Abstract
Chrysanthemum indicum is widely used to treat immune-related and infectious disorders in East Asia. C. indicum flower oil contains 1,8-cineole, germacrene D, camphor, α-cadinol, camphene, pinocarvone, β-caryophyllene, 3-cyclohexen-1-ol, and γ-curcumene. We evaluated the safety of C. indicum flower oil by conducting acute oral toxicity, bone marrow micronucleus, and bacterial reverse mutation tests. Mortality, clinical signs and gross findings of mice were measured for 15 days after the oral single gavage administration of C. indicum flower oil. There were no mortality and clinical signs of toxicity at 2,000 mg/kg body weight/day of C. indicum flower oil throughout the 15 day period. Micronucleated erythrocyte cell counts for all treated groups were not significantly different between test and control groups. Levels of 15.63~500 μg C. indicum flower oil/plate did not induce mutagenicity in S. Typhimurium and E. coli, with or without the introduction of a metabolic activation system. These results indicate that ingesting C. indicum flower oil produces no acute oral toxicity, bone marrow micronucleus, and bacterial reverse mutation.
Keywords: Chrysanthemum indicum flower oil, acute oral toxicity, bone marrow micronucleus, bacterial reverse mutation
INTRODUCTION
Chrysanthemum indicum L. (C. indicum), belonging to the Compositae family, is a perennial garden herb in many countries. C. indicum has been used to treat various immune-related disorders, hypertension symptoms, and several infectious diseases in Korean and Chinese medicine (1–3). C. indicum flowers are also commonly used as tea to treat antiinflammation, headache, and eye diseases (2,4). The pharmaceutical benefits of C. indicum were reportedly due to its antioxidant, antiviral, antibacterial, and immunomodulatory properties (5,6). In previous reports, the major volatile compounds of Chrysanthemum sp. were 1,8-cineole, germacrene D, camphor, α-cadinol, camphene, pinocarvone, β-caryophyllene, 3-cyclohexen-1-ol, and γ-curcumene (7–10).
The micronucleus test, an in vivo short-term screening test developed by Schmid (11) and Heddle (12), is useful in investigating the toxicity of compounds. The frequency of micronucleated polychromatic erythrocytes (MPCE) induced by mutagens increases after exposure to carbon monoxide (13), bleeding (14), or anoxia (15). Well-known, carbon monoxide causes tissue anoxia by producing a carbon monoxide-hemoglobin complex.
Abnormalities in chromosomal structures such as increased chromosomal breakage or chromosomal loss are associated with enhanced risk of carcinogenesis and in the progression of neoplastic transformation (16). Detection of chromosomal aberrations has been widely used as a tool to indicate carcinogen induced DNA damage as well as to assess the antigenotoxic effect of natural or synthetic chemopreventive agents (11,17,18).
The available toxicological information on C. indicum flower oil is insufficient to assure its safe use in dietary supplements or functional materials. We evaluated the mutagenicity and toxicity of C. indicum flower oil based on acute oral toxicity, bone marrow micronucleus, and bacterial reverse mutation tests.
MATERIALS AND METHODS
Extraction of C. indicum flower oil
Flowers of C. indicum were harvested in the fall of 2006 from Namwon (Jeollabuk-do) province, in western Korea, and purchased at Gyeongdong herbal market (Seoul, Korea) in the spring of 2007. Samples were stored at −70°C in airtight bags for later analysis. Oils of C. indicum flowers were extracted by steam distillation method (19). The obtained oils were dried over anhydrous sodium sulfate for 24 hr, and then stored in hermetically sealed glass containers at −4°C for later testing.
Acute oral toxicity study in mice
Both 10 female and 10 male ICR mice (specific pathogen free, 6 weeks of age) were purchased from Orient Bio (Orient Bio Inc., Seongnam, South Korea). Mice were assigned to each group (n=10) and acclimated for a week in the housing. Temperature was maintained at 23±3°C and relative humidity was 50±10%. A 12 hr light/dark cycle was observed and air was changed 10~20 times per day. Mice were freely accessed to stock rodent pellet (PMI Nutritional International, Richmond, IN, USA) and tap water. The study protocol was reviewed by IACUC (Institutional Animal Care and Use Committee, Daejeon, South Korea) and conducted in compliance with the Association for Assessment and internationally recognized Accreditation of Laboratory Animal Care (AAALAC, Daejeon, South Korea) (20). Body weight of the mice at the beginning of the study was 29.6±1.1 g for males and 21.5±1.3 g for females. Mice were fasted for 4 hr prior to oral administration of C. indicum flower oil dissolved in carboxymethyl cellulose (CMC). Constant volume (10 mL/kg body weight) containing 200 mg/mL of C. indicum flower oil was orally administered to each of the five mice per sex to achieve a dose of 2,000 mg/kg body weight. CMC was used as negative control. Feeds and water were provided after 4 hr of oral administration. Overall symptoms, including mortality, clinical signs, and gross findings, were observed once a day for 15 days. Body weight was weighed just before oral administration (day 1) and after dosing (days 2, 4, 8, and 15). On day 15, all animals were euthanized under CO2 gas overdose and examined for internal organ abnormalities.
Animal test subjects for bone marrow micronucleus test
Female and male Institute for Cancer Research (ICR) mice (30 each, specific pathogen free, 6 weeks old) were obtained (Orient Bio Inc.) and divided into 10 groups (five male, five female) of six mice each. Average body weight at the beginning of the study was about 35.5 g for males and 25.7 g for females. Six of the mice groups (three male, three female) were administered C. indicum flower oils orally at doses of 500, 1,000 and 2,000 mg/kg body weight for 2 days. Two groups (one male, one female) were given oral doses of corn oil as a negative control, and the remaining two groups were given cyclophosphamide monohydrate as a positive control. Animals were euthanized within 24 hr of final dose administration by an overdose of CO2 gas.
Bone marrow micronucleus test
Micronucleus slides were prepared using the method described by Schmid (21). The femur bones of each mouse were separated and cleaned of surrounding muscle tissue. The upper end of the femur was cut until a small opening was visible. About 0.5 mL bovine albumin was injected into the opening of the bone via syringe, flushing the bone marrow into a clean, dry centrifuge tube. The flushing was repeated to ensure a homogeneous cell suspension. The suspension volume was increased to 3 mL and then centrifuged at 1,000 rpm for 5 min. The supernatant was discarded and the collected cells were disturbed by gently tapping the tube, then mixed well in a minimum quantity of bovine albumin.
One drop of the cell suspension was placed on a clean, dry slide and spread with another slide held at a 45° angle. Both slides were air dried and fixed in absolute methanol for 5 min. The slides were placed for 20 min in 5% Giemsa staining solution in 0.01 M phosphate buffer, with pH adjusted to 6.8. The slides were washed in running tap water, air dried, mounted with DPX (synthetic resin of distrene plasticiser xylene), then examined under oil immersion at 1,000× magnification using bright-field illumination. All slides were randomly coded prior to microscopic analysis, and 2,000 bone marrow polychromatic erythrocytes (PCE) were examined from each animal, with the number of micronucleated PCE recorded. The percentage of PCE among 500 normochromatic erythrocytes (NCE) was determined for a given sample to evaluate treatment cytotoxicity. Mann-Whitney’s U-test was used to evaluate results from the treatment and positive control groups with respect to micronucleus formation and PCE/(PCE+NCE) ratios.
Bacterial reverse mutation assay
Mutagenic activity of C. indicum flower oil was evaluated using the Ames protocol (22). Histidine-requiring Salmonella Typhimurium (TA100, TA1535, TA98, and TA1537) and tryptophan-requiring Escherichia coli (WP2uvrA) strains were obtained from Molecular Toxicology Inc. (Boone, NC, USA). C. indicum flower oil was dissolved at a concentration of 2,000 μg/mL in dimethyl sulfoxide (DMSO, 99.99% pure, Sigma-Aldrich Chemical Co., St. Louis, MO, USA). This base-concentration solution was further diluted with DMSO into a series of lower concentration test solutions (i.e., 500, 250, 125, 62.5, 31.25 and 15.63 μg/mL) in accordance with Organization for Economic Cooperation and Development (OECD) guidelines (23). Triplicate mutagenicity assays were conducted with and without the rat liver S9 mix (Molecular Toxicology Inc.) metabolic activation system, and with negative and positive controls in accordance with OECD TG 471 guidelines (24). The sources and grades of positive control materials were as follows: sodium azide (100.1% pure, Sigma-Aldrich Chemical Co.), 2-nitrofluorene (98.1% pure, Aldrich Chemical Co., Milwaukee, WI, USA), 9-aminoacridine (97.7% pure, Merck Chemical Co., Whitehouse Station, NJ, USA), 4-nitroquinoline 1-oxide (99% pure, Sigma Chemical Co., St. Louis, MO, USA), 2-aminoanthracene (99.8% pure, Aldrich Chemical Co.), and benzo[a]pyrene (99.8% pure, Sigma-Aldrich Chemical Co.).
Statistical analysis
Changes in body weight and clinical signs observed for 15 days after oral administration were analyzed using the Path/Tox system (V4.2.2; Xybion Medical Systems Corp., Cedar Knolls, NJ, USA). All data were evaluated using the Statistical Analysis System (SAS, v8.2; SAS Institute Inc., Cary, NC, USA). Differences between treatment and control group averages were assessed using a t-test. Differences in the frequency of chromosome aberration between negative control and treatment groups were evaluated using chi-square and Fisher’s exact tests. The Cochran-Armitage trend test was used to assess dose-response relationships. Differences between negative and positive control group means were assessed using the Fisher’s exact test.
RESTULS AND DISCUSSION
We previously determined the main components of C. indicum flower oil (Table 1) (7). Flower oil of C. indicum was characterized as having prominent (>3%) contents of α-pinene (14.63%), 1,8-cineol (10.71%), germacrene D (5.25%), (−)-sinularene (3.95%), β-bisabolene (3.95%), bornyl acetate (3.64%), β-elemene (3.18%), and borneol (3.02%). Zhang et al. (8) analyzed the volatile composition of C. indicum essential oils by GC/MS from eight populations in China. They found a total of 169 compounds and the predominant components were 1,8-cineole (0.62~7.34%), (+)-(1R, 4R)-camphor (0.17~27.56%), caryophyllene oxide (0.54~5.8%), β-phellandrene (0.72~1.87%), 2-methyl-6-(p-tolyl)hept-2-ene (0.3~8.6%), and hexadecanoic acid (0.72~15.97%). These results indicate that major compounds in C. indicum were varied with growing region, species, analytical tools, etc.
Table 1.
Major components (%) of Chrysanthemum indicum flower oil
| Components | R.I.1) | Contents (Relative %) |
|---|---|---|
| α-Pinene | 940 | 14.63 |
| 1,8-Cineol | 1,020 | 10.71 |
| Camphor | 1,152 | 2.64 |
| Borneol | 1,186 | 3.02 |
| Bornyl acetate | 1,289 | 3.64 |
| β-Elemene | 1,382 | 3.18 |
| Germacrene D | 1,502 | 5.25 |
| (−)-Sinularene | 1,514 | 3.95 |
| β-Bisabolene | 1,516 | 3.95 |
| β-Sesquiphellandrene | 1,532 | 1.19 |
Retention indices (R.I.) were calculated using n-alkanes (C8–C22) as external references on an HP-5MS capillary column.
Acute oral toxicity test
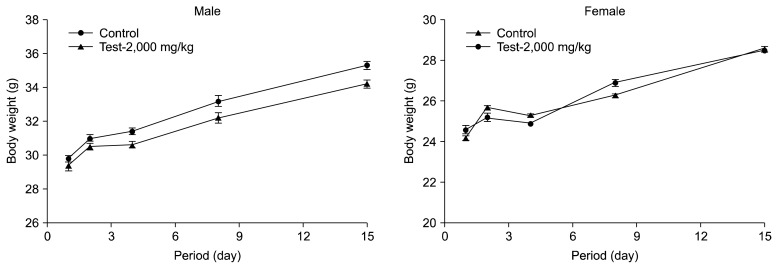
Mortality, clinical signs and gross findings of mice were measured for 15 days after the oral single gavage administration of C. indicum flower oil. No mortality and clinical signs of toxicity were observed throughout the 15 day period (Table 2). Therefore, the approximate lethal doses of C. indicum flower oil in male and female mice are higher than 2,000 mg/kg. Mean body weights for male and female rats at 2,000 mg/kg of C. indicum flower oil group were compared to control groups (Fig. 1). Slight body weight loss was observed from male mice in test group during the experimental periods but not statistically different from the control group. Slight body weight loss occurred among female mice at 4 days in both control and 2,000 mg/kg of C. indicum flower oil group but recovered after 8 days. After fifteen days of study, the mean body weights of control and C. indicum flower oil groups there were not significantly different.
Table 2.
Mortality of mice during 15 days after oral administration of Chrysanthemum indicum flower oil
| Dose (mg/kg) | Dosing phase | Mortality | |||
|---|---|---|---|---|---|
|
| |||||
| 1 day | ≤5 day | ≤10 day | ≤15 day | ||
| Male | |||||
| 0 | 0 | 0 | 0 | 0 | 0/5 |
| 2,000 | 0 | 0 | 0 | 0 | 0/5 |
| Female | |||||
| 0 | 0 | 0 | 0 | 0 | 0/5 |
| 2,000 | 0 | 0 | 0 | 0 | 0/5 |
Fig. 1.
Body weight changes for male and female rats after administered by C. indicum flower oil at 2,000 mg/kg body weight. Each value is expressed as mean±SD of measurement.
Bone marrow micronucleus test
Micronucleated erythrocyte cell counts were not significantly different between test groups and negative control groups (Table 3). PCE content in relation to total erythrocytes was evaluated to assess erythropoiesis rate, a measure of cytotoxicity. The estimated PCE : NCE ratio in the bone marrow preparations showed no statistically significant difference in hematopoiesis alterations as a result of extract treatment, indicating no cytotoxic effects.
Table 3.
Micronucleus test of Chrysanthemum indicum flower oil in mice
| Chemical tested | Dose (mg/kg) | No. of animals | MNPCE/2000 PCEs (mean±SD) | PCE/(PCE+NCE) (mean±SD) |
|---|---|---|---|---|
| Male | ||||
| Vehicle1) | 0 | 6 | 0.67±0.82 | 0.49±0.01 |
| Test item2) | 500 | 6 | 0.83±1.17 | 0.48±0.03 |
| Test item | 1,000 | 6 | 0.67±0.82 | 0.48±0.04 |
| Test item | 2,000 | 6 | 1.17±0.75 | 0.46±0.05 |
| CPA | 70 | 6 | 67.00±7.24* | 0.43±0.02* |
| Female | ||||
| Vehicle | 0 | 6 | 1.17±0.75 | 0.54±0.03 |
| Test item | 500 | 6 | 0.67±1.03 | 0.54±0.03 |
| Test item | 1,000 | 6 | 0.50±0.55 | 0.51±0.03 |
| Test item | 2,000 | 6 | 0.67±0.52 | 0.47±0.01* |
| CPA | 70 | 6 | 52.00±5.22* | 0.47±0.03* |
Significantly different from the control at P<0.01.
Vehicle: corn oil.
Test item: C. indicum flower oil.
MNPCE, PCE with one or more micronuclei; PCE, polychromatic erythrocyte; NCE, normochromatic erythrocyte; CPA, cyclophosphamide monohydrate (positive control article).
Bacterial reverse mutation assay
Mutagenicity of C. indicum flower derived essential oil was evaluated in a bacterial reverse mutation assay using histidine requiring S. Typhimurium (TA100, TA1535, TA98, and TA1537) and tryptophan-requiring E. coli (WP2uvrA) (Table 4). Regardless of S9 mix presence or absence, the number of reverting colonies for all treatment concentrations was not significantly different from negative control. No cytotoxicity was observed in any bacterial systems used in the mutation assay. A marked increase in the number of revertant colonies was observed in the positive controls compared to the negative control. No mutagenic activity was observed for any test concentration of the C. indicum flower oil. According to a previous study, d-limonene does not elicit mutagenicity in four strains of S. Typhimurium (25), nor chromosomal aberrations or sister chromatid exchange in cultured Chinese hamster ovary cells, or cell mutations in the livers or kidneys of rats (26).
Table 4.
Bacterial reverse mutation assay with Chrysanthemum indicum flower oil
| Test strain | Chemical treated | Dose (μg/plate) | Revertant colonies/plate (mean) [Factor]1) | |
|---|---|---|---|---|
|
| ||||
| Without S9 mix | With S9 mix | |||
| TA 100 | Test item2) | 0 | 120±9 | 133±6 |
| 15.63 | 131±11 [1.1] | |||
| 31.25 | 118±3 [1.0] | 132±19 [1.0] | ||
| 62.5 | 121±7 [1.0] | 145±5 [1.1] | ||
| 125 | 116±13 [1.0] | 144±12 [1.1] | ||
| 250 | 112±17 [0.9] | 133±15 [1.0] | ||
| 500 | 76±5 [0.6] | 137±6 [1.0] | ||
| 750 | 133±12 [1.0] | |||
| TA 1535 | Test item | 0 | 13±3 | 12±1 |
| 15.63 | 13±2 [1.0] | |||
| 31.25 | 13±2 [1.0] | 9±2 [0.8] | ||
| 62.6 | 15±1 [1.2] | 10±1 [0.8] | ||
| 125 | 15±2 [1.2] | 10±3 [0.8] | ||
| 250 | 12±2 [0.9] | 9±1 [0.8] | ||
| 500 | 10±1 [0.8] | 10±3 [0.8] | ||
| 750 | 9±7 [0.8] | |||
| TA 98 | Test item | 0 | 29±5 | 35±3 |
| 15.63 | 23±3 [0.8] | |||
| 31.25 | 29±6 [1.0] | 36±1 [1.0] | ||
| 62.5 | 22±3 [0.8] | 34±3 [1.0] | ||
| 125 | 24±2 [0.8] | 42±1 [1.2] | ||
| 250 | 24±1 [0.8] | 34±3 [1.0] | ||
| 500 | 17±2 [0.6] | 33±6 [0.9] | ||
| 1,000 | 37±5 [1.1] | |||
| TA 1537 | Test item | 0 | 5±1 | 18±3 |
| 15.63 | 7±1 [1.4] | |||
| 31.25 | 6±2 [1.2] | 17±2 [0.9] | ||
| 62.5 | 6±2 [1.2] | 17±3 [0.9] | ||
| 125 | 6±1 [1.2] | 21±3 [1.2] | ||
| 250 | 6±0 [1.2] | 16±2 [0.9] | ||
| 500 | 3±2 [0.6] | 14±3 [0.8] | ||
| 750 | 7±2 [0.4] | |||
| E. coli | Test item | 0 | 32±4 | 35±7 |
| WP2uvrA | 125 | 34±1 [1.1] | 40±3 [1.1] | |
| 250 | 31±3 [1.0] | 35±1 [1.0] | ||
| 500 | 29±5 [0.9] | 32±3 [0.9] | ||
| 1,000 | 28±4 [0.9] | 33±3 [0.9] | ||
| 2,000 | 19±1 [0.6] | 23±3 [0.7] | ||
| 2,500 | 15±2 [0.5] | 18±0 [0.5] | ||
| Positive controls | ||||
| TA 100 | SA | 0.5 | 426±11 [3.6] | |
| TA 1535 | SA | 0.5 | 232±23 [17.8] | |
| TA 98 | 2-NF | 2 | 443±40 [15.3] | |
| TA 1537 | 9-AA | 50 | 467±42 [93.4] | |
| WP2uvrA | 4NQO | 0.5 | 13±16 [4.3] | |
| TA 100 | BP | 2 | 1,081±48 [8.1] | |
| TA 1535 | 2-AA | 2 | 11±2 [0.8] | 110±35 [9.2] |
| TA 98 | BP | 2 | 24±3 [0.8] | 457±11 [13.1] |
| TA 1537 | BP | 2 | 112±17 [6.2] | |
| WP2uvrA | 2-AA | 4 | 185±13 [5.3] | |
Number of relevant colonies on treated plate/Number of relevant colonies on vehicle control plate.
Test item: C. indicum flower oil.
SA, sodium azide; 2-NF, 2-nitrofluorene; 4NQO, 4-nitroquinoline 1-oxide; 2-AA, 2-aminoanthracene; BP, benzyl[a]pyrene.
Our results suggest that C. indicum flower oil produces no bone marrow micronucleus abnormalities, mutagenicity, or chromosomal aberrations, and thus might be considered a functional food or medicinal ingredient. However, further detailed studies, such as in vivo animal studies that further define toxicological properties, are required to understand the potential of adverse health effects from routine ingestion by humans.
ACKNOWLEDGMENTS
The authors wish to thank the Institute of Toxicology for technical assistance in conducting the animal and in vitro research studies. This study was supported by the Agriculture R&D Promotion Center, Ministry of Agriculture and Forestry, Republic of Korea (204024-03-3-SB010), and through a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2005-005-J13001).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Yu DO, Xie FZ, He WY, Liang XT. Application of 2D NMR techniques in the structure determination of chrysanthetriol. Acta Pharmaceutica Sinica. 1992;27:191–196. [PubMed] [Google Scholar]
- 2.Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M. Medicinal flowers. VI. absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull. 2002;50:972–975. doi: 10.1248/cpb.50.972. [DOI] [PubMed] [Google Scholar]
- 3.Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J Ethnopharmacol. 2005;101:334–337. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Kim HG, Kim YE, Do JR, Lee YC, Lee BY. Antioxidative activity and physiological activity of some Korean medicinal plants. Korean J Food Sci Technol. 1995;27:80–85. [Google Scholar]
- 5.Lee JH, Chang KM, Kim GH. Anti-inflammatory activities of Chopi (Zanthoxylum piperitum A.P. DC) essential oil: suppression of the inducible nitric oxide synthase and cellular adhesion. Food Sci Biotechnol. 2008;18:123–132. [Google Scholar]
- 6.Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96:151–158. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Chang KM, Kim GH. Comparative chemical composition of domestic and imported Chrysanthemum indicum L. flower oils. Food Sci Biotechnol. 2009;18:1288–1292. [Google Scholar]
- 8.Zhang C, Qin MJ, Shu P, Hong JL, Lu L, He DX. Chemical variations of the essential oils in flower heads of Chrysanthemum indicum L. from China. Chem Biodiver. 2010;7:2951–2962. doi: 10.1002/cbdv.201000034. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Li F, Zeng S. Capillary GC determination of β-elemene, camphor and borneol in Chrysanthemum morifolium. Yaowu Fenxi Zazhi. 2005;25:508–511. [Google Scholar]
- 10.Wang Y, Yang X. GC-MS analysis of essential oil of the flower of the Chrysanthemum morifolium by the different processing methods. Zhongguo Zhongyao Zazhi. 2006;31:456–459. [PubMed] [Google Scholar]
- 11.Schmid W. Chemical mutagen testing on in vivo somatic mammalian cells. Agents Act. 1973;3:77–85. doi: 10.1007/BF01986538. [DOI] [PubMed] [Google Scholar]
- 12.Heddle LA. A rapid in vivo test for chromosomal damage. Mutat Res. 1973;18:187–190. doi: 10.1016/0027-5107(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Nagashima T, Shimizu H. Occupational Health in the Chemical Industry. WHO Regional Office for Europe; Copenhagen, Denmark: 1998. Effects of carbon monoxide on the micronucleus test; pp. 156–166. [Google Scholar]
- 14.Heddle JA, Gimino MC, Hayashi M, Romagna F. Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen. 1991;18:277–291. doi: 10.1002/em.2850180414. [DOI] [PubMed] [Google Scholar]
- 15.Nagashima T, Okonogi H, Suzuki Y. Effects of anoxia on chromosomal aberration. Tokyo Jikeikai Med J. 1993;108:71–78. [Google Scholar]
- 16.Guerin MR. Energy sources of polycyclic aromatic hydrocarbons. In: Gelboin HV, Ts’o POP, editors. Polycyclic Hydrocarbons and Cancer: Chemistry, Molecular Biology and Environment. Academic Press; New York, NY, USA: 1978. pp. 1–42. [Google Scholar]
- 17.Miyamoto CT, Santanna JR, Franco CC, Castro-Prado MA. Genotoxicity (mitotic recombination) of the cancer chemotherapeutic agents’ cisplatin and cytosine arabinoside in Aspergillus indulans. Food Chem Toxicol. 2007;45:1091–1095. doi: 10.1016/j.fct.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Han EH, Hwang YP, Jeong TC, Lee SS, Shin JG, Jeong HG. Eugenol inhibit 7,12-dimethylbenz[a]anthracene-induced genotoxicity in MCF-7 cells: Bifunctional effects on CYP1 and NAD(P)H: quinine oxidoreductase. FEBS Lett. 2007;581:749–756. doi: 10.1016/j.febslet.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 19.Gomez NE, Witte L. A simple method to extract essential oils from tissue samples by using microwave radiation. J Chem Ecol. 2001;27:2351–2359. doi: 10.1023/a:1012295307740. [DOI] [PubMed] [Google Scholar]
- 20.AAALAC international. Association for Assessment and Accreditation of Laboratory Animal Care. 1998. [accessed July 2006]. http://www.aaalac.org.
- 21.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 22.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 23.OECD. OECD Principles on Good Laboratory Practice. Organization for Economic Cooperation and Development; 1997. [Google Scholar]
- 24.OECD. TG No. 471 ‘Bacterial reverse mutation test’. Organization for Economic Cooperation and Development; 1997. Guidelines for the testing of chemicals. [Google Scholar]
- 25.Sun J. D-Limonene: Safety and clinical applications. Alternative Med Rev. 2007;12:259–264. [PubMed] [Google Scholar]
- 26.Turner SD, Tinwell H, Piegorsch W, Schmezer P, Ashby J. The male rat carcinogens limonene and sodium saccharine are not mutagenic to male big blue rats. Mutagen. 2001;16:329–332. doi: 10.1093/mutage/16.4.329. [DOI] [PubMed] [Google Scholar]