Abstract
In this study, we evaluated the hypolipidemic and antioxidative effects of biotransformed soybean powder (BTS; Phellinus baumii-fermented soybean) on lipid metabolism in rats. Sprague-Dawley (SD) male rats were divided into basal diet group (BA), high fat diet group (HF), high fat diet containing 10% BTS group (10 BTS), and high fat diet containing 20% BTS group (20 BTS). Changes in the content of various isoflavones, including daidzein and genistein, within the soybean after fermentation to BTS were investigated. The levels of daidzein and genistein were 149.28 μg/g and 364.31 μg/g, respectively. After six weeks experimental period, Food efficiency ratio in the 10 and 20 BTS group was significantly lower than the HF group (P<0.05). Total serum levels of cholesterol, triglycerides, low-density lipoprotein cholesterol, and atherogenic index ratio in the 10 or 20 BTS group were significantly lower than the HF group. The levels of alanine aminotransferase, aspartate aminotransferase and thiobarbituric acid reactive substance were significantly lower in the groups that received 10% and 20% BTS than the HF. The activities of SOD and CAT were significantly higher in the 10 and 20 BTS group than the HF group. The activity of XO in the 10 and 20 BTS group was significantly lower than in the HF group by 20% and 23%, respectively. In conclusion, these data suggest that BTS is an effective agent in improving lipid metabolism and antioxidant enzyme system.
Keywords: biotransformed soybean, Phellinus baumii, hypolipidemia
INTRODUCTION
The incidence of patients with cardiovascular risk factors such as hypertension and obesity is on the rise, presumably due to poor lifestyle habits, including a lack of exercise and a high-calorie diet (1). As previously shown, a diet comprised of vegetables, fish oil, and seaweed may decrease serum cholesterol levels (2,3). Biochemically speaking, dietary fiber, a well-balanced amino acid composition, chlorophyll, saponin, phytosterols, and poly-unsaturated fatty acids have been shown to improve serum cholesterol levels (4).
The dietary intake of soybean has also been linked to decreased levels of cholesterol, cancer, osteoporosis, and cardiovascular disease (5–8). Several studies have shown that the important components of soybean include proteins, isoflavones, phytic acid, anthocyanin pigment, saponin, and unsaturated fatty acids (9,10). Indeed, mice that were fed a high-fat diet in addition to soybean products exhibited a decreased incidence of type 2 diabetes and atherogenic injury compared with mice fed only a high-fat diet (11). Cassidy (12) suggested that this effect was due to isoflavones; therefore, several studies have focused on these molecules. However, most components found in nature that induce isoflavone production exist in the form of glycosides, such as genistein, daidzein, and glycitin. Since these types of isoflavones cannot be digested by stomach acid, they are not absorbed directly. Absorption occurs only after an enzyme from a resident microorganism in the colon hydrolyzes the isoflavone; therefore, absorption rates are very low.
Isoflavones can also be converted by fermentation to aglycone, which can be absorbed directly by the stomach and small intestine without further alteration. Therefore, Aglycone isoflavones from fermented food are more useful than isoflavones from non-fermented food (13). Many techniques to produce aglycone isoflavones have been developed, including biotransformation (14). In biotransformation, an isoflavone is broken down to aglycone using a microorganism, which can produce β-glucosidase. Recently, it has been reported that dietary soy isoflavone has beneficial effect on antioxidant parameters in vivo(15,16).
In this study, soybean isoflavones were biotransformed using Phellinus baumii, which produces large amounts of β-glucosidase, to soybean aglycone, which is easily absorbed. To examine the effects of the product on lipid metabolism, rats were fed a high-fat diet comprised of 20% fat and 1% cholesterol to induce hyperlipidemia. Following the administration of BTS (Biotransformed soybean), weight change, anti-oxidative enzyme activation, diet efficiency, and total serum lipid levels were monitored in the animals.
MATERIALS AND METHODS
Materials
Soybean was obtained from local market in Andong, South Korea. Daidzein and genistein were purchased from Sigma Chemical Co. (St. Louis, MO, USA). High performance liquid chromatography (HPLC)-grade acetonitrile and water were purchased from Merck Co. (Darmstadt, Germany). All other reagents were of the highest grade available unless otherwise indicated.
Preparation of BTS
Soybean was washed, and soaked in the water for 12 h. Then, 50 g of soybeans were divided into each Erlenmeyer with 50 g, sterilize under a controlled environment (121ºC, 1.5 hPa). P. baumii cultured in Potato Dextrose Broth for 7 days, it (5%, v/v) was inoculated into soybean and cultured at 24ºC for 2 weeks. The soybean cultured was dried at 80ºC for 48 h and pulverized for use in this experiment. Contents of BTS were the following: moisture (4.65%), crude ash (4.9%), crude protein (40%), crude lipid (21%), crude fiber (1.45%), and carbohydrate (28%).
Measurement of total isoflavone and aglycone
The contents of total isoflavone and aglycone in BTS were determined by HPLC. BST powder 0.5 g was added in 2 mL of 1M HCl and extracted in 95ºC water bath for 2 h. After 2 h, the mixture was cooling, mixed with 8 mL of acetonitrile, and leaved for 2 h. The supernatant was collected to measure total isoflavone and aglycone. The samples were dried at 105ºC for 24 h and the solid contents were analyzed for isoflavone levels. The supernatant of extract was filtered through a syringe filter (0.22 μm, Waters Co., Milfore, MA, USA) prior to HPLC analysis. Reversed phase HPLC analysis was carried out with Yonglin SP930D system (Yonglin, Anyang, Korea), using a Merck RP-8 column (4×250 mm, Merck Co.). The aglycones were analyzed using an isocratic solvent system with the mixture of 0.1% phosphoric acid in 0.05 M KH2PO4 (solvent B) and acetonitrile (solvent A) (75:25, v/v) as the mobile phase. Following the injection of 20 μL of sample, solvent flow rate was 1.0 mL/min, and eluted statins were detected at UV 260 nm. Daidzein and genistein (Sigma Chemical Co.) were used as standard, the isoflavone contents were calculated from standard curve.
Animal treatment
Sprague-Dawley (SD) male rats (Orient Bio Inc., Gyeonggi-do, South Korea), 4 week-old (80~120 g), were used in the present study. The rats were housed individually in cages under a 12 h light/dark cycle in a temperature and a relative humidity controlled environment (21±2 ºC, 55±10%), freely access water and feed. All animal protocols were approved by the Animal Care Committee of Kyungpook National University. In the first week, for preliminary breeding, deionized water and the basal diet were provided daily. Then, the rats were randomized into four groups and six rats each [named as basal diet group (BA), high fat diet group (HF), high fat diet containing 10% BTS (10 BTS), and high fat diet containing 20% BTS (20 BTS)]. For the seven weeks, BA group was fed basal diet and experimental groups (HF, BTS) were fed high fat diet. After the seven weeks, HF group was fed high fat diet and BTS group was fed high fat diet containing BTS for additional six-weeks. The basal diet (BA) of rat was purchase from the Cargil Agri Purina Inc. (Gyeonggi-do, South Korea). The diet compositions are listed in Table 1. After the six weeks experimental period, the rats fasted for 14 h, and blood was collected from the abdominal aorta and centrifuged (3,000 rpm, 30 min) after standing for 30 min at room temperature. The supernatant was used to biochemical analysis.
Table 1.
Composition of experimental diets
| Composition | BA | HF | BTS 10 | BTS 20 |
|---|---|---|---|---|
| Casein1) | 20.0 | 20.0 | 20.0 | 20.0 |
| DL-Methionine2) | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline bitartrate2) | 0.25 | 0.25 | 0.25 | 0.25 |
| Corn starch3) | 62.95 | 48.95 | 41.45 | 33.45 |
| Cellulose2) | 5.0 | 5.0 | 5.0 | 5.0 |
| Mineral mix4) | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix4) | 1.0 | 1.0 | 1.0 | 1.0 |
| Lard5) | 7.0 | 20.0 | 17.5 | 15.5 |
| Cholesterol6) | – | 1.0 | 1.0 | 1.0 |
| BTS | – | 10.0 | 20.0 | |
| Total | 100 | 100 | 100 | 100 |
| Calorie | 400 | 461 | 408.5 | 358.5 |
Fonterra Co. (New Zealand).
Sigma Co. (USA).
Samyang genex Co. (Korea).
Dyets Co. (USA).
Cheiljedang Co. (Korea).
Kanto Co. (Japan).
Measurement of weight gain and diet intake
The experimental animals weight was measured once a week and diet intakes were calculate by subtracting the remainder from the supply. The gained weight through the experimental period was divided by cumulative dietary intake to obtain the food efficiency ratio (FER). The formula for FER as follow:
Blood analysis
Alanine amino transferase (ALT), aspartate amino transferase (AST), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglyceride (TG) in serum were measured using Automatic Chemistry Analyzer (KONELAB 20XT, Finland). An atherogenic index (AI) was calculated by the method of Yang et al. (17): AI= (TC–HDL-C)/HDL-C.
Thiobarbutric acid reactive substance (TBARS) content (nmole/mg) was measured using the method described by Ohkawa et al. (18). An absorbance at 532 nm was calculated for TBARS. The blood glutathione peroxidase (GSH) activity was determined by a modified dynamic method (17).
Antioxidant enzyme activities in liver
The liver tissue was homogenized in 0.25 M sucrose solution using a tissue homogenizer with a Teflon pestle at 4ºC to give 20% homogenate (w/v). The homogenate was centrifuged at 600×g for 10 min to remove any cell debris and then the supernatant was further centrifuged at 10,000×g for 20 min to remove the mitochondria pellet. Finally, the supernatant was ultracentrifuged at 105,000×g for 60 min to obtain the cytosol supernatant. The amounts of protein in the mitochondria and cytosolic fractions were measured by the method of Lowry et al. (19) with bovine serum albumin as the standard. The activity of Catalase (CAT) was assayed by measuring the hydrogen peroxide (H2O2) decreasing rate by the methods of Aebi (20). The activity of superoxide dismutase (SOD) was measured spectrophotometrically by the inhibition rate of auto-oxidation of hematoxylin by the methods of Martin et al. (21). The activity of xanthine oxidase (XO) was measured by the methods of Stripe and Della (22).
Statistical analysis
Values were given as mean±SD of 6 rats in each group. Data were analyzed using one-way ANOVA of variance followed by Duncan’s post hoc multiple test with SPSS (SPSS Inc., Chicago, IL, USA) for significance. The limit of statistical significance was set at P<0.05.
RESULTS AND DISCUSSION
BTS preparation and the isoflavone content
The isoflavones in 1 M HCl extracts of the BTS were analyzed by HPLC. As shown in Table 2, 74% of the total isoflavone was transformed to aglycones, including daidzein (149.28 μg/g) and genistein (364.31 μg/g).
Table 2.
Contents of isoflavones in soybeans biotransformed with P. banmii KCTC16882
| Total isoflavone (μg/g) | Aglycone isoflavone (μg/g) | Conversion yield (%)1) | |
|---|---|---|---|
|
| |||
| Daidzein | Genistein | ||
| 696.3 | 149.3 | 364.3 | 74.0 |
Conversion yield (%)=(contents of diadzein and genistein/contents of total isoflavone) × 100.
Previously, Pyo and Seong (23) reported that Monascus- fermented soybean extracts contained mostly aglycones and mevinolin; however, the fermentation method they used is time consuming. Interestingly, P. baumii displayed an increased rate of transformation, and thus required less time overall for the conversion to aglycone isoflavones. Together, these data provide a simple and efficient way to produce BTS.
Effect of BTS on body weight gain and diet efficiency
To determine the effect of BTS on diet efficiency, we monitored weight gain in animals that had been administered BTS along with a high-fat diet. Table 3 shows the body weight gain during the experimental period, including the initial body weight, final body weight after fasting for 12 h, food intake, and food efficiency ratio (FER).
Table 3.
The body weight, food intake, and food efficiency ratio in rats fed the experimental diets for 6 weeks
| Groups1) | Body weight | Food intake (g/day) | FER (%) | ||
|---|---|---|---|---|---|
|
| |||||
| Initial (g) | Final (g) | Gain (g/day) | |||
| BA | 459.2±37.5 | 502.0±29.4 | 0.9±0.4a | 24.5±1.7b | 3.8±0.4a |
| HF | 495.6±36.6 | 553.2±5.3 | 2.0±0.4b | 16.8±1.1a | 12.1±1.8b |
| BTS 10 | 489.2±26.6 | 536.7±32.5 | 0.9±0.3a | 18.7±1.0a | 4.8±1.5a |
| BTS 20 | 489.2±23.9 | 524.7±23.1 | 0.6±0.2a | 20.4±1.8a | 3.1±0.2a |
The values are mean±SD of 6 rats.
BA: basal diet group, HF: high fat diet group, BTS 10: high fat diet containing 10% BTS, BTS 20: high fat diet containing 20% BTS.
Values within a column with different superscript letters are significantly different each other groups at P<0.05.
The body weight gain of the rats in the HF group was increased compared to those in the BA group (P<0.05). However, both groups of BTS-treated animals (10 BTS and 20 BTS) exhibited less body weight gain compared to the animals in the HF group. Moreover, body weight gain in the 20 BTS group was lower than the 10 BTS group by 33%. The body weight change observed coincided with a collateral decrease in the FER.
Our results are similar to those of Jung and Wang (24) and Kyselova et al. (25). The former study reported that rats fed Chunggugjang for eight weeks slowly gained weight compared to control rats, whereas the latter study suggested that rats lost weight depending on the level of genistein ingested. Together with these results, our study indicates that isoflavones and other functional materials in soybean may assist in weight loss.
We also observed a difference in food intake among the experimental groups. Food intake in the BA group (24.5±1.7 g/day) was higher than in the other groups. However, in the BTS-treated groups, food intake was increased compared to that in the HF group. One study suggested that a high-fat diet affected the taste of food as well as the calorie density, so that the overall intake quantity was decreased (26). Similarly, the taste of food, and therefore the amount ingested, can be influenced by the types of proteins present (27). In this study, food consumption in the BTS groups was slightly higher than the HF group. However, food efficiency ratio in the BTS groups was significantly lower than the HF group. These results suggest that BTS can prevent high-fat diet-induced weight gain, since it decreases the food efficiency to that of a basal-level diet. Therefore, BTS could be an efficient agent in the treatment of obesity.
Effect of BTS on lipid metabolism
Table 4 shows the effect of BTS powder administration for seven weeks on the serum lipid levels of rats fed a high-fat diet. The HF group showed a significant increase in TC, LDL-C, TG, and AI levels to 137.6±7.1 mg/dL, 21.1±9.2 mg/dL, 180.7±9.4 mg/dL and 0.9±0.1 mg/dL, respectively, compared to the BA group. However, the 10 BTS group showed reductions in the levels of TC, TG, and AI. The 20 BTS group showed reductions in the levels of TC, LDL-C, TG, and AI. Furthermore, the administration of 20% BTS resulted in a significant reduction in the levels of TC (1.8-fold), LDL-C (1.5-fold), TG (2.0-fold) and AI (3.0-fold) compared with the HF group (Table 3).
Table 4.
Effect on serum lipid levels in rats fed the experimental diets
| Groups | TC (mg/dL) | TG (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | AI (mg/dL) |
|---|---|---|---|---|---|
| BA | 81.6±10.7a | 123.2±12.8ab | 64.9±14.6ab | 15.4±6.8a | 0.3±0.1a |
| HF | 137.6±7.1b | 180.7±19.4b | 73.5±17.2b | 21.1±9.2b | 0.9±0.1b |
| BTS 10 | 87.6±6.8ab | 93.0±10.1a | 75.3±16.1ab | 23.8±9.3b | 0.2±0.1a |
| BTS 20 | 74.6±8.9a | 90.5±11.2a | 70.9±10.2a | 14.5±5.7a | 0.3±0.1a |
The values are mean±SD of 6 rats.
Values within a column with different superscript letters are significantly different each other groups at P<0.05.
Many studies have reported the hyperlipidemic effects of soybean and fermented soybean in animals and humans. Furthermore, aglycone isoflavones (i.e., fermented soybean) seem to have greater activity than do glycoside isoflavones (11). Kawakami et al. (28) reported that the level of total serum isoflavones in male SD rats fed an aglycone isoflavone-rich diet was significantly higher than that in rats fed a glycoside isoflavone-rich diet. Moreover, an aglycone isoflavone-rich diet reduced total liver and serum cholesterol levels, as well as liver triglyceride levels in rats fed cholesterol. Thus, isoflavonoid-rich BTS treatment may be more effective in controlling lipid metabolism due to the aglycone isoflavone treatment.
Effect of BTS on the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
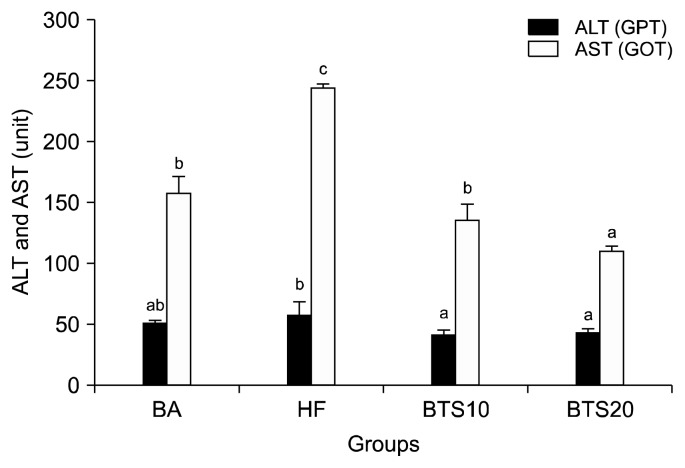
ALT and AST are critical for the transformation of amino acids. They reside in the liver and are vigorously activated in response to liver damage. Thus, ALT and AST levels are important indicators of liver function (24). To assess the effect of BTS on hepatic function, AST and ALT levels were measured following treatment. The levels of ALT in the HF group (58.0±11.7 dL) was higher than the BA group by 10.9%; however, it was significantly lower in the groups that received 10% and 20% BTS than the HF group by 28.3% and 24.0%, respectively. The levels of AST in the HF group (244.3± 4.2 dL) was higher than the BA group by 54.3%; however, it was significantly lower in the groups that received 10% and 20% BTS than the HF group by 44.2% and 54.8%, respectively (Fig. 1).
Fig. 1.
Hepatic function parameters in hyperlipidemic rats treated with BTS 10 and 20 powders. The values are mean±SD of 6 rats. a–cValues within a column with different superscript letters are significantly different each other groups at P<0.05.
The liver is generally considered to be the primary organ responsible for the maintenance of cholesterol homeostasis; therefore, the activities of serum ALT and AST as known markers of liver damage were evaluated (24). In this study, ALT and AST were markedly elevated in hyperlipidemic animals compared to the BA group, but the 10 and 20 BST group showed significantly reduced serum levels. The reduction in ALT and AST in the BTS 10 and BTS 20 groups is likely due to the inhibition of dietary cholesterol absorption.
Effect of BTS on lipid peroxidation and antioxidant enzyme activities in rat
To examine the effect of BTS treatment on lipid peroxidation and antioxidant enzyme activity, we monitored the levels of thiobarbituric acid reactive substance (TBARS) and glutathione (GSH). The level of TBARS in the HF group was significantly higher than the BA group and it was significantly lower in the 10 and 20 BTS group than the HF group by 86% and 82%, respectively. The activity of GSH in the HF group was lower than the BA group and it wasn’t significant differences between two groups. Moreover, it was significantly higher in the 10 and 20 BTS group than the HF group by 50% and 43%, respectively (Table 5). The activities of SOD and CAT were significantly higher in the 10 and 20 BTS group than the HF group. The activity of XO in the 10 and 20 BTS group was significantly lower than in the HF group by 20% and 23%, respectively (Table 6).
Table 5.
Effect of BTS on thiobarbituric acid reactive substance level and glutathione activity in rats
| Groups | TBARS1) | GSH2) |
|---|---|---|
| BA | 0.07±0.01a | 1.83±0.22ab |
| HF | 0.28±0.03b | 1.54±0.28a |
| BTS 10 | 0.04±0.01a | 2.31±0.15b |
| BTS 20 | 0.05±0.02a | 2.20±0.38b |
The values are mean±SD of 6 rats.
Thiobarbituric acid reactive substance (TBARS) unit: nmole/mg protein.
Glutathione (GSH) unit: μmole/mg protein.
Values within a column with different superscript letters are significantly different each other groups at P<0.05.
Table 6.
Effect of BTS on SOD, CAT and XO activities in rats
| Groups | SOD1) | CAT2) | XO3) |
|---|---|---|---|
| BA | 3.25±0.86c | 430.87±25.93c | 0.87±0.05a |
| HF | 1.87±0.16a | 317.32±20.01a | 1.28±0.10c |
| BTS 10 | 2.58±0.20b | 379.35±23.99b | 1.02±0.03b |
| BTS 20 | 2.63±0.20b | 390.87±20.93b | 0.98±0.06ab |
The values are mean±SD of 6 rats.
Superoxide dismutase (SOD) unit: nmole H2O2 reduced/mg protein/min.
Catalase (CAT) unit: nmole H2O2 reduced/mg protein/min.
Xanthine oxidase (XO) unit: nmole H2O2 reduced/mg protein/min.
Values within a column with different superscript letters are significantly different each other groups at P<0.05.
Increased reactive oxygen species levels are a causative factor linking hyperlipidemia with the pathogenesis of atherosclerosis. Oxidative stress occurs when the production of free radicals exceeds the capacity of the natural antioxidant system. High-fat diets have been shown to increase free radical production in vivo. Therefore, a high-fat diet leads to increased free radical production, which results in increased lipid peroxide levels (17). Antioxidative enzymes, however, are capable of eliminating free radicals and the products of lipid peroxidation, thereby protecting cells and tissues from oxidative damage. In the current study, the BTS groups showed decreased serum TBARS activity and increased blood GSH activity. Commonly known, GSH is a major defensive antioxidant agent that scavenges oxygen free radicals (29). SOD accelerates dismutation of superoxide anion radical to hydrogen peroxide that in turn is removed by CAT and GPx (30). The activities of SOD and CAT in HF group were significantly lower than the BA group, which could be due to the increased utilization of the enzyme for scavenging of oxygen free radicals overproduced in HF group. The activity of XO in BTS groups was significantly lower than the HF group, suggesting that BTS treatment acts upon decreasing in production of oxygen free radicals. The mechanisms by which the BTS treatment up-regulate antioxidant enzyme activity merit further investigation.
Our data suggest that BTS treatment may aid in lipid metabolism and antioxidant enzyme activities in both the serum and liver. We conclude that BTS has definite cardio-protective potential; moreover, we believe that this study constitutes a valid scientific basis for consuming BTS as a functional dietary supplement and in medicinal applications.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.JuŸwiak S, Wójcicki J, Mokrzycki K, Marchlewicz M, Białecka M, Wenda-Różewicka L, Gawrońska-Szklarz B, DroŸdzik M. Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol Rep. 2005;57:604–609. [PubMed] [Google Scholar]
- 2.Kobatake Y, Kuroda K, Jinnouchi H, Nishide E, Innami S. Differential effects of dietary eicosapentaenoic and docosahexaenoic fatty acid on lowering of triglyceride and cholesterol levels in the serum of fats on hypercholesterolemic diet. J Nutr Sci Vitaminol. 1984;30:357–372. doi: 10.3177/jnsv.30.357. [DOI] [PubMed] [Google Scholar]
- 3.Mcdonald BE, Gerrard JM, Bruce VM, Corner EJ. Comparison of the effect of canola oil and sunflower oil on plasma lipids and lipoproteins and on in vivo thromboxan A2 and prostacyclin production in healthy young men. Am J Clin Nutr. 1989;50:1382–1388. doi: 10.1093/ajcn/50.6.1382. [DOI] [PubMed] [Google Scholar]
- 4.Shon MY, Lee J, Choi JH, Choi SY, Nam SH, Seo KI, Lee SW, Sung NJ, Park SK. Antioxidant and free radical scavenging activity of methanol extract of chungkukjang. J Food Compost Anal. 2007;20:113–118. [Google Scholar]
- 5.Adlercreutz H. Phytoestrogens: epidemiology and a possible role in cancer protection. Environ Health Perspect. 1995;103:103–112. doi: 10.1289/ehp.95103s7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BA. Eating to beat breast cancer potential role for soy supplements. Ann Oncol. 1997;8:223–225. doi: 10.1023/a:1008237505645. [DOI] [PubMed] [Google Scholar]
- 8.Yugarani T, Tan BKH, Teh M, Das NP. Effects of polyphenolic natural products on the lipid profiles of rats fed high fat diets. Lipids. 1992;27:181–186. doi: 10.1007/BF02536175. [DOI] [PubMed] [Google Scholar]
- 9.Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001;172:1–6. doi: 10.1016/s0304-3835(01)00627-9. [DOI] [PubMed] [Google Scholar]
- 10.Shin EC, Hwang CE, Lee BW, Kim HT, Ko JM, Baek IY, Lee YB, Choi JS, Cho EJ, Seo WT, Cho KM. Chemometric approach to fatty acid profiles in soybean cultivars by principal component analysis (PCA) Prev Nutr Food Sci. 2012;17:184–191. doi: 10.3746/pnf.2012.17.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon DY, Daily JW, III, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy A. Physiological effects of phytoestrogens in relation cancer and other human health risks. Proc Nutr Soc. 1996;55:399–417. doi: 10.1079/pns19960038. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994;42:1666–1673. [Google Scholar]
- 14.Moon SW, Park MS, Ahn JB, Ji GE. Quality characteristics of chocolate blended with Bifidobacterium-fermented isofalvone powder. Korean J Food Sci Technol. 2003;6:1162–1168. [Google Scholar]
- 15.Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Viña J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 16.Kamboh AA, Zhu WY. Effect of increasing levels of bioflavonoids in broiler feed on plasma anti-oxidative potential, lipid metabolites, and fatty acid composition of meat. Poult Sci. 2013;92:454–461. doi: 10.3382/ps.2012-02584. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Yang L, Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol. 2010;48:2374–2379. doi: 10.1016/j.fct.2010.05.074. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohisi N, Yagi K. Assay for lipid peroxide in animal tissue by thiobabutiric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosenbrough NJ, Far AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Martin JP, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987;255:329–336. doi: 10.1016/0003-9861(87)90400-0. [DOI] [PubMed] [Google Scholar]
- 22.Stirpe F, Della CE. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O) J Biol Chem. 1969;244:3855–3863. [PubMed] [Google Scholar]
- 23.Pyo YH, Seong KS. Hypolipidemic effects of Monascus-fermented soybean extracts in rats fed a high-fat and -cholesterol diet. J Agric Food Chem. 2009;57:8617–8622. doi: 10.1021/jf901878c. [DOI] [PubMed] [Google Scholar]
- 24.Jung MJ, Wang MH. Effect of fermented soybean- derived Chungkookjang on diet-induced hyperlipidemia in BioF1B hamsters. Food Biotechnol. 2009;23:73–81. [Google Scholar]
- 25.Kyselova V, Peknicova J, Boubelik M, Buckiova D. Body and organ weight, sperm acrosomal status and reproduction after genistein and diethylstilbestrol treatment of CD1 mice in a multigenerational study. Theriogenology. 2004;61:1307–1325. doi: 10.1016/j.theriogenology.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Cho WK, Park HJ. Effect of chitosan treated with enzymatic methods on glucose and lipid metabolism in rats. Korean J Nutr. 1998;31:1112–1120. [Google Scholar]
- 27.Mitchell GV, Jenkins MY, Grundel E. Protein efficiency ratios and net protein ratios of selected protein foods. Plant Foods Hum Nutr. 1989;39:53–58. doi: 10.1007/BF01092401. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami Y, Tsurugasaki W, Nakamura S, Osada K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J Nutr Biochem. 2005;16:205–212. doi: 10.1016/j.jnutbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhou CY, Ran L, Zhang X. The effects on the serum lipids, fatty liver and lipid peroxidation of vitamin E in experimental hyperlipemia rats. Chinese J Clin Pharmacol Therapeutics. 2004;9:202–215. [Google Scholar]
- 30.Del Maestro RF. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]