Abstract
In this study, we investigated the inhibitory effects of Baechu kimchi added Ecklonia cava on the activities of α-glucosidase and α-amylase and its alleviating effect on the postprandial hyperglycemia in STZ-induced diabetic mice. Baechu kimchi added Ecklonia cava (BKE, 15%) was fermented at 5°C for 28 days. Optimum ripened BKE was used in this study as it showed the strongest inhibitory activities on α-glucosidase and α-amylase by fermentation time among the BKEs in our previous study. The BKE was extracted with 80% methanol and the extract solution was concentrated, and then used in this study. The BKE extract showed higher inhibitory activities than Baechu kimchi extract against α-glucosidase and α-amylase. The IC50 values of the BKE extract against α-glucosidase and α-amylase were 0.58 and 0.35 mg/mL, respectively; BKE exhibited a lower α-glucosidase inhibitory activity but a higher α-amylase inhibitory activity than those of acarbose. The BKE extract alleviated postprandial hyperglycemia caused by starch loading in normal and streptozotocin-induced diabetic mice. Furthermore, the BKE extract significantly lowered the incremental area under the curve in both normal and diabetic mice (P<0.05). These results indicated that the BKE extract may delay carbohydrate digestion and thus glucose absorption.
Keywords: Baechu kimchi, Ecklonia cava, α-glucosidase, α-amylase, postprandial hyperglycemia
INTRODUCTION
Diabetes mellitus has become an abiding interest of all modern societies with recent increases in the prevalence of risk factors such as abdominal obesity, dislipidemia and hypertension. The prevalence of diabetic patients worldwide has dramatically increased due to modern lifestyle and an increase of consumption in high carbohydrate diets (1). Diabetes sufferers have high blood glucose levels due to either not enough insulin production or cells of the liver, muscles and fat tissue do not respond to insulin in a normal way (2). One important approach for suppressing postprandial hyperglycemia is to reduce or slow dietary carbohydrate digestion and absorption. Starch digestion primarily occurs in the small intestine through the action of pancreatic α-amylase, yielding both linear maltose and branched isomaltose oligosaccharides, which are further hydrolyzed by intestinal α-glucosidase (3). By inhibiting the enzymes involved in carbohydrate digestion, postprandial hyperglycemia can be significantly delayed, which may have beneficial effects on insulin resistance and glycemic index control in diabetic patients (4). Current pharmacotherapeutics insufficiently reverse hyperglycemia, have limited tolerability and induced side effects (5). Hence, much effort has been extended in search of effective α-amylase and α-glucosidase inhibitors from natural sources in order to develop physiologically functional foods and introduce natural medicines (6,7).
Ecklonia cava, a marine brown alga, is widely distributed in the subtidal zone off the southern coasts of Korea and Japan and is plentifully produced, about 30,000 tons per year, for commercial purpose on Jeju Island of Korea. E. cava is utilized as food ingredients, animal feed, fertilizers as well as raw materials to produce fucoidanor phlorotannin. According to numerous investigations. Ecklonia cava exhibits various bioactivities, such as anti-allergic, antiviral, and anti-diabetic (8–10).
Baechu kimchi is a salt fermented cabbage widely consumed in traditional Korean foods. Various unique microorganisms and bioactive components present in Baechu kimchi show antioxidant ability (11), an elevated immune response (12) and anti-cancer (13) and anti-diabetic effects (14). A previous study indicated that the supplement of Baechu kimchi improved fasting blood glucose and insulin resistance in a high-fat diet-fed rat model (14). However, a research of Baechu kimchi about the inhibitory effect of α-glucosidase and postprandial hyperglycemia has not been carried out yet. Thus, this study was conducted to investigate the inhibitory effects of Baechu kimchi added Ecklonia cava on the activities of α-glucosidase and α-amylase and its alleviating effect on postprandial hyperglycemia in STZ-induced diabetic mice.
MATERIALS AND METHODS
Materials
Ecklonia cava was collected along the coast of Jeju Island, Korea. The samples were washed three times with tap water to remove the salt, epiphytes, and sand attached to the surface, then carefully rinsed with fresh water. Ecklonia cava was boiled with 0.3% citric acid in water for 10 min for softening. Baechu (Korean cabbage) was divided in four pieces and then 3% salt (w/w) compared to Baechu was evenly sprinkled over the cabbage. Spliced Baechu was pickled in 10% salty water until reaching salinity at 1.8±0.1. After the salted cabbages were washed twice under running water, it was naturally dehydrated for 2 h. Baechu kimchi was prepared by mixing salted cabbage with spice ingredients and 15% of Ecklonia cava (Table 1). Baechu kimchi added Ecklonia cava (BKE) was fermented at 5°C for 28 days. Each sample was made with fresh, optimum ripened and over ripened BKE. Optimum ripened BKE (pH 4.28, acidity 0.71), which had been fermented for 20 days at 5°C, was used in this study since optimum ripened BKE had the strongest inhibitory activities against α-glucosidase and α-amylase by fermentation time among the BKEs in our previous study. The BKE was extracted with 80% methanol and the extracts solution was concentrated. Then, dried BKE extract was stored in a deep freezer (−80°C).
Table 1.
Recipes of Baechu kimchi added Ecklonia cava and Baechu kimchi used in this study
| Ingredients | Baechu kimchi added Ecklonia cava (%) | Baechu kimchi (%) |
|---|---|---|
| Korean cabbage | 85.00 | 97.75 |
| Ecklonia cava | 12.75 | - |
| Green onion | 2.30 | 2.30 |
| Garlic | 2.50 | 2.50 |
| Ginger | 0.50 | 0.50 |
| Red pepper powder | 2.60 | 2.60 |
| Fermented shrimp juice | 1.70 | 1.70 |
| Sand eel fermented juice | 1.30 | 1.30 |
| Glutinous rice paste | 3.60 | 3.60 |
| Sugar | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
Inhibition assay for α-glucosidase activity in vitro
The α-glucosidase inhibitory assay was done by the chromogenic method developed by Watanabe et al. using a readily available yeast enzyme (15). Briefly, yeast α-glucosidase (0.7 U, Sigma, St. Louis, MO, USA) was dissolved in 100 mM phosphate buffer (pH 7.0) containing 2 g/L bovine serum albumin and 0.2 g/L NaN3 and used as an enzyme solution. 5 mM p-nitrophenyl-α-D-glucopyranoside in the same buffer (pH 7.0) was used as a substrate solution. The 50 μL of enzyme solution and 10 μL of sample dissolved in dimethylsulfoxide at a 5 mg/mL concentration were mixed in a well, and absorbance at 405 nm was measured using a microplate reader (Medel 680, BioRad, Hercules, CA, USA). After incubation for 5 min, substrate solution (50 μL) was added and incubated for another 5 min at room temperature. The increase in absorbance from zero time was measured. Inhibitory activity was expressed as 100 minus relative absorbance difference (%) of test compounds to absorbance change of the control, where carrier solvent replaced the test solution. The measurements were performed in triplicate and IC50 value, i.e., the concentration of the extracts that results in 50% inhibition of maximal activity, was determined.
Inhibition assay for α-amylase activity in vitro
The α-amylase inhibitory activity was assayed in the same way (15) as described for the α-glucosidase inhibitory assay above, except that porcine pancreatic amylase (100 U, Sigma) and blocked p-nitrophenyl-α-D-malto-pentoglycoside (Sigma) were used as enzyme and substrate, respectively.
Experimental animals
Four-week old male mice (ICR, Orient Bio Inc., Seoul, Korea) were kept under a 12 h light/12 h dark cycle with room temperature controlled. The animals were maintained with pelleted food, while tap water was ad libitum. After an adjustment period of 2 weeks, diabetes was induced by intraperitoneal injection of STZ (60 mg/kg) freshly dissolved in citrate buffer (0.1 M, pH 4.5) in the fasted (18 h) animals. After seven days, tail bleeds were performed and animals with a blood glucose concentration above 300 mg/dL were considered to be diabetic.
Measurement of blood glucose level
Both normal mice and STZ-induced diabetic mice fasted overnight were randomly divided into four groups. Fasted animals were deprived of food for at least 12 h but allowed free access to water. After overnight fasting, the mice were administered orally either soluble starch (2 g/kg body weight) alone (control) or starch with BKE extract (300 mg/kg body weight). Blood samples were taken from the tail vein at 0, 30, 60, and 120 min. Blood glucose was measured using a glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Areas under the curve (AUC) were calculated using the trapezoidal rule (16). All procedures were approved by the animal ethics committee of our university (PNU-2012-0039).
Data statistical analysis
The data were represented as mean±SD. The statistical analysis was performed using SAS 9.1 software (SAS Institute Inc, Cary, NC, USA). The Student’s t-test was used for comparisons between control and sample groups. The values were evaluated by one-way analysis of variance (ANOVA) followed by post-hoc Duncan’s multiple range tests.
RESULTS AND DISCUSSION
Inhibitory effect of BKE extract on α-glucosidase and α-amylase in vitro
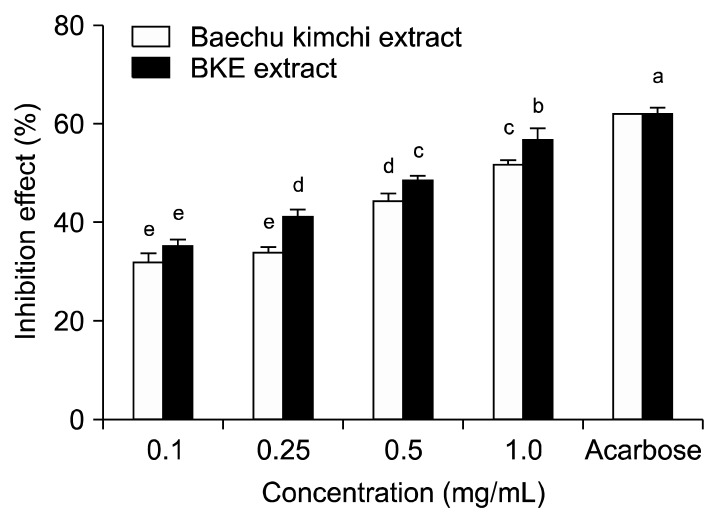
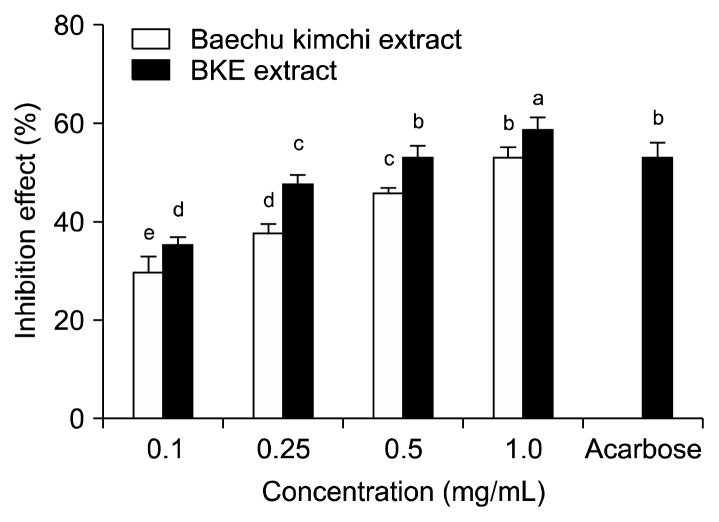
The inhibitory effect of Baechu kimchi added Ecklonia cava (BKE) extract against α-glucosidase is shown in Fig. 1. BKE extract inhibited α-glucosidase activity in a dose dependent manner (35.43, 41.23, 48.71 and 56.92% at 0.1, 0.25, 0.5, and 1.0 mg/mL concentrations, respectively). Moreover, α-glucosidase inhibitory activity of BKE extract was higher than that of Baechu kimchi extract. Acarbose, an α-glucosidase inhibitor, used as an oral hypoglycemic agent, inhibited the enzyme activity by 62.03% at a concentration of 0.5 mg/mL. The inhibitory effect of BKE extract against α-amylase is shown in Fig. 2. The BKE extract inhibited α-amylase by 35.24, 47.81, 53.13 and 58.96% at concentrations of 0.1, 0.25, 0.5, and 1.0 mg/mL, respectively. The BKE extract inhibited the α-amylase more effectively than Baechu kimchi extract. The α-amylase inhibitory activity of BKE extractat the concentration of 0.5 mg/mL was similar to that of acarbose at the same concentration. The IC50 values of the BKE extract against α-glucosidase and α-amylase were 0.58 and 0.35 mg/mL, respectively, which were stronger than those of Baechu kimchi extract. Further, the IC50 values of the BKE extract showed a lower α-glucosidase inhibitory activity but a higher α-amylase inhibitory activity compared to acarbose (Table 2).
Fig. 1.
Inhibitory activity of BKE extract on α-glucosidase. Each value is expressed as mean±SD in triplicate experiments. a–eValues with different alphabets are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. The concentration of acarbose used as positive control was 0.5 mg/mL. BKE extract: Baechu kimchi added Ecklonia cava extract.
Fig. 2.
Inhibitory activity of BKE extract on α-amylase. Each value is expressed as mean±SD in triplicate experiments. a–eValues with different alphabets are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. The concentration of acarbose used as positive control was 0.5 mg/mL. BKE extract: Baechu kimchi added Ecklonia cava extract.
Table 2.
IC50 values for the inhibitory effects of BKE extract on α-glucosidase and α-amylase
| Sample | IC50 (mg/mL)1) | |
|---|---|---|
|
| ||
| α-Glucosidase | α-Amylase | |
| BKE extract | 0.58±0.01b | 0.35±0.01c |
| Baechu kimchi extract | 0.88±0.02a | 0.80±0.02a |
| Acarbose | 0.34±0.02C | 0.45±0.04b |
IC50 value is the concentration of sample required for 50% inhibition. Each value is expressed as mean±SD (n=3).
Values with different alphabets in a column are significantly different at P<0.05 as analyzed by Duncan’s multiple range test.
BKE extract: Baechu kimchi added Ecklonia cava extract.
Starch digestion primarily occurs in the small intestine through the action of pancreatic α-amylase, yielding both linear maltose and branched isomaltose oligosaccharides, which are further hydrolyzed by intestinal α-glucosidases to release absorbable monosaccharides. The α-glucosidase inhibitors impede carbohydrate hydrolysis by inhibiting glucosidase enzymes in the brush border of the small intestine. The low speed of digestion and absorption of carbohydrates reduces the postprandial rise in plasma glucose (17). Thus the α-amylase and α-glucosidase inhibitors prevent high glucose concentration in the blood after a meal (3). Controlling both fasting and postprandial hyperglycemia are the major targets of diabetic therapy (18). Previously known, postprandial hyperglycemia might have a stronger correlation with cardiovascular morbidity and mortality than fasting hyperglycemia (19). Hence, the decrease in postprandial hyperglycemia by retarding the absorption of glucose through the inhibition of carbohydrate-hydrolyzing enzymes, such as α-amylase and α-glucosidase, would play a key role in the control of diabetes (20,21).
Baechu kimchi contains several ingredients in addition to Chinese cabbage, such as onion, ginger, garlic, and red pepper, which have proven hypoglycemic (22,23), insulinotropic (24) and antidiabetic effects (25,26). Our data showed that BKE extract had higher inhibitory activities than Baechu kimchi extract on α-glucosidase and α-amylase, suggesting that addition the of E. cava in Baechu kimchi may delay the digestion and absorption of carbohydrates.
Effect of BKE extract on blood glucose level in vivo
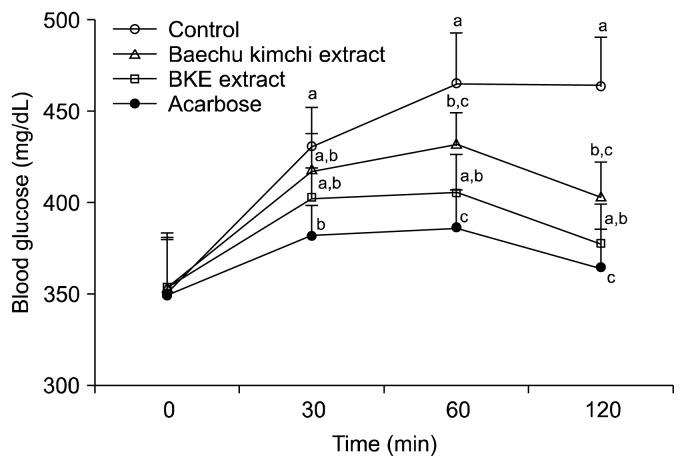
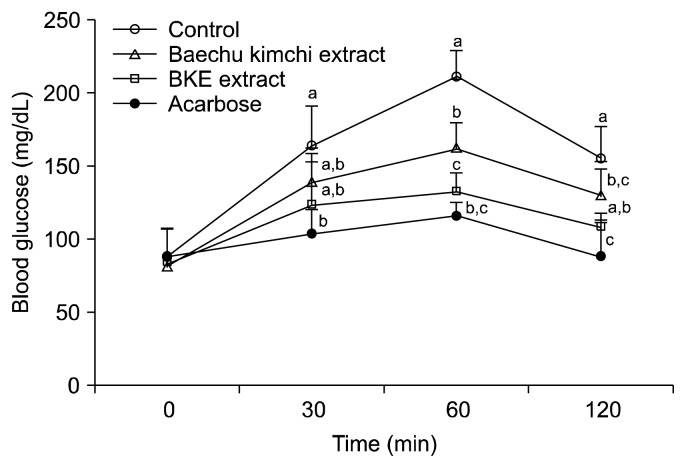
The alleviating effect of BKE extract on hyperglycemia after a meal was investigated in STZ-induced diabetic and normal mice. Postprandial blood glucose levels in the BKE extract-administered diabetic mice were significantly lower than those of the mice in the control group (Fig. 3). The blood glucose levels of diabetic mice in the control group were 430.5, 465.0 and 464.0 mg/dL at 30, 60 and 120 min, respectively; however, the increase in postprandial blood glucose levels was significantly reduced (P<0.05) in diabetic mice administered BKE extract (402.4, 405.0 and 377.0 mg/dL at the same respective time points), which were lower than those of the Baechu kimchi extract. The postprandial blood glucose level was also significantly decreased when normal mice were orally administered starch with BKE extract (Fig. 4). The area under the two-hour blood glucose response curve (AUC) of the BKE extract-administered group (790.08±69.22 mg·h/dL) was significantly lower (P<0.05) than the control group (883.50±71.08 mg·h/dL) in the diabetic mice (Table 3).
Fig. 3.
Blood glucose levels after the administration of BKE extract in streptozotocin-induced diabetic mice. Control (distilled water), BKE extract (300 mg/kg), Baechu kimchi (300 mg/kg) and acarbose (100 mg/kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of seven mice (n=21). a–cValues with different alphabets are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. BKE extract: Baechu kimchi added Ecklonia cava extract.
Fig. 4.
Blood glucose levels after the administration of BKE extract in normal mice. Control (distilled water), BKE extract (300 mg/kg), Baechu kimchi (300 mg/kg) and acarbose (100 mg/kg) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of seven mice (n=21). a–cValues with different alphabets are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. BKE extract: Baechu kimchi added Ecklonia cava extract.
Table 3.
Area under the curve (AUC) of postprandial glucose responses of normal and streptozotocin-induced diabetic mice
| Group1) | AUC (mg·h/dL) | |
|---|---|---|
|
| ||
| Normal mice | Diabetic mice | |
| BKE extract | 236.01±62.33b | 790.08±69.22ab |
| Baechu kimchi extract | 275.75±31.75b | 836.65±58.99ab |
| Acarbose | 204.68±33.35b | 749.10±32.76b |
| Control | 339.98±4.23a | 883.50±71.08a |
Baechu kimchi added Ecklonia cava extract (BKE extract, 300 mg/kg), Baechu kimchi extract (300 mg/kg), acarbose (100 mg/kg), and control (distilled water) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of 7 mice (n=42).
Values with different alphabets in a column are significantly different at P<0.05 as analyzed by Duncan’s multiple range test.
Postprandial hyperglycemia is the earliest metabolic abnormality to take place in type 2 diabetes (27). The treatment goal for patients with type 2 diabetes is generally agreed to maintain near-normal levels of glycemic control, both in the fasting and postprandial states (28). Fig. 3 and 4 suggest that the hypoglycemic effect of BKE extract was higher than that of Baechu kimchi extract on starch loading. The increase in postprandial blood glucose level was significantly suppressed by BKE extract administration in both STZ-induced diabetic and normal mice. These results indicate that BKE extract may delay the absorption of dietary carbohydrates consumed, resulting in suppressing an increase in postprandial blood glucose levels. Management of diabetes is not only related to the increased release of insulin and its action for glycemic control, but also important in controlling the glycemic index and postprandial hyperglycemia (29). Postprandial hyperglycemia is involved in a variety of metabolic disorders and other diseases such as cardiac dysfunction (30) and cancer (31). Since α-glucosidase inhibitors delay the rate of transition from disaccharide to monosaccharide, the postprandial blood glucose is maintained at a lower level (32). Acarbose, an anti-diabetic drug used to treat type 2 diabetes by inhibiting the α-glucosidase in the brush border of the small intestine, has demonstrated that long-term treatment decreases the risk of diabetes, hypertension, and cardiovascular diseases (33). Interestingly, acarbose treatment helps to reduce progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance, a state of high risk for diabetes and atherosclerosis (34). Despite the multiple benefits of acarbose, this drug causes gastrointestinal problems such as sour stomach, belching, nausea, vomiting, indigestion, and diarrhea (35). Therefore, a necessary need exists to discover and develop safe and more effective antidiabetic agents. Lately, alternative therapies, including the functional food for treating diabetes, is showing a growing interest (36).
In conclusion, the present study demonstrates that BKE extract alleviated postprandial hyperglycemia by inhibiting the activities of α-glucosidase and α-amylase in STZ-induced diabetic mice. Thus, BKE extract may be helpful in improving blood glucose level after a meal.
ACKNOWLEDGMENTS
This study was supported by Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, which is gratefully appreciated.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 2.Muoio DM, Newgard CB. Obesity-related derangement in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 3.Akkarachiyasit S, Charoenlertkul P, Yibchok-Anun S, Adisakwattana S. Inhibitory activities of cyaniding and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int J Mol Sci. 2010;11:3387–3396. doi: 10.3390/ijms11093387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron AD. Postprandial hyperglycemic and alpha-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40:51–55. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 5.Eurich DT, McAlicter FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, Johnson JA. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito N, Sakai H, Suzuki S, Sekihara H, Yajima Y. Effect of an alpha-glucosidase inhibitor (voglibose), in combination with sulphonylureas, on glycaemic control in type 2 diabetes patients. J Int Med Res. 1998;26:219–232. doi: 10.1177/030006059802600501. [DOI] [PubMed] [Google Scholar]
- 7.Stand E, Baumgartl HJ, Fuchtenbusch M, Stemplinger J. Effect of acarbose on addition insulin therapy in type 2 diabetes patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999;1:215–220. doi: 10.1046/j.1463-1326.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Lee SH, Le QT, Kim MM, Kim SK. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcɛRI. J Agric Food Chem. 2008;56:12073–12080. doi: 10.1021/jf802732n. [DOI] [PubMed] [Google Scholar]
- 9.Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH, Kim TG, Kim SH, Kim NG, Hun H, Kim J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga, Ecklonia cava. Biol Pharm Bull. 2004;27:544–547. doi: 10.1248/bpb.27.544. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Li Y, Karadeniz F, Kim MM, Kim SK. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J Sci Food Agric. 2009;89:1552–1558. [Google Scholar]
- 11.Park JM, Shin JH, Gu JG, Yoon SJ, Song JC, Jeon WM, Suh HJ, Chang UJ, Yang CY, Kim JM. Effect of antioxidant activity in kimchi during a short-term and over-ripening fermentation period. J Biosci Bioeng. 2011;112:356–359. doi: 10.1016/j.jbiosc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Kim MJ, Kwon MJ, Song YO, Lee EK, Youn HJ, Song YS. The effects of kimchi on hematological and immunological parameters in vivo and in vitro. J Korean Soc Food Sci Nutr. 1997;26:1208–1214. [Google Scholar]
- 13.Nan HM, Park JW, Song YJ, Yun HY, Park JS, Hyun T, Youn SJ, Kim YD, Kang JW, Kim H. Kimchi and soybean pastes are risk factors of gastric cancer. World J Gastroenterol. 2005;11:3175–3181. doi: 10.3748/wjg.v11.i21.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam MS, Choi H. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabetes model of rats. J Med Food. 2009;12:292–297. doi: 10.1089/jmf.2008.0181. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of alpha-glucosidase inhibitors from tochu-cha (Eucommia ulmoides) Biosci Biotechnol Biochem. 1997;61:177–178. doi: 10.1271/bbb.61.177. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS. Effect of Rhemanniae radix on the hyperglycemic mice induced with streptozotocin. J Korean Soc Food Sci Nutr. 2004;33:1133–1138. [Google Scholar]
- 17.Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163:1306–1316. doi: 10.1001/archinte.163.11.1306. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004;164:486–491. doi: 10.1001/archinte.164.5.486. [DOI] [PubMed] [Google Scholar]
- 19.Haller H. The clinical importance of postprandial glucose. Diabetes Res Clin Pract. 1998;40:43–49. doi: 10.1016/s0168-8227(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 20.Bhandari MR, Anurakkun NJ, Hong G, Kawabata J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem. 2008;106:247–252. [Google Scholar]
- 21.Puls W, Keup U, Krause HP, Thomas G, Hoffmeister F. Glucosidase inhibition. A new approach to the treatment of diabetes, obesity, and hyperlipoproteinaemia. Naturwissenschaften. 1977;64:536–537. doi: 10.1007/BF00483562. [DOI] [PubMed] [Google Scholar]
- 22.Babu PS, Srinivasan K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol Cell Biochem. 1997;175:49–57. doi: 10.1023/a:1006881027166. [DOI] [PubMed] [Google Scholar]
- 23.El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Akhani SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2004;56:101–105. doi: 10.1211/0022357022403. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya K, Ohtani A, Kusano S. Enhancement of insulin sensitivity in adipocytes by ginger. Biofactors. 2004;22:153–156. doi: 10.1002/biof.5520220130. [DOI] [PubMed] [Google Scholar]
- 26.Tolan I, Ragoobirsing D, Morrison EY. The effect of capsaicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother Res. 2001;15:391–394. doi: 10.1002/ptr.750. [DOI] [PubMed] [Google Scholar]
- 27.Lebovitz HE. Postprandial hyperglycemic state: importance and consequences. Diabetes Res Clin Pract. 1998;40:27–28. [PubMed] [Google Scholar]
- 28.Ratner RE. Controlling postprandial hyperglycemia. Am J Cardiol. 2001;88:26–31. doi: 10.1016/s0002-9149(01)01834-3. [DOI] [PubMed] [Google Scholar]
- 29.Mosihuzzman M, Naheed S, Hareem S, Talib S, Abbas G, Khan SN, Choudhary MI, Sener B, Tareen RB, Israr M. Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci. 2013;92:187–192. doi: 10.1016/j.lfs.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Akhtar S, Benter IF. The role of epidermal growth factor receptor in diabetes-induced cardiac dysfunction. Bioimpacts. 2013;3:5–9. doi: 10.5681/bi.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 32.Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;18:CD003639. doi: 10.1002/14651858.CD003639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki Y, Katakami N, Hayaishi-Okano R, Matsuhisa M, Kajimoto Y, Kosugi K, Hatano M, Hori M. Alpha-glucosidase inhibitor reduces the progression of carotid intima-media thickness. Diabetes Res Clin Pract. 2005;67:204–210. doi: 10.1016/j.diabres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:115–123. doi: 10.1002/dmrr.357. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan K. Plant foods in the management of diabetes mellitus: spices as beneficial antidiabetic food adjuncts. Int J Food Sci Nutr. 2005;56:399–414. doi: 10.1080/09637480500512872. [DOI] [PubMed] [Google Scholar]