factors IX and X. Biochem J. 2007;405:351–357. doi: 10.1042/BJ20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett NE, Holbrook L, Jones S, Kaiser WJ, Moraes LA, Rana R, Sage T, Stanley RG, Tucker KL, Wright B, Gibbins JM. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154:918–939. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson SP, Schoenwaelder SM. Antiplatelet therapy: in search of the ‘magic bullet’. Nat Rev Drug Discov. 2003;2:775–789. doi: 10.1038/nrd1198. [DOI] [PubMed] [Google Scholar]
- 13.Walenga JM, Thethi I, Lewis BE. Nonhemostatic adverse effects of anticoagulants and antiplatelet agents. Semin Thromb Hemost. 2012;38:884–892. doi: 10.1055/s-0032-1325615. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Lim KM, Kim CW, Shin HJ, Seo DB, Lee SJ, Noh JY, Bae ON, Shin S, Chung JH. Black soybean extract can attenuate thrombosis through inhibition of collagen-induced platelet activation. J Nutr Biochem. 2011;22:964–970. doi: 10.1016/j.jnutbio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003;78:5445–5451. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- 16.Zhao QC, Kiyohara H, Yamada H. Anti-complementary neural polysaccharides from leaves of Artemisia princeps. Phytochemistry. 1993;35:73–77. doi: 10.1016/s0031-9422(00)90512-0. [DOI] [PubMed] [Google Scholar]
- 17.Han JM, Kim MJ, Baek SH, An S, Jin YY, Chung HG, Baek NI, Choi MS, Lee KT, Jeong TS. Antiatherosclerotic effects of Artemisia princeps Pampanini cv. Sajabal in LDL receptor deficient mice. J Agric Food Chem. 2009;57:1267–1274. doi: 10.1021/jf802639y. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Han JM, Jin YY, Baek NI, Bang MH, Chung HG, Choi MS, Lee KT, Sok DE, Jeong TS. In vitro antioxidant and anti-inflammtory acitivities of jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res. 2008;31:429–437. doi: 10.1007/s12272-001-1175-8. [DOI] [PubMed] [Google Scholar]
- 19.Jung UJ, Baek NI, Chung HG, Jeong TS, Lee KT, Lee MK, Choi MS. Antilipogenic and hypolipidemic effects of ethanol extracts from two variants of Artemisia princeps Pampanini in obese diabetic mice. J Med Food. 2009;12:1238–1244. doi: 10.1089/jmf.2009.0039. [DOI] [PubMed] [Google Scholar]
- 20.Kang YJ, Jung UJ, Lee MK, Kim HJ, Jeon SM, Park YB, Chung HG, Baek NI, Lee KT, Jeong TS, Choi MS. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic beta-cell function in type 2 diabetic mice. Diabetes Res Clin Pract. 2008;82:25–32. doi: 10.1016/j.diabres.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Choi JY, Shin SK, Jeon SM, Baek NI, Chung HG, Jeong TS, Lee KT, Lee MK, Choi MS. Dose-response study of sajabalssuk ethanol extract from Artemisia princeps Pampanini on blood glucose in subjects with impaired fasting glucose or mild type 2 diabetes. J Med Food. 2011;14:101–107. doi: 10.1089/jmf.2010.1266. [DOI] [PubMed] [Google Scholar]
- 22.Clutton P, Folts JD, Freedman JE. Pharmacological control of platelet function. Pharmacol Res. 2001;44:255–264. doi: 10.1006/phrs.2001.0861. [DOI] [PubMed] [Google Scholar]
- 23.Halushka PV, Allan CJ, Davis-Bruno KL. Thromboxane A2 receptors. J Lipid Mediat Cell Signal. 1995;12:361–378. doi: 10.1016/0929-7855(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 24.Horn PT, Kohli JD, LeBreton GC, Venton DL. Antagonism of prostanoid-induced vascular contraction by 13-azaprostanoic acid (13-APA) J Cardiovasc Pharmacol. 1984;6:609–613. doi: 10.1097/00005344-198407000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Maree AO, Fitzgerald DJ. Aspirin and coronary artery disease. Thromb Haemost. 2004;92:1175–1181. doi: 10.1160/TH04-02-0127. [DOI] [PubMed] [Google Scholar]
- 26.Wang XH, Shao DH, Liang GW, Zhang R, Xin Q, Zhang T, Cao QY. Cyclooxygenase inhibitors in some dietary vegetables inhibit platelet aggregation function induced by arachidonic acid. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:1260–1263. [PubMed] [Google Scholar]
- 27.Yoon JH, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46:585–596. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009;125:497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost. 2002;88:186–193. [PubMed] [Google Scholar]
- 30.Dormann D, Clemetson KJ, Kehrel BE. The GPIb thrombin-binding site is essential for thrombin-induced platelet procoagulant activity. Blood. 2000;96:2469–2478. [PubMed] [Google Scholar]
- 31.Brass LF. More pieces of the platelet activation puzzle slide into place. J Clin Invest. 1999;104:1663–1665. doi: 10.1172/JCI8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee W, Yang EJ, Ku SK, Song KS, Bae JS. Anticoagulant activities of oleanolic acid via inhibition of tissue factor expressions. BMB Rep. 2012;45:390–395. doi: 10.5483/bmbrep.2012.45.7.065. [DOI] [PubMed] [Google Scholar]