Abstract
Recent studies have shown that Rosa canina L. and tiliroside, the principal constituent of its seeds, exhibit anti-obesity and anti-diabetic activities via enhancement of fatty acid oxidation in the liver and skeletal muscle. However, the effects of rosehip, the fruit of this plant, extract (RHE), or tiliroside on lipid accumulation in adipocytes have not been analyzed. We investigated the effects of RHE and tiliroside on lipid accumulation and protein expression of key transcription factors in both in vitro and in vivo models. RHE and tiliroside inhibited lipid accumulation in a dose-dependent manner in 3T3-L1 cells. We also analyzed the inhibitory effect of RHE on white adipose tissue (WAT) in high-fat diet (HFD)-induced obesity mice model. Male C57BL/6J mice were fed HFD or HFD supplemented with 1% RHE (HFDRH) for 8 weeks. The HFDRH-fed group gained less body weight and had less visceral fat than the HFD-fed group. Liver weight was significantly lower in the HFDRH-fed group and total hepatic lipid and triglyceride (TG) content was also reduced. A significant reduction in the expression of peroxisome proliferator-activated receptor gamma (PPARγ) was observed in epididymal fat in the HFDRH-fed group, in comparison with controls, through Western blotting. These results suggest that downregulation of PPARγ expression is involved, at least in part, in the suppressive effect of RHE on lipid accumulation in WAT.
Keywords: rosehip, tiliroside, 3T3-L1 cells, lipid accumulation, PPARγ
INTRODUCTION
Obesity is a global problem caused by an imbalance in energy intake and consumption and carries a strong risk for type 2 diabetes associated with insulin resistance. Excessive lipid accumulation in white adipose tissue (WAT), particularly visceral fat, can cause dysbolisms such as hyperlipidemia, hypertension, and arteriosclerosis, and these disorders can induce fatal cardiovascular disease (1). The primary care for these disorders is enhancement of energy consumption by exercise and optimization of energy intake by diet modification. Decrease in visceral fat content is important for control of these metabolic diseases.
Recently, various food materials or natural compounds isolated from edible plants that show anti-obesity effect have been screened for use as functional foods or dietary supplements. In our search for novel food materials having anti-obesity effect, we have screened many herbs collected from all parts of the world. We found that tiliroside, a principal constituent of rosehip seed, accelerates fat metabolism in the liver and improves glucose clearance (2).
Rosa canina L. (Rosaceae), known as dog rose, is widely distributed in and around Europe. The fruit of this plant, rosehip, has been used as a diuretic, laxative, anti-gout, and anti-rheumatic remedy in traditional European medicine. The fruit is rich in anti-oxidants such as ascorbic acid (3), phenolic compounds (4), and carotenoids (5). Ninomiya et al. (2) reported that the 80% aqueous acetone extract, containing the active constituent trans-tiliroside, a glycosidic flavonoid, from the whole fruit of Rosa canina L. showed a substantial inhibitory effect on body weight gain in non-obese mice (Fig. 1). In mice, a single oral administration of tiliroside at a dose of 10 mg/kg upregulated the expression of PPARalpha mRNA in the liver; thus, tiliroside may stimulate fatty acid oxidation. Using the obese-diabetic mouse model KK-Ay, tiliroside administration ameliorated dyslipidemia, insulin resistance, and hypoadiponectinemia by activation of adiponectin signaling in the liver and skeletal muscle (6). Moreover, tiliroside and its derivatives significantly enhance glucose consumption in HepG2 cells via activation of acetyl-CoA carboxylase and adenosine 5′-monophosphate-activated protein kinase (AMPK) (7). Andersson et al. have shown that high doses of rosehip powder can both prevent and reverse the increase in body weight and decrease in glucose tolerance caused by a high-fat diet in the C57BL/6J mouse model (8). From these studies, rosehip extract and tiliroside are considered to have anti-obesity and anti-diabetic effects.
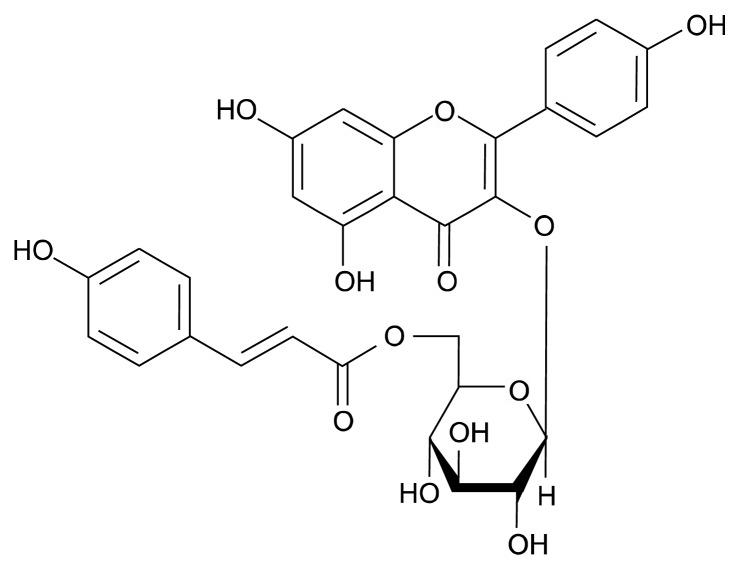
Fig. 1.
The structure of tiliroside.
Tiliroside, an acylated kaempferol glycoside, is the principal polyphenol in seeds of Rosa canina L. (2) and is also present in several medicinal and dietary plants such as lindens, strawberries, and raspberries (9–11). Tiliroside possesses anti-inflammatory, antioxidant, anti-carcinogenic, cytochrome P450 inhibitory, tyrosinase inhibitory, glucose absorption inhibitory, and hepatoprotective activities (9–15). However, almost all the studies about analyzing the effects of rosehip or tiliroside in metabolic disorders have focused on fatty acid metabolism in non-adipose tissues such as liver or skeletal muscle; no reports focus on adipocytes or adipose tissue. In this study, we evaluated the effect of rosehip extract and tiliroside on lipid accumulation in 3T3-L1 preadipocytes. We also investigated whether rosehip extract affects the expression of key transcriptional factors involved in lipogenesis in a mouse model of high-fat-induced obesity.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), penicillin, and streptomycin were purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from Life Technologies (Carlsbad, CA, USA). Dimethyl sulfoxide (DMSO) and other chemicals, unless otherwise indicated, were purchased from Wako (Osaka, Japan) or Sigma. The chemicals were of guaranteed grade or tissue culture grade. Rosehip extract (RHE), also known as rosehip polyphenol EX (Morishita Jintan, Osaka, Japan), consists of aqueous ethanol extract of whole rosehip containing seeds, dextrin, and cyclodextrin. RHE is guaranteed to contain more than 0.1% of tiliroside as the active ingredient. Tiliroside was extracted and purified from the seeds of Rosa canina L. as previously described (2).
Cell culture and adipocyte differentiation
3T3-L1 preadipocytes were obtained from the Human Science Research Resources Bank (HSRRB, Osaka, Japan). Cells were maintained in DMEM supplemented with 10% FBS containing 100 units/mL of penicillin and 100 μg/mL of streptomycin. Cells were seeded into 24-well plates, cultured for 4 days, and induced to differentiate into adipocytes by changing the culture medium to a differentiation/induction medium containing 0.5 mM isobutylmethylxanthine (IBMX), 0.25 μM dexamethasone, 10 μg/mL insulin, and 10% FBS in DMEM (day 5). Three days later, the culture medium was changed to fresh differentiation/induction medium and the culture was maintained for the next 4 days. RHE and tiliroside dissolved in DMSO were added to the differentiation/induction medium. Cells were treated with 1 μg/mL of berberine chloride (BBR) or 125, 250, and 500 μg/mL of RHE or 31.3, 62.5, and 125 μg/mL of tiliroside during days 5–11. BBR was used as a positive control.
Oil red O staining
Oil red O staining and absorbance measurements were performed in accordance with the manufacturer’s protocol (Adipogenesis Assay Kit, Cayman Chemical Company, Ann Arbor, MI, USA). Briefly, at day 7, the culture medium was removed by aspiration and the cells were fixed using fixative solution. The cells were gently washed with phosphate-buffered saline (PBS) and stained with oil red O solution. After staining, the staining solution was removed and the plates were rinsed with distilled water and PBS. The stained lipid droplets were visualized under an Olympus microscope (IX 50, Tokyo, Japan) and photographed using a digital camera (G10, Canon, Tokyo, Japan). The droplets were extracted using an extraction solution and the lipids were quantified by absorbance (520 nm) measurements. Accumulation rate was calculated as follows:
where S and C represent the absorbance of sample solution and untreated control solution, respectively, and BLave represents the average absorbance of undifferentiated preadipocytes.
Animals
Nine-week-old male C57BL/6J mice (Japan SLC, Inc., Hamamatsu, Japan) were housed at a constant temperature of 23±1°C with a 12 h light-dark cycle and fed a standard laboratory chow (AIN-93M, Oriental Yeast, Tokyo, Japan) for a week. All mice were fasted for 20 h prior to the experiment. The mice were randomly divided into two groups (n=5) and fed either a high-fat diet (HFD-60, Oriental Yeast; HFD) or a high-fat diet containing 1% RHE (HFDRH) for 8 weeks. The composition of the experimental diets is shown in Table 1. The mice had ad libitum access to food and water. Food intake and body weight were measured twice a week. At day 55, the mice were deprived of food for 18 h and were sacrificed by exsanguination from abdominal aorta under anesthesia. Blood samples were immediately centrifuged to separate serum and stored at −80°C until further analysis. White adipose tissue (WAT) from mesenteric, perirenal, and epididymal sites and liver were removed and weighed. A part of the excised epididymal fat was immediately immersed in Allprotect Tissue Reagent (Qiagen, Valencia, CA, USA) to stabilize the proteins and stored at −80°C until immunoblot analysis. The excised liver was also stored at −80°C until lipid analysis. Plasma glucose, triglycerides (TG), and free fatty acid (FFA) concentrations were determined using commercial kits (Wako). Plasma insulin, high molecular weight (HMW) adiponectin, and leptin (Shibayagi, Gunma, Japan) concentrations were measured using an enzyme-linked immunosorbent assay kit in accordance with the manufacturer’s instructions. The mice were all treated in strict accordance with Doshisha Women’s College of Liberal Arts guidelines for the care and use of laboratory animals.
Table 1.
Composition of experimental diets (g/100 g)
| Ingredient | HFD1) | HFDRH2) |
|---|---|---|
| Casein | 25.60 | 25.60 |
| l-Cystine | 0.36 | 0.36 |
| Maltodextrin | 6.00 | 6.00 |
| α-Corn starch | 16.00 | 15.00 |
| Sucrose | 5.50 | 5.50 |
| Soybean oil | 2.00 | 2.00 |
| Lard | 33.00 | 33.00 |
| Cellulose | 6.61 | 6.61 |
| AIN-93G mineral mix | 3.50 | 3.50 |
| AIN-93 vitamin mix | 1.00 | 1.00 |
| Calcium carbonate | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 |
| RHE3) | - | 1.00 |
| Total | 100.00 | 100.00 |
| Energy (kcal/g) | 5.062 | 5.063 |
High-fat diet.
High-fat diet containing 1% rosehip extract.
Rosehip extract.
Lipid measurements
Hepatic TG content was determined by the Folch method with slight modifications (16). Briefly, the harvested liver samples were lyophilized and ground to powder in test tubes. Chloroform : methanol (2:1, v/v) extraction solution was added and the samples were vortexed vigorously. After centrifugation, the supernatant was rinsed with distilled water. After additional centrifugation, the organic layer was collected and evaporated under reduced pressure and the resulting residue was weighed as total lipids. The samples were resuspended in isopropyl alcohol containing 10% Triton X-100 and TG contents were measured enzymatically using Triglyceride E-test Wako (Wako). The IC50 values were calculated by probit analysis.
SDS-PAGE and immunoblotting
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed following the standard Laemmli method (17). A sample buffer (TEFCO, Tokyo, Japan) containing 5% 2-mercaptoethanol was added to the protein samples. The samples were denatured at 95°C for 5 min and subjected to SDS-PAGE on a 7~20% gradient gel (TEFCO). For immunoblotting, proteins separated by SDS-PAGE were electrophoretically transferred to polyvinylidene fluoride membranes (TEFCO). The membranes were blocked with SuperBlock Blocking Buffer (Thermo Fisher Scientific, Waltham, MA, USA) and incubated overnight at 4°C with a specific antibody diluted in PBS containing 0.1% Tween 20. After washing, the membranes were incubated with 1:20,000 diluted horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibodies (GE Healthcare, Piscataway, NJ, USA) for 1 h at room temperature and then washed thoroughly. Each protein was detected using ECL Prime (GE Healthcare) and analyzed with LAS-3000 mini image analyzer (Fujifilm, Tokyo, Japan).
Analysis of PPARγ expression
Western blotting analysis was performed to determine the level of PPARγ expression in epididymal WAT. Briefly, stored WAT were homogenized in extraction buffer (50 mM Tris-HCl, 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1.0% NP-40) containing protease and phosphatase inhibitor cocktails (Roche Diagnostics, Mannheim, Germany) using a high-throughput sample disruptor (TissueLyser II, Qiagen). The homogenate was centrifuged at 15,000×g for 30 min at 4°C and the resulting supernatant was recovered. The protein concentration in the samples was determined using the BCA Protein Assay Reagent Kit (Thermo Fisher Scientific) and 20 μg protein samples were applied to SDS-PAGE. PPARγ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, internal standard) were visualized using specific antibodies against PPARγ (Cell Signaling Technology, Danvers, MA, USA) and GAPDH (GeneTex, Irvine, CA, USA), respectively. The band quantifications were performed using Multi Gauge Ver. 3.0 software (Fujifilm).
Statistical analysis
All data are presented as mean±SEM. For statistical analysis, one-way analysis of variance followed by the Dunnett’s test or Student’s t test was performed using JMP 9 (SAS Institute, Cary, NC, USA). Probability (P) values of less than 0.05 were considered statistically significant.
RESULTS
Inhibitory effect of RHE and tiliroside on lipid accumulation in 3T3-L1 cells
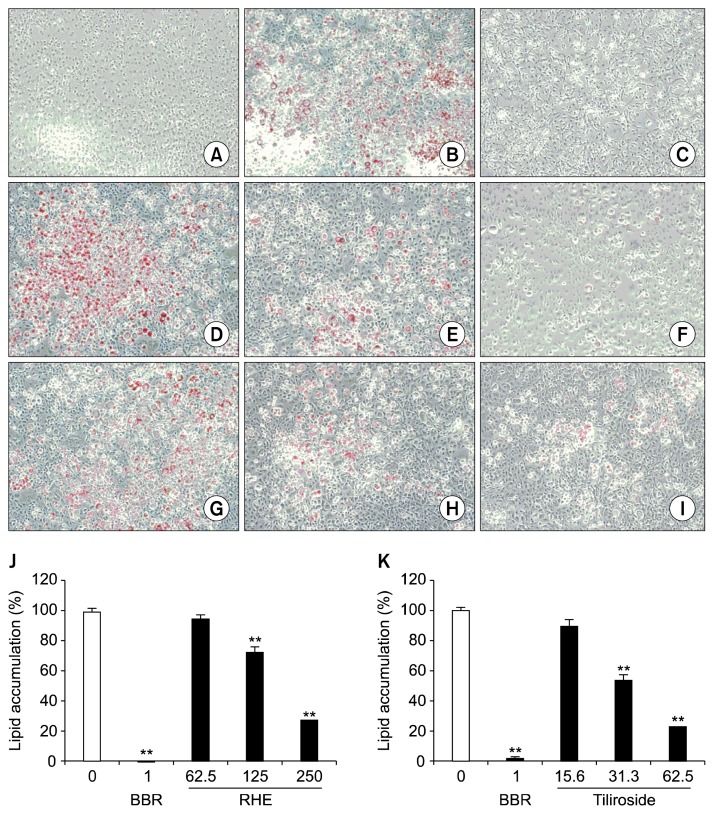
To evaluate whether RHE and tiliroside affect lipid accumulation in adipocytes, 3T3-L1 preadipocytes were treated with 1 μg/mL of BBR, 62.5, 125, or 250 μg/mL of RHE, or 15.6, 31.3, or 62.5 μg/mL of tiliroside in the presence of differentiation/induction medium. As shown in Fig. 2, lipid droplets stained by oil red O were not observed in undifferentiated cells, compared to many stained lipid droplets in the differentiated 3T3-L1 adipocytes (Fig. 2A, B). BBR completely inhibited lipid accumulation (Fig. 2C), and RHE and tiliroside treatment also suppressed lipid accumulation in differentiated adipocytes (Fig. 2D~I). In comparison with vehicle-treated 3T3-L1 adipocytes, the number of lipid droplets in RHE- or tiliroside-treated cells decreased in a concentration-dependent manner. RHE and tiliroside significantly inhibited lipid accumulation at concentrations above 125 and 31.3 μg/mL, respectively (Fig. 2J, K). Calculated IC50 values were 185.1 μg/mL (RHE) and 34.3 μg/mL (tiliroside).
Fig. 2.
Effect of BBR, RHE, or tiliroside on lipid accumulation in 3T3-L1 adipocytes. 3T3-L1 preadipocytes were cultured in differentiation medium for 7 days, supplemented with BBR (1 μg/mL), RHE (62.5, 125, and 250 μg/mL), or tiliroside (15.6, 31.3, and 62.5 μg/mL). Oil red O staining was performed to confirm lipid accumulation visually and to determine the lipid content. Absorbance of the extracted oil red O was measured at 520 nm. (A) Undifferentiated preadipocytes, (B) Untreated control, (C) Adipocytes treated with BBR at a concentration of 1 μg/mL, (D~F) Adipocytes treated with RHE at concentrations of 62.5, 125, and 250 μg/mL, (G~I) Adipocytes treated with tiliroside at concentrations of 15.6, 31.3, and 62.5 μg/mL, (J, K) Lipid accumulation rates of RHE- and tiliroside-treated adipocytes, respectively. Each column represents mean±SEM. All experiments were performed in triplicate. Significantly different from untreated control (white column), **P<0.01 (Dunnett’s test). BBR, berberine chloride; RHE, rosehip extract.
Effect of RHE on mouse body weight and adiposity
Table 1 shows the composition of the experimental diets. One gram of α-corn starch per 100 g of HFD was replaced with the same amount of RHE to prepare HFD containing 1% RHE (HFDRH). To examine the anti-obesity effect of RHE, the C57BL/6J mice were fed HFD or HFDRH for 8 weeks. At the end of the experimental period, the body weight of mice in the HFDRH group was significantly lower than that of mice in the HFD group (Table 2, P<0.05). The amount of visceral fat, particularly the mesenteric and perirenal fat, decreased significantly in the HFDRH-fed group. Moreover, liver weight was significantly lowered by administration of HFDRH. Hepatic lipid and TG content of the HFDRH-fed group tended to be lower than that of the HFD-fed group, at 54.3% and 32.5%, respectively, however, these two differences were not statistically significant. Several biochemical parameters of blood were determined using commercial determination kits (Table 3). In the HFDRH-fed group, the concentration of insulin and HMW adiponectin was significantly increased, whereas that of leptin was not altered. No changes were observed in other parameters such as blood glucose, FFA, and TG concentrations.
Table 2.
Effect of RHE on body weight, hepatic weight, and hepatic lipid content
| HFD | HFDRH | |
|---|---|---|
| Final body weight (g) | 41.5±1.5 | 37.3±1.0* |
| Body weight gain (g) | 15.9±1.3 | 12.2±0.7* |
| Mesenteric fat (mg/100 g B.W.) | 1,024±135 | 579±58* |
| Perirenal fat (mg/100 g B.W.) | 1,237±68 | 878±56** |
| Epididymal fat (mg/100 g B.W.) | 2,476±114 | 2,021±167 |
| Total visceral fat (mg/100 g B.W.) | 4,737±305 | 3,478±261* |
| Liver weight (mg) | 1,354±96 | 1,068±65* |
| Total hepatic lipid (mg/liver) | 466.2±128.6 | 212.9±26.5 |
| Hepatic TG content (mg/liver) | 16.6±2.8 | 11.2±1.9 |
Values are represented as mean±SEM and n=5. Significantly different from HFD group, *P<0.05, **P<0.01 (Student’s t test). RHE, rosehip extract; HFD, high-fat diet group; HFDRH, high-fat diet containing 1% rosehip extract group; TG, triglyceride.
Table 3.
Effect of RHE on blood parameters
| HFD | HFDRH | |
|---|---|---|
| Glucose (mg/dL) | 252.6±48.3 | 196.9±6.8 |
| FFA (mEq/L) | 1.0±0.1 | 0.9±0.1 |
| TG (mg/dL) | 80.6±5.8 | 76.7±1.7 |
| Insulin (ng/mL) | 0.52±0.01 | 0.57±0.02* |
| HMW adiponectin (ng/mL) | 738.4±38.6 | 901.2±34.6* |
| Leptin (ng/mL) | 1.96±0.20 | 1.96±0.35 |
Values are represented as mean±SEM and n=5. Significantly different from HFD group, *P<0.05 (Student’s t test). RHE, rose-hip extract; HFD, high-fat diet group; HFDRH, high-fat diet containing 1% rosehip extract group; FFA, free fatty acid; TG, triglyceride; HMW, high molecular weight.
PPARγ expression in WAT
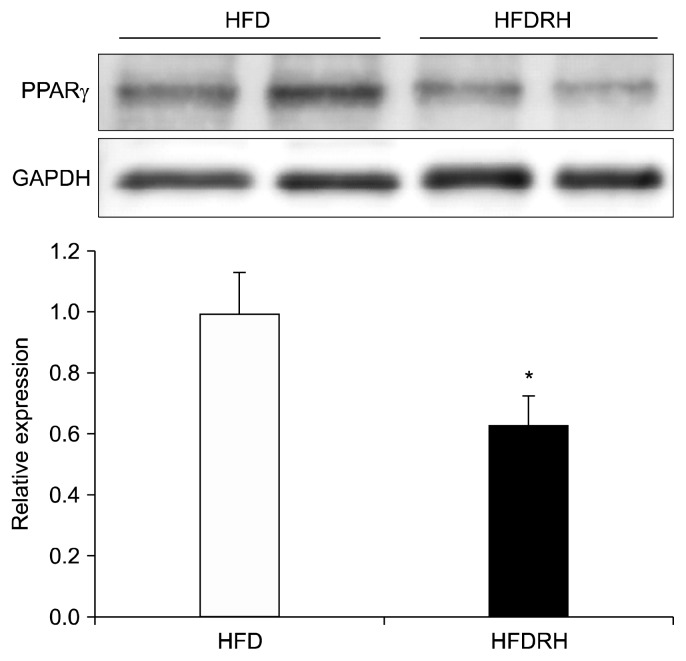
To examine PPARγ expression in WAT, an epididymal fat portion was collected and total protein was extracted from the harvested adipose tissue for PPARγ protein expression analyzed by Western blotting. The intensity of PPARγ bands was normalized against that of GAPDH bands. As shown in Fig. 3, PPARγ protein expression in the HFDRH-fed group was significantly lower than that in the HFD-fed group (38.8%).
Fig. 3.
Effect of RHE on PPARγ expression in epididymal fat. Male C57BL/6 J mice were fed HFD or HFDRH for 8 weeks. On the final day, a part of epididymal fat was collected and total protein extracted. The amount of PPARγ was quantified by Western blotting. Protein expression of PPARγ was quantified and normalized against GAPDH. Each column represents mean±SEM and n=5. Significantly different from the HFD-fed group (white column), *P<0.05 (Student’s t test). RHE, rosehip extract; HFD, high-fat diet group; HFDRH, high-fat diet containing 1% rosehip extract group; PPARγ, peroxisome proliferator-activated receptor gamma; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Nuclear receptors are responsible for the transcriptional regulation of the vast majority of the genes encoding metabolic enzymes. These receptors are transcription factors that respond to small lipophilic hormones, vitamins, and metabolites. PPAR family of nuclear receptors take part in the genetic regulation of complex pathways involved in mammalian metabolism such as fatty acid oxidation and lipogenesis that occur in response to nutritional and physiological stimuli (18).
Development of obesity is characterized by the growth of adipose tissue mass. One strategy to reduce adiposity would be to inhibit lipogenesis in WAT. PPARγ, predominantly expressed in the adipose tissue and immune system, performs the key role as regulator of adipogenesis, lipogenesis, and adipose insulin sensitivity (19,20). Thiazolidinediones (TZDs), a class of clinical drugs for the treatment of insulin resistance, act as PPARγ ligands (21). PPARγ stimulated by TZDs increases the number of small adipocytes, induces apoptosis of large adipocytes, and promotes adiponectin production, resulting in improved insulin resistance. However, the use of TZDs has been beset by severe side effects including weight gain, edema, cardiovascular toxicity (22), and bone loss (23). Several plant extracts and compounds that show an inhibitory effect on body weight gain via suppression of PPARγ expression have been reported (24–27). As previously suggested, kaempferol, a type of flavonol shown to be a PPARγ antagonist, exerts its anti-obesity effects partly through the down-regulation of PPARγ (28). BBR potently inhibits the differentiation of 3T3-L1 preadipocytes by inhibiting the mRNA and protein levels of PPARγ and C/EBPα and their upstream regulators (29). Therefore, we selected BBR as the positive control in our in vitro study.
In our study, lipid accumulation in 3T3-L1 preadipocytes, differentiated in the presence of insulin, dexamethasone, and IBMX, was suppressed by BBR, RHE, and tiliroside. The IC50 values were 185.1 (RHE) and 34.3 μg/mL (tiliroside) (Fig. 2). Feeding the mice the high-fat diet containing 1% RHE prevented body weight gain and decreased visceral fat accumulation. We analyzed protein expression in visceral fat to examine the anti-obesity effect of RHE and found that PPARγ expression decreased in RHE-treated animals (Fig. 3).
Adenosine 5′-monophosphate-activated protein kinase (AMPK) is an energy sensor that is activated when the cell energy level is low (30). AMPK regulates the uptake and metabolism of glucose and fatty acids, as well as the synthesis and oxidation of fatty acids, cholesterol, glycogen, and protein (31,32). Activated AMPK inhibits lipogenesis by phosphorylating PPARγ (33), and reduces PPARγ mRNA levels (34,35). In a previous report, Goto et al. revealed that a 21-day administration of tiliroside enhanced AMPK phosphorylation in the liver and muscle (6), suggesting that repression of PPARγ expression by RHE can be attributed to AMPK activation in adipose tissue.
The RHE used in this study contained aqueous ethanol extract from rosehips and excipients, and the standardized tiliroside content was more than 0.1% (w/w). If tiliroside was the only compound inhibiting lipid accumulation, the effective concentration of tiliroside would need to be approximately 200 ng/mL. However, our results showed that IC50 value of tiliroside was 34.1 μg/mL, which is much higher than the estimated effective concentration. This result suggests that components other than tiliroside are involved in the inhibitory effect of RHE on lipid accumulation in 3T3-L1 cells. Further investigation is needed to find all active constituents.
In our study, blood concentration of HMW adiponectin in the high-fat diet containing 1% rosehip extract (HFDRH)-fed group was higher than that in the high-fat diet (HFD)-fed group. This phenomenon may be explained by the inhibition of lipid accumulation by RHE, which may alleviate the decrease in adiponectin secretion often observed in adipocyte hypertrophy. Blood concentration of insulin was also significantly higher in the HFDRH-fed group, although the change was suspected to be in the range of physiological variation. In contrast, liver weight of the HFDRH-fed group was significantly decreased and hepatic lipid and TG contents also had decreased. Andersson et al. reported that rosehip powder reduces hepatic TG and cholesterol contents as a result of downregulation of the hepatic lipogenic program (8). Our results did not contradict their conclusions.
In conclusion, RHE and tiliroside suppressed lipid accumulation associated with the differentiation of 3T3-L1 preadipocytes. In the HFD-induced obesity mice model, RHE administration inhibited body weight gain and decreased the amount of visceral fat. Moreover, the expression of PPARγ in WAT had significantly decreased with RHE administration. These results suggested that PPARγ suppression is involved, at least in part, in the mechanism of RHE inhibiting lipid accumulation. RHE may be a good candidate food material for preventing obesity.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Crowley VE. Overview of human obesity and central mechanisms regulating energy homeostasis. Ann Clin Biochem. 2008;45:245–255. doi: 10.1258/acb.2007.007193. [DOI] [PubMed] [Google Scholar]
- 2.Ninomiya K, Matsuda H, Kubo M, Morikawa T, Nishida N, Yoshikawa M. Potent anti-obese principle from Rosa canina: structural requirements and mode of action of trans-tiliroside. Bioorg Med Chem Lett. 2007;17:3059–3064. doi: 10.1016/j.bmcl.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 3.Stralsjo L, Alklint C, Olsson ME, Sjoholm I. Total folate content and retention in rosehips (Rosa ssp.) after drying. J Agric Food Chem. 2003;51:4291–4295. doi: 10.1021/jf034208q. [DOI] [PubMed] [Google Scholar]
- 4.Daels-Rakotoarison DA, Gressier B, Trotin F, Brunet C, Luyckx M, Dine T, Bailleul F, Cazin M, Cazin JC. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother Res. 2002;16:157–161. doi: 10.1002/ptr.985. [DOI] [PubMed] [Google Scholar]
- 5.Hodisan T, Culea M, Cimpoiu C, Cot A. Separation, identification and quantitative determination of free amino acids from plant extracts. J Pharm Biomed Anal. 1998;18:319–323. doi: 10.1016/s0731-7085(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 6.Goto T, Teraminami A, Lee JY, Ohyama K, Funakoshi K, Kim YI, Hirai S, Uemura T, Yu R, Takahashi N, Kawada T. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese-diabetic mice. J Nutr Biochem. 2012;23:768–776. doi: 10.1016/j.jnutbio.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Qin N, Li CB, Jin MN, Shi LH, Duan HQ, Niu WY. Synthesis and biological activity of novel tiliroside derivants. Eur J Med Chem. 2011;46:5189–5195. doi: 10.1016/j.ejmech.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 8.Andersson U, Henriksson E, Ström K, Alenfall J, Göransson O, Holm C. Rose hip exerts antidiabetic effects via a mechanism involving downregulation of the hepatic lipogenic program. Am J Physiol Endocrinol Metab. 2011;300:E111–E121. doi: 10.1152/ajpendo.00268.2010. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda H, Ninomiya K, Shimoda H, Yoshikawa M. Hepatoprotective principles from the flowers of Tilia argentea (linden): structure requirements of tiliroside and mechanisms of action. Bioorg Med Chem. 2002;10:707–712. doi: 10.1016/s0968-0896(01)00321-2. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto S, Tomise K, Aburatani M, Onuki H, Hirota H, Ishiharajima E, Ohta T. Isolation of cytochrome P450 inhibitors from strawberry fruit, Fragaria ananassa. J Nat Prod. 2004;67:1839–1841. doi: 10.1021/np0400104. [DOI] [PubMed] [Google Scholar]
- 11.Lu YH, Chen J, Wei DZ, Wang ZT, Tao XY. Tyrosinase inhibitory effect and inhibitory mechanism of tiliroside from raspberry. J Enzyme Inhib Med Chem. 2009;24:1154–1160. doi: 10.1080/14756360802694252. [DOI] [PubMed] [Google Scholar]
- 12.Sala A, Recio MC, Schinella GR, Máñez S, Giner RM, Cerdá-Nicolás M, Rosí JL. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur J Pharmacol. 2003;461:53–61. doi: 10.1016/s0014-2999(02)02953-9. [DOI] [PubMed] [Google Scholar]
- 13.Tomczyk M, Drozdowska D, Bielawska A, Bielawski K, Gudej J. Human DNA topoisomerase inhibitors from Potentilla argentea and their cytotoxic effect against MCF-7. Pharmazie. 2008;63:389–393. [PubMed] [Google Scholar]
- 14.Rao YK, Geethangili M, Fang SH, Tzeng YM. Anti-oxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study. Food Chem Toxicol. 2007;45:1770–1776. doi: 10.1016/j.fct.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Goto T, Horita M, Nagai H, Nagatomo A, Nishida N, Matsuura Y, Nagaoka S. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Mol Nutr Food Res. 2012;56:435–445. doi: 10.1002/mnfr.201100458. [DOI] [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Moreno M, Lombardi A, Silvestri E, Senese R, Cioffi F, Goglia F, Lanni A, Lange P. PPARs: Nuclear receptors controlled by, and controlling, nutrient handling through nuclear and cytosolic signaling. PPAR Res. 20102010 doi: 10.1155/2010/435689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 20.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans R. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas A, Blandon J, Rude J, Elfar A, Mukherjee D. PPAR-γ agonist in treatment of diabetes: cardiovascular safety considerations. Cardiovasc Hematol Agents Med Chem. 2012;10:124–134. doi: 10.2174/187152512800388948. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 23.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-g agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women. A randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 24.Rayalam S, Della-Fera MA, Yang JY, Park HJ, Ambati S, Baile CA. Resveratrol potentiates genistein’s antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J Nutr. 2007;137:2668–2673. doi: 10.1093/jn/137.12.2668. [DOI] [PubMed] [Google Scholar]
- 25.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 26.Roh C, Park MK, Shin HJ, Jung U, Kim JK. Buddleja officinalis Maximowicz extract inhibits lipid accumulation on adipocyte differentiation in 3T3-L1 cells and high-fat mice. Molecules. 2012;17:8687–8695. doi: 10.3390/molecules17078687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Kim KA, Kim DH. Ginsenoside Rh1 ameliorates high fat diet-induced obesity in mice by inhibiting adipocyte differentiation. Biol Pharm Bull. 2013;36:102–107. doi: 10.1248/bpb.b12-00558. [DOI] [PubMed] [Google Scholar]
- 28.Park UH, Jeong JC, Jang JS, Sung MR, Youn H, Lee SJ, Kim EJ, Um SJ. Negative regulation of adipogenesis by kaempferol, a component of Rhizoma Polygonati falcatum in 3T3-L1 cells. Biol Pharm Bull. 2012;35:1525–1533. doi: 10.1248/bpb.b12-00254. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, Zhang Y, Gong Z, Sheng X, Li Z, Zhang W, Qin Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARγ pathway. Biochem Biophys Res Commun. 2006;348:571–578. doi: 10.1016/j.bbrc.2006.07.095. [DOI] [PubMed] [Google Scholar]
- 30.Yun CL, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carling D. The AMP-activated protein kinase cascade – a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase – development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65:3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habinowski SA, Witters LA. The effect of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2001;286:852–858. doi: 10.1006/bbrc.2001.5484. [DOI] [PubMed] [Google Scholar]
- 35.Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by elF2alpha in adipocytes. Biochem Biophys Res Commun. 2006;340:43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]