Abstract
The objective of the present study was to evaluate the physicochemical and microbial properties of the Korean traditional rice wine Makgeolli, supplemented with banana during 6 day fermentation. The alcohol contents of the control and banana Makgeolli were 17.0 and 16.5%, respectively. The pH values decreased while total acidity, total soluble solids, and color values increased throughout the fermentation process. An increase in microorganism counts throughout the 6-day fermentation period was noted in all samples. The major free sugar and organic acid detected in all samples were glucose and succinic acid, respectively. There were 39 volatile compounds detected in the control and banana Makgeolli. The major ester detected was ethyl acetate (20.037 and 22.604% for the control and banana Makgeolli, respectively). The major alcohol compounds detected were 3-methylbutanol (20.933%) and 3-methyl-1-butanol (34.325%) in the control. 2-mtehyl-1-propanol (22.289%) and 3-methyl-1-butanol (39.851%) were the highest alcohol compounds detected in the banana Makgeolli.
Keywords: Makgeolli, banana, fermentation, physicochemical properties, microbial properties
INTRODUCTION
Traditionally, Korean rice wine, or Makgeolli, has been brewed with nuruk, cooked rice, yeast, and some medicinal plants or herbs (1,2). Nuruk has been prepared by inoculating moistened ground wheat or rice with a mixture of airborne microorganisms, such as fungi, yeast, and a variety of other bacteria (3–7). The nuruk in the Makgeolli plays a role in breaking down the starch so the resulting sugar can be utilized by the yeast to create the Makgeolli. Makgeolli has been recorded in Chinese literature since the Koguryeo dynasty (8–10) and continues to be a part of Korea’s culture. However, due to a lack of unique characteristics, inferior acceptability, and functionality, Makgeolli has been declining in popularity. Recently, different varieties of cocktail Makgeollis were developed and many research groups have been studying how to improve the quality of Makgeolli through the study of its microbial activities, functional characteristics, utilization of raw materials, manufacture processes, extension of shelf life, and more (4,5,9,11,12).
Bananas are a group of monocot plants in the Musaceae family that originated from Southern and Southeast Asia (13). Bananas are an excellent tropical fruit with an agreeable flavor and a high nutritional value. When consumed, the intake of sugars, fiber, vitamins, and minerals are high with very low contribution to fat intake (14). Overall bananas are not only a nutritious fruit for carbohydrates, vitamins, and minerals, but they also contain many secondary metabolites. Phenolics are a group of secondary metabolites in bananas which have been highlighted recently for their health benefits, such as treating diarrhea, promoting blood clotting and wound healing, and antimicrobial and anti-inflammatory activities (13). Bananas are the world’s leading fruit crop considering their nutritional aspect, and economically the 5th most agricultural crop traded worldwide (15). However, no studies have reported evaluating the product quality and physicochemical properties of Makgeolli that incorporates banana. Thus, the objective of the present study was to investigate the physicochemical and microbial properties of Makgeolli supplemented with bananas during fermentation.
MATERIALS AND METHODS
Materials
Rice was purchased from a local market (Yongin, Korea). Nuruk and yeast were obtained from Korea Enzyme, Co. (Hwaseong, Korea) and Saf-instant (Lesaffre, Marcq-en-Baroeul, France), respectively. Banana used in this study was purchased from E-mart (Yongin, Korea).
Preparation of the control and banana Makgeolli
The preparation of the Makgeolli (control) and banana Makgeolli is illustrated in Fig. 1. Two kg of rice was rinsed and soaked in tap water for 3 h. After soaking, the rice was then drained of water for 40 min and immediately steamed for 40 min. After the rice was steamed, the heat was turned off and left to sit for 20 min. The rice was quickly cooled by spreading it out thinly on an aluminum pan. Once cooled, the rice was placed in a 10 L glass bottle, along with the nuruk (80 g), yeast (28 g), and distilled water (3.5 L). For the banana Makgeolli, 400 g of bananas were peeled, grinded, and added to the mixture of rice, nuruk, yeast and distilled water (3.12 L). The top was secured with a plastic wrap and a rubber band and placed in a water bath that was set at 28°C and allowed to ferment for 6 days. The samples were filtered (Advantec 5B, Tokyo Roshi Kaisha, Ltd., Tokyo, Japan) before being tested.
Fig. 1.
Procedure for the control and banana Makgeolli.
Alcohol content
The alcohol contents of the Makgeollis were taken using the procedures adapted from Woo et al. (16). Briefly, 100 mL of the sample was run through a distiller until around 70 mL was collected. The collected sample set to 100 mL with distilled water and the alcohol content (%) was measured using an alcohol hydrometer. The alcohol-temperature correction table was used with the sample’s alcohol content and temperature.
pH, total acidity, and total soluble solid content
The pH was measured using an Orion 3 Star pH Benchtop (Thermo Electron Co., Beverly, MA, USA). After measuring the pH, 10 mL of the sample combined with the indicator Bromothymol blue and neutral red and titrated with a 0.1 N NaOH solution. The amount of NaOH (mL) was then converted to tartaric acid (16). The total soluble solid content was measured using a digital refractometer (Model Dr−103L, Bellingham+ Stanley Ltd., Tumbridge Wells, UK) and measured in Brix (°Bx).
Color
The color values were obtained using the Tri-Stimulus Colorimeter (Model JC801, Color Techno System Co., Tokyo, Japan), which was calibrated with the original value from a standard plate (L=98.48, a=0.14, and b= 0.41). The L (lightness), a (redness), and b (yellowness) values were obtained.
Total viable cells, lactic acid bacteria, and yeast
Total viable cells, lactic acid bacteria, and yeast counts were measured according to the Korean Food and Drug Association (17). The standard plate count was used on the pour plate method for each sample. The samples were diluted in a 10 fold dilution with sterile saline (0.85% NaCl). In the total viable cell count, 1 mL of the diluted samples were mixed with 20 mL of PCA (plate count agar; Difco™, Lawrence, KA, USA) and incubated at 37°C for 48 h. In the lactic acid bacteria count, 1 mL of the diluted samples were mixed with 20 mL of BCP (bromocresol purple agar, Eiken Chemical, Tokyo, Japan) and incubated at 37°C for 72 h. In the yeast count, 1 mL of the diluted samples were mixed with 20 mL of PDA (potato dextrose agar; Difco™) and incubated at 25°C for 120 h. Each dilution was duplicated and the values were averaged. The number of microorganisms was counted by colony forming units (CFU).
Free sugar
Free sugar contents were obtained using the method adapted from Kerem et al. (18). The samples were filtered through a 0.2 μm membrane filter and analyzed by HPLC system (JASCO Co., Tokyo, Japan). Using the external standard method, the standards, fructose, glucose, sucrose, and maltose, were used and the curve was quantified. The HPLC conditions were set as follows: the flow rate through the carbohydrate high performance column (4.0 μm, 4.6×250 mm, Waters, Milford, MA, USA) was 1.4 mL/min, the column temperature was set at 35°C, the mobile phase was 75% acetonitrile, and the detector was RI-930 detector (JASCO Co.).
Organic acid
Organic acid contents were obtained using the method adapted from Kerem et al. (18). The samples were filtered through a 0.2 μm membrane filter and analyzed by HPLC system (JASCO Co.). Using the external standard method, the standards, oxalic acid, citric acid, malic acid, succinic acid, formic acid, and acetic acid, were used and the curve was quantified. The HPLC conditions were set as follows: the flow rate through the Supelcogel C-610H column (9 μm, 7.8×300 mm, Sigma, St. Louis, MO, USA) was 0.5 mL/min, the column temperature was set at 30°C, the mobile phase was 0.1% phosphoric acid, and the detector was the multi-wavelength detector (MD-2010 Plus, JASCO Co.).
Volatile compounds
Octanal (15 μL) was used as the standard for the analysis of volatile compounds, and 20 mL of the samples were obtained in a 240 mL screw amber bottle (Sigma-Aldrich Co.). The samples were placed in a 50°C dry oven for 10 min and the volatile compounds were collected. The volatile compounds were analyzed using the GC-MS (GCMS-QP2010, Shimadzu, Kyoto, Japan), and the condition at which they were analyzed were as follows: GC was run with AT1 column (60×0.25 mm, Shimadzu), the rate of temperature increase was 8°C/min between 5°C to 120°C, 12°C/min to 180°C, 15°C/min to 230°C, the carrier gas used was N2, and the injection temperature was 200°C while the detection temperature was 250°C.
Statistical analysis
All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) was performed using the general linear models (GLM) procedure to determine significant differences among the samples. Means were compared by using Fisher’s least significant difference (LSD) test. Significance was defined at the 5% level.
RESULTS AND DISCUSSION
Alcohol content, pH, total acidity, and total soluble solid content
The alcohol contents of the control and banana Makgeolli on the final day of the fermentation period were 17 and 16.5%, respectively (Table 1). These values fall within the range of alcohol content given by Jin et al. of 15~18% (19). Alcohol content is one of the factors that affect the quality of Takju and can also be used to show the degree of fermentation throughout the fermentation process (20). Starch is converted to glucose by hydrolysis throughout the fermentation process. Glucose is then used by yeast to produce alcohol and CO2. Therefore, formation of bubbles can also be seen throughout the process of fermentation.
Table 1.
Alcohol contents of the control and banana Makgeolli on day 6 of the fermentation period
| Sample | Alcohol contents (%) |
|---|---|
| Control | 17.0 |
| Banana Makgeolli | 16.5 |
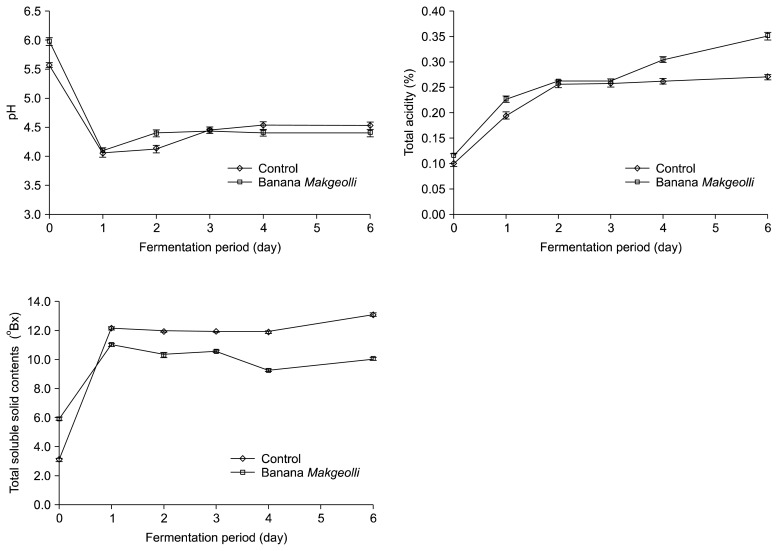
The pH, total acidity, and total soluble solid contents of the control and banana Makgeolli can be seen in Fig. 2. The pH values of the control and banana Makgeolli were similar throughout the 6 day fermentation period. A sharp decrease in pH on day 1 of the fermentation was noted, which then slightly increased to a pH of around 4.4. The final pH values of the control and banana Makgeolli fall within the range of 3.4 to 4.5 (1,8,19).
Fig. 2.
pH values, total acidity values and total soluble solid contents of the control and banana Makgeolli throughout the 6-day fermentation period.
In the analysis of total acidity, all the samples sharply increased on day 1 of the fermentation period and slowly increased to a final value of 0.26 and 0.35% for the control and banana Makgeolli, respectively. Production of organic acids by microorganisms affect the total acidity of the Makgeolli, and the change of total acidity is a factor for knowing that compounds are changing in the Makgeolli(20). A previous study showed the combination of total acidity and volatile compounds affect the quality of flavor and shelf life of Makgeollis (21).
Due to the addition of bananas, the banana Makgeolli had a higher initial total soluble content than the control. A sharp increase in soluble contents was observed on day 1 of the fermentation period, and the final total soluble contents of the control and banana Makgeolli were 13.2 and 10.1 °Bx, respectively. Due to the sharp changes on day 1 in the pH, total acidity, and total soluble contents, we speculated that most of the microbial activities occur on the first day of fermentation.
Color
Table 2 shows the values of the color analysis. When compared to the banana Makgeolli, the control had a higher L value after the final fermentation day. The banana Makgeolli had the higher a and b values than the control after the fermentation period. Also, the L, a, and b values increased throughout the fermentation process. Kim et al. (20), Lee et al. (22) and Park et al. (23) also reported similar results.
Table 2.
Color values (L, a and b) of the control and banana Makgeolli throughout the 6 day fermentation period
| Sample | Fermentation period (day) | Color | ||
|---|---|---|---|---|
|
| ||||
| L | a | b | ||
| Control | 0 | 64.29 | 0.12 | 0.22 |
| 1 | 66.40 | 0.11 | 3.30 | |
| 3 | 68.66 | 0.67 | 7.50 | |
| 4 | 68.93 | 0.62 | 8.30 | |
| 6 | 69.01 | 0.79 | 9.70 | |
| Banana Makgeolli | 0 | 59.85 | 1.18 | 4.14 |
| 1 | 63.07 | 1.43 | 9.84 | |
| 3 | 65.63 | 1.91 | 9.98 | |
| 4 | 65.84 | 1.87 | 10.62 | |
| 6 | 65.22 | 2.10 | 11.85 | |
Microbial changes
The microbial cell counts for the total viable cells, lactic acid bacteria, and yeast can be seen in Table 3. The final total viable cells, lactic acid bacteria, and yeast counts for the control and banana Makgeolli were 2.88×107 and 3.57×107, 1.50×106 and 1.84×106, and 1.90×107 and 2.17×107 CFU/mL, respectively, with an increase of all microorganisms in all samples throughout the fermentation period. Seo et al. (8) and Rhee et al. (24) also reported an increase of total microorganisms throughout the fermentation process.
Table 3.
Microbial cell counts of the control and banana Makgeolli throughout the 6 day fermentation period (Unit: CFU/mL)
| Sample | Microorganism | Fermentation period (day) | |||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1 | 3 | 6 | ||
| Control | Total viable cells | 1.23×105 | 2.50×106 | 4.24×106 | 2.88×107 |
| Lactic acid bacteria | 1.10×105 | 1.70×105 | 1.15×106 | 1.50×106 | |
| Yeasts | 1.26×105 | 2.38×106 | 1.56×107 | 1.90×107 | |
| Banana Makgeolli | Total viable cells | 3.36×105 | 4.50×106 | 5.67×106 | 3.57×107 |
| Lactic acid bacteria | 2.02×105 | 3.50×105 | 1.30×106 | 1.84×106 | |
| Yeasts | 3.74×105 | 4.86×106 | 1.67×107 | 2.17×107 | |
Free sugar
The free sugar contents of the control and banana Makgeolli are displayed in Table 4. The major free sugar detected in both the control and banana Makgeolli was glucose. Glucose significantly decreased after the 6 day fermentation period. Glucose plays an important role in fermentation as its production from starch allows for the production of alcohol in Makgeolli. Glucose is formed through the hydrolysis of starch by amylases in the nuruk. Therefore, through α-amylase, glucose increases in Makgeolli. Glucose was also found to be a major sugar in a study by Park et al. (23). Fructose, glucose and maltose were found to be the major reducing sugars used by yeast to create alcohol, and the reduction of these sugars was found to give Makgeolli its particular taste (19).
Table 4.
Free sugar contents of the control and banana Makgeollis on day 1 and 6 of the fermentation period
| Sample | Storage period (day) | Fructose | Glucose | Sucrose | Maltose | Lactose |
|---|---|---|---|---|---|---|
| Control | 1 | ND1) | 49.855a | 0.114c | 0.834c | 0.326b |
| 6 | 0.103c | 29.831c | 0.679a | 1.686a | ND | |
| Banana Makgeolli | 1 | 0.140a | 38.74b | nd | 0.723d | 0.285b |
| 6 | 0.119b | 25.060d | 0.535b | 1.477b | 1.021a |
Units: mg/mL.
Values with different letters within the same column differ significantly (P<0.05).
ND: not detected.
Organic acid
Malic, succinic, lactic, and acetic acids were significantly elevated throughout the fermentation period in all the samples (Table 5). Banana Makgeolli had a significant increase in tartaric acid as well. Succinic acid contents were highest in both the control and banana Makgeolli. Jeon et al. (3) suggests that the production of organic acid may be dependent on several factors, including nuruk fermentation conditions, or other circumstances. Rhee et al. (24) explained that the suppression of lactic acid may be due to the high colony forming unit of yeast in the initial stage of the brewing, which may suppress the acid forming bacteria but allowed the high content of succinic acid.
Table 5.
Organic acid contents of the control and banana Makgeollis on day 1 and 6 of the fermentation period
| Sample | Storage period (day) | Oxalic acid | Citric acid | Tartaric acid | Malic acid | Succinic acid | Lactic acid | Acetic acid |
|---|---|---|---|---|---|---|---|---|
| Control | 1 | ND1) | 0.155c | 0.037c | 0.174d | 7.698d | 0.082c | 1.256d |
| 6 | 0.003a | 0.127d | 0.035c | 0.201c | 24.587b | 0.440a | 1.696c | |
| Banana Makgeolli | 1 | 0.002a | 0.660a | 0.096b | 0.315b | 9.118c | 0.057c | 2.626b |
| 6 | 0.002a | 0.374b | 0.105a | 0.362a | 25.698a | 0.373b | 2.860a |
Units: mg/mL.
Values with different letters within the same column differ significantly (P<0.05).
ND: not detected.
Volatile compounds
The relative peak areas of volatile compounds of the control and the banana Makgeolli are shown in Table 6. A total of 37 volatile compounds were detected in the control and the banana Makgeolli. The major volatile compounds in the samples were esters and alcohols, which was also reported in studies by Lee et al. (1) and Rhee et al. (24). Lee et al. (1) reported that all the major components seem to be the production of yeast fermentation. The major ester compound detected was ethyl acetate. The amount detected for the control and the banana Makgeolli was 20.037 and 22.604%, respectively. Ethyl acetate is a major volatile compound that gives Makgeolli its fruity taste (25). Kwon et al. (26) reported that the major esters found were (E)-cinnamaldehyde, ethyl linoleaste, ethyl linolenate, ethyl caprate, and ethyl palmitate. The differences of compounds may be due to the differences in ingredients and yeasts used. The major alcohol compounds in the control were 3-methylbutanol (22.469%) and 3-methyl-1-butanol (34.325%). For the banana Makgeolli, the major alcohol compounds were 2-methyl-1-propanol (23.150%) and 3-methyl-1-butanol (39.851%). Acetaldehyde was also detected in the samples. In general, acetaldehyde provokes the smell and was also detected in Korean traditional soju and Chonju(24). Han et al. (27) analyzed 9 different alcohols, excluding ethanol, and found that iso-amyl alcohol and iso-butyl alcohol were the main alcohols detected. Lee et al. (28) studied the effects of different yeasts on 8 different alcohols; 4 out of 5 Makgeollis were observed to have iso-amyl alcohol as its major alcohol (28). The differences of volatile compounds detected may be due to the differences in yeast and ingredients. Han et al. (27) stated that a greater amount of 2-methyl-1-propanol than 3-methyl-1-butanol in Makgeolli resulted in a low quality Makgeolli.
Table 6.
Volatile compounds of the control and banana Makgeolli on day 6 of the fermentation period
| No | RI1) | Compounds | Sample | |
|---|---|---|---|---|
|
| ||||
| Control | Banana Makgeolli | |||
| 1 | 362 | Acetaldehyde | 0.572 | 0.844 |
| 2 | 367 | Methanol | 0.165 | 0.053 |
| 3 | 373 | Ethanol | 3.050 | 0.667 |
| 4 | 600 | Hexane | 4.338 | 0.046 |
| 5 | 603 | 1-Propanol | 4.208 | 0.211 |
| 6 | 623 | Ethyl acetate | 20.037 | 22.604 |
| 7 | 650 | 3-Methylbutanal | 22.469 | 0.097 |
| 8 | 670 | 2-Methyl-1-propanol | 0.057 | 23.150 |
| 9 | 676 | Acetic acid | 0.005 | 0.007 |
| 10 | 699 | 1-Butanol | 0.018 | 0.226 |
| 11 | 727 | Propanoic acid, ethyl ester | 0.386 | 0.719 |
| 12 | 730 | Acetic acid, propyl ester | 0.076 | 0.092 |
| 13 | 770 | 3-Methyl-1-butanol | 34.325 | 39.851 |
| 14 | 775 | Ethyl isobutyrate | 0.928 | 1.135 |
| 15 | 786 | Acetic acid, 2-methylpropyl ester | 0.325 | 0.433 |
| 16 | 794 | 3-Methyl-1-hexanol | 0.274 | 0.581 |
| 17 | 795 | Hexanal | 0.274 | 0.581 |
| 18 | 799 | Isobutyric acid | 0.096 | 0.244 |
| 19 | 801 | 2,4-Dimethyl-hexane | 0.094 | 0.171 |
| 20 | 806 | Butanoic acid, ethyl ester | 0.530 | 0.821 |
| 21 | 831 | 2,4-Dimethyl-heptane | 0.004 | 0.000 |
| 22 | 860 | Ethyl 2-methyl butyrate | 0.571 | 0.577 |
| 23 | 864 | 3-Ethoxy-1-propanol | 0.019 | 0.571 |
| 24 | 870 | 4-Methyl octane | 0.658 | 0.228 |
| 25 | 885 | 2-Methyl butyl acetate | 0.278 | 0.219 |
| 26 | 901 | Pentanoic acid, ethyl ester | 0.028 | 0.017 |
| 27 | 961 | Benzaldehyde | 0.240 | 0.129 |
| 28 | 996 | Octanal | 5.052 | 5.222 |
| 29 | 1,046 | 1,8-Cineole | 0.009 | 0.000 |
| 30 | 1,061 | 3-Ethyl-3-methyl heptane | 0.001 | 0.001 |
| 31 | 1,068 | 3,8-Dimethyl decane | 0.010 | 0.002 |
| 32 | 1,100 | Undecane | 0.033 | 0.021 |
| 33 | 1,107 | 3,8-Dimethyl-undecane | 0.020 | 0.012 |
| 34 | 1,124 | Phenethyl alcohol | 0.161 | 0.000 |
| 35 | 1,195 | Octanoic acid, ethyl ester | 0.606 | 0.428 |
| 36 | 1,799 | Tetradecanoic acid, ethyl ester | 0.014 | 0.020 |
| 37 | 1,995 | Hexadecanoic acid, ethyl ester | 0.065 | 0.020 |
| Total | 100 | 100 | ||
Units: Peak area (%).
RI: Retention index.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Lee SJ, Kwon YH, Kim HR, Ahn BH. Chemical and sensory characterization of Korean commercial rice wines (Yakju) Food Sci Biotechnol. 2007;16:374–380. [Google Scholar]
- 2.Lee MY, Sung SY, Kang HK, Byun HS, Jung SM, Song JH, Lee JS. Quality characteristics and physiological functionality of traditional rice wines in Chungnam province of Korea. Korean J Microbiol Biotechnol. 2010;38:177–182. [Google Scholar]
- 3.Jeon BY, Seo HN, Yun A, Lee IH, Park DH. Effect of glasswort (Salicornia herbacea L.) on Nuruk-making process and Makgeolli quality. Food Sci Biotechnol. 2010;19:999–1004. [Google Scholar]
- 4.Lee SS, Kim KS, Eom AH, Sung CK, Hong IP. Production of Korean traditional rice-wines made from cultures of the single fungal isolates under laboratory conditions. Korean J Mycol. 2002;30:61–65. [Google Scholar]
- 5.Han EH, Lee TS, Noh BS, Lee DS. Quality characteristics of mash of Takju prepared by using different Nuruk during fermentation. Korean J Food Sci Technol. 1997;29:555–562. [Google Scholar]
- 6.Lee DH, Kim JH, Lee JS. Effect of pears on the quality and physiological functionality of Makgeoly. Korean J Food Nutr. 2009;22:606–611. [Google Scholar]
- 7.Lee HH, Lee JH, Ko YJ, Park MH, Lee JO, Ryu CH. Changes in allergenicity and quality of Nuruk during fermentation. J Korean Soc Food Sci Nutr. 2009;38:76–82. [Google Scholar]
- 8.Seo DH, Jung JH, Kim HY, Kim YR, Ha SJ, Kim YC, Park CS. Identification of lactic acid bacteria involved in traditional Korean rice wine fermentation. Food Sci Biotechnol. 2007;16:994–998. [Google Scholar]
- 9.Song JC, Park HJ. Takju brewing using the uncooked germed brown rice at second stage mash. J Korean Soc Food Sci Nutr. 2003;32:847–854. [Google Scholar]
- 10.Kim YJ, Han YS. The use of Korean traditional liquors and plan for encouraging it. Korean J Food Culture. 2006;21:31–41. [Google Scholar]
- 11.Kim JH, Lee DH, Lee SH, Choi SY, Lee JS. Effect of Ganoderma lucidum on the quality and functionality of Korean traditional rice wine, Yakju. J Biosci Bioeng. 2004;97:24–28. doi: 10.1016/S1389-1723(04)70160-7. [DOI] [PubMed] [Google Scholar]
- 12.Cho EK, Kim HY, Byeon HJ, Kim SW, Choi YJ. Nitrite scavenging and alcohol metabolizing activites of hot water extract from Makgeoly and its angiotensin converting enzyme inhibitory effect. J Life Sci. 2010;20:768–774. [Google Scholar]
- 13.Pothavorn P, Kitdamrongsont K, Swangpol S, Wongniam S, Atawongsa K, Savasti J, Somana J. Sap phytochemical compositions of some bananas in thailand. J Agric Food Chem. 2010;58:8782–8787. doi: 10.1021/jf101220k. [DOI] [PubMed] [Google Scholar]
- 14.Veneziano A, Vacca G, Arana S, Simone FD, Rastrelli L. Determination of carbendazim, thiabendazole and thiophanate-methyl in banana (Musa acuminate) samples imported to Italy. Food Chem. 2004;87:383–386. [Google Scholar]
- 15.Aurore G, Parfait B, Fahrasmane L. Bananas, raw materials for making processed food products. Trends Food Sci Tech. 2009;20:78–91. [Google Scholar]
- 16.Woo KS, Ko JY, Song SB, Lee JS, Oh BG, Kang JR, Nam MH, Ryu IS, Jeong HS, Seo MC. Physicochemical characteristics of Korean traditional wines prepared by addition of sorghum (Sorghum bicolor L. Moench) using different Nuruks. J Korean Soc Food Sci Nutr. 2010;39:548–553. [Google Scholar]
- 17.KFDA. Code food. Korea Food and Drug Association; Seoul, Korea: 2010. [Google Scholar]
- 18.Kerem Z, Bravdo B, Shoseyov O, Tugendhaft Y. Rapid liquid chromatography-ultraviolet determination of organic acids and phenolic compounds in red wine and must. J Chromatogr A. 2007;1052:211–215. doi: 10.1016/j.chroma.2004.08.105. [DOI] [PubMed] [Google Scholar]
- 19.Jin J, Kim SY, Jin Q, Eom HJ, Han NS. Diversity analysis of lactic acid bacteria in Takju, Korean rice wine. J Microbiol Biotechnol. 2008;18:1678–1682. [PubMed] [Google Scholar]
- 20.Kim JY, Sung KW, Bae HW, Yi YH. pH, acidity, color, reducing sugar, total sugar, alcohol and organoleptic characteristics of puffed rice powder added Takju during fermentation. Korean J Food Sci Technol. 2007;39:266–271. [Google Scholar]
- 21.Lee TJ, Hwang DY, Lee CY, Son HJ. Changes in yeast cell number, total acid and organic acid during production and distribution processes of Makgeolli, traditional alcohol of Korea. Korean J Microbiol. 2009;45:391–396. [Google Scholar]
- 22.Lee SS, Kim KS, Eom AH, Sung CK, Hong IP. Production of Korean traditional rice-wines made from cultures of the single fungal isolates under laboratory conditions. Korean J Mycol. 2002;30:61–65. [Google Scholar]
- 23.Park CS, Oh EH, Jeong HS, Yoon HS. Quality characteristics of the germinated brown rice wine added with red pepper. J Korean Soc Food Sci Nutr. 2009;38:1090–1096. [Google Scholar]
- 24.Rhee SJ, Lee Chung-Yun Jetty, Kim KK, Lee CH. Comparison of the traditional (Samhaeju) and industrial (Chongju) rice wine brewing in Korea. Food Sci Biotechnol. 2003;12:242–247. [Google Scholar]
- 25.So MH, Lee YS, Han SH, Noh WS. Analysis of major flavor compounds in Takju mash brewed with a modified Nuruk. J Korean Soc Food Sci Nutr. 1999;12:421–426. [Google Scholar]
- 26.Kwon YH, Jo SJ, Kim HR, Lee HJ, Kim JH, Ahn BH. Physicochemical properties and volatile compounds in Jeonju Moju. Korean J Food Sci Technol. 2009;41:503–508. [Google Scholar]
- 27.Han EH, Lee TS, Noh BS, Lee DS. Quality characteristics in mash of Takju prepared by using different Nuruk during fermentation. Korean J Food Sci Technol. 1997;29:555–562. [Google Scholar]
- 28.Lee HS, Park CS, Choi JY. Quality characteristics of the mashes of Takju prepared using different yeasts. Korean J Food Sci Technol. 2010;42:56–62. [Google Scholar]