Abstract
A total of 48 different volatile oils were identified form P. brevitarsis larvae by gas chromatography/mass spectrometry (GC/MS). Acids (48.67%) were detected as the major group in P. brevitarsis larvae comprising the largest proportion of the volatile compounds, followed by esters (19.84%), hydrocarbons (18.90%), alcohols (8.37%), miscellaneous (1.71%), aldehydes (1.35%) and terpenes (1.16%). The major volatile constituents were 9-hexadecenoic acid (16.75%), 6-octadecenoic acid (14.88%) and n-hexadecanoic acid (11.06%). The composition of fatty acid was also determined by GC analysis and 16 fatty acids were identified. The predominant fatty acids were oleic acid (C18:1, 64.24%) followed by palmitic acid (C16:0, 15.89%), palmitoleic acid (C16:1, 10.43%) and linoleic acid (C18:2, 4.69%) constituting more than 95% of total fatty acids. The distinguished characteristic of the fatty acid profile of P. brevitarsis larvae was the high proportion of unsaturated fatty acid (80.54% of total fatty acids) versus saturated fatty acids (19.46% of total fatty acids). Furthermore, small but significant amounts of linoleic, linolenic and γ-linolenic acids bestow P. brevitarsis larvae with considerable nutritional value. The novel findings of the present study provide a scientific basis for the comprehensive utilization of the insect as a nutritionally promising food source and a possibility for more effective utilization.
Keywords: Protaetia brevitarsis, fatty acid, volatile oil, simultaneous distillation extraction (SDE), GC, GC/MS
INTRODUCTION
Over 1,000 species of insects are widely consumed as an important source of nutrition in Asia, Africa, South America and Australia (1,2). Most of the edible insects are rich in proteins with essential amino acids comparable to the major sources of protein such as meat products (3). In general, consumption of edible insects may contribute to not only the total intake of protein but also significant nutritional value such as fat, minerals and vitamins (4). The fat contents of edible insects varied with species, which contained diverse fatty acids including essential fatty acids such as linoleic (C18:2) and linolenic acid (C18:3) (1). In addition, edible insects have higher feed conversion efficiencies than conventional livestock (5).
The order Coleoptera (beetles) contains edible insects and the larvae and/or adult beetles were used as an important food source in Asian countries due to their nutritional values (6). Pemberton (7) reported an overview of insects as traditional medicine, which showed evidence that larvae belonging to the Scarabaeidae family is one of the most important traditional medicine in Korea. Despite the fact that edible insects possess both various nutrients and bioactivities, the studies of insects as food sources and/or functional foods have been very limited compared with those of plants and animals due to their repulsive appearance. Only grasshopper and silkworm are approved as edible insects in Korea (4,8).
The white spotted flower chafer, Protaetia brevitarsis (P. brevitarsis), part of the family of Scarabaeidae belonging to the order Coleoptera, are primarily found in Eastern Asia and the larvae stage have been used as a traditional medicine (9). P. brevitarsis larvae possess therapeutic effects in the treatment and prevention of breast cancer, inflammatory disease and liver-related diseases such as hepatic cancer, liver cirrhosis, and hepatitis (10,11). High antioxidant effect of P. brevitarsis at different growth stages were reported (9) and the antimicrobial peptides protaetins have been purified from P. brevitarsis larvae (12). Despite the fact that P. brevitarsis larvae demonstrated various biological activities, composition analysis of P. brevitarsis larvae have not been studied yet. Furthermore, the majority of the previous studies on P. brevitarsis larvae were mainly focused on the biochemistry and the physiology of the insect itself (13–17).
Our preliminary analyses suggest that P. brevitarsis larvae possess significant amounts of unsaturated fatty acids. However, only limited studies on nutritional characteristics and profiles of lipid soluble components of P. brevitarsis larvae have been studied. Therefore, in the present study, we have analyzed both the composition of the essential oils and fatty acid profiles of P. brevitarsis larvae to give the possibility for application as a valuable lipid source and also providing a scientific basis for further comprehensive utilization of insects as well.
MATERIALS AND METHODS
Sample preparation
P. brevitarsis larvae were supplied by World Way Corp (Chungnam, Korea) in February, 2012. In brief, massive growth of P. brevitarsis larvae were randomly collected and washed with distilled water. After freeze dried at −20°C, the samples were milled using a food grinder and passed through a 30-mesh sieve. The powder of P. brevitarsis larvae was sealed in airtight containers and stored at −70°C before analysis.
Proximate composition
The proximate composition (ash, moisture, lipid, protein and carbohydrate) of P. brevitarsis larvae was determined by following standard methods of the Association of Official Analytical Chemists (AOAC) (18). Briefly, the crude protein content was determined by the Kjeldahl method and calculated by multiplying the nitrogen content using a factor of 6.25. Crude fat was extracted from P. brevitarsis larvae powder in a Soxhlet apparatus with ethyl ether as the solvent. Moisture content was determined by drying the sample in an oven at 105°C until a constant weight was obtained. Ash content was determined by dry-ashing in a furnace at 550°C for 5 hr. Carbohydrate content had been determined by subtracting the sum of the weights including ash, moisture, protein and lipid.
Fatty acid analysis
Fatty acid composition was determined using GC of fatty acid methyl esters (FAME). The preparation of FAME was slightly modified according to Van Wijngaarden (19). Briefly, 3 g of sample was mixed with 5 mL of tetrahydrofuran (THF) and 30 mL of 1 N ethanolic potassium hydroxide solution for saponification in a flask. Reflux condenser was attached to the flask and heated at 85°C for 90 min on the heating mantle. The mixture was cooled to room temperature and filtered using Whatman filter paper (No. 2). The filtered solvent acidified with HCl and petroleum ether:distilled water (40:60 v/v) were added. The ether extract layer was collected and dried over Na2SO4. After Na2SO4 was filtered out, solvent was removed by using a sand bath. For the methylation of sample, approximately 40 mL of methanol and 0.5 mL of H2SO4 were added and the mixture was refluxed for 3 hr. After cooling, diethyl ether (30 mL) and distilled water (50 mL) were added. The ether layer, which contained fatty acid methyl esters, was drawn off and the aqueous layer was backwashed with another 30 mL of ether. The ether was removed by using a rotary vacuum evaporator, and the methyl esters were dissolved in methylene chloride for GC. Analysis of FAME was performed on a HP 6890N GC-FID (Hewlett-Packard Co., Wilmington, DE, USA), equipped with a Supelco™ SP-2560 capillary column (100 m×0.25 mm×0.20 μm) (Sigma-Aldrich, St. Louis, MO, USA). The split ratio was 50:1 and 1 μL of solution was injected into the column. Helium was used as the carrier gas with flow rate of 1 mL/min. The oven temperature was kept at 140°C for 5 min, increased at a rate of 3°C/min to 240°C, and held at 240°C for 10 min. The injector and detector temperatures were maintained at 260°C. The fatty acids were identified by comparing their retention times with those of the FAME standards under the same conditions.
Simultaneous distillation extraction (SDE) of volatile compounds
The volatile compounds were extracted with a modified Likens-Nickerson apparatus as described by Schultz (20). Ten grams of sample was placed in a 2 L round bottom flask with 1 L of deionized water. Sample was extracted using 100 mL of redistilled n-pentane : diethyl ether (1:1, v/v). Extraction time was set for 4 hr after the distilled water started to boil in the sample flask. The extract was dehydrated by anhydrous sodium sulfate at −4°C for 24 hr and concentrated to a final volume of approximately 0.5 mL using a rotary vacuum evaporator (N-1100, EYELA, Tokyo, Japan).
Analysis of volatile compounds by GC/MS
The analysis of volatile compounds was carried out on an Agilent 7890A gas chromatograph equipped with a HP-5MS capillary column (30 m×0.25 mm id, film thickness 0.25 μm) (Aglient Technologies Inc., Santa Clara, CA, USA). The capillary column was directly coupled to an Agilent 5975C mass spectrometer (Agilent Technologies Inc.). The carrier gas was helium with a flow rate of 1 mL/min. The sample was injected with a split ratio of 1:10 into the capillary column. Injector and detector temperatures were set at 250°C and 230°C, respectively. The GC oven temperature was held at 80°C for 2 min, and then programmed from 60 to 300°C for 10 min at a rate of 5°C/min.
Identification of fatty acids and volatile compounds
The volatile compounds were identified by comparing their retention time with those of known compounds and also by comparing their mass spectra with those stored in the National Institute of Standards and Technology 11 (NIST 11) Mass Spectral Library. Some of the identification was confirmed by injecting the chemical standards into the GC/MS system. The fatty acids were identified by comparing their retention times with those of the FAME standards under the same conditions.
RESULTS AND DISCUSSION
Proximate composition of P. brevitarsis larvae
Table 1 shows the results of the proximate composition analysis of P. brevitarsis larvae. P. brevitarsis larvae contained 54.25±1.22% crude protein, 26.70±1.77% crude fat, 10.61±1.22% carbohydrate, 4.45±0.03% crude ash and 3.99±0.16% moisture. The crude protein content was similar to other edible insects such as Oecophylla smaragdina (53.46±0.98%) and Copris nevinsoni (54.43± 0.26%) (21). P. brevitarsis larvae with high protein might be used as a valuable alternative dietary source in developing countries faced with a nutritional imbalance. The crude fat content of P. brevitarsis larvae was comparable to those of Brachytrupes portentosus (20.60±0.60%) and Tessaratoma papillosa (23.55±0.78%) (21). The overall results demonstrated that P. brevitarsis larvae are a good nutritional source especially for fat and protein.
Table 1.
Proximate composition of Protaetia brevitarsis larvae
| Component | Composition (%) |
|---|---|
| Moisture | 3.99±0.16 |
| Crude protein | 54.25±1.22 |
| Crude fat | 26.70±1.77 |
| Crude ash | 4.45±0.03 |
| Carbohydrate | 10.61±1.22 |
Data are expressed as mean±SD (n=3) on a dry weight basis.
Fatty acids composition of P. brevitarsis larvae
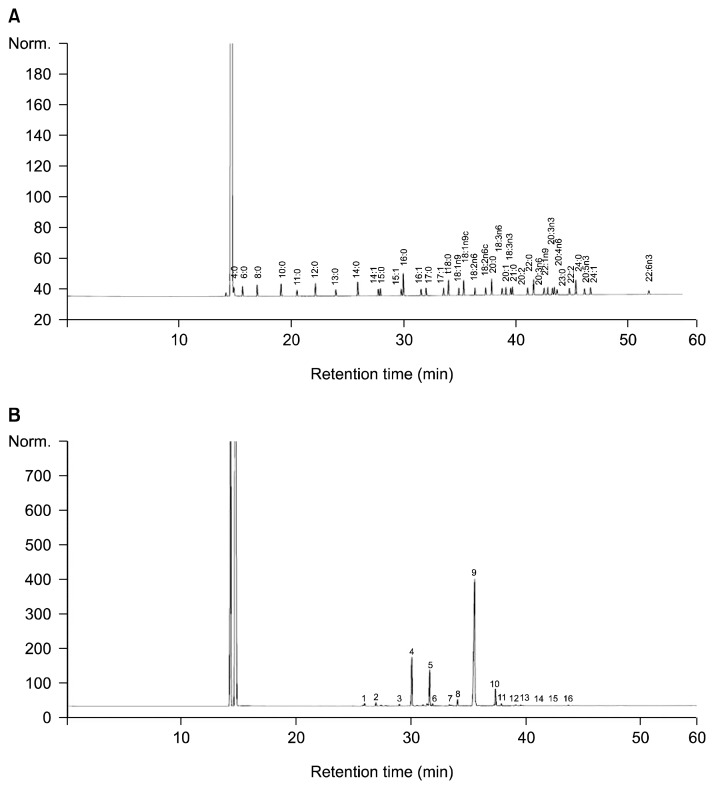
Our preliminary study suggested that P. brevitarsis larvae contained relatively high content of unsaturated lipid and therefore gas chromatography (GC) analysis was performed in order to investigate the fatty acid profile of P. brevitarsis larvae. The chromatograms shown in Fig. 1 correspond to the standard fatty acid methyl ester (A) and fatty acids in P. brevitarsis larvae (B). The detailed fatty acid composition and its retention time of P. brevitarsis larvae are presented in Table 2, in which the resulting data were expressed as both grams of each fatty acid per 100 grams of P. brevitarsis larvae on a dry weight basis and percentage of total fatty acids.
Fig. 1.
Gas chromatogram of standard fatty acids (A) and fatty acids of Protaetia brevitarsis larvae (B). Identification of major peaks: 4, palmitic acid; 5, palmitoleic acid; 9, oleic acid; 10, linoleic acid. For peak number, see Table 3.
Table 2.
Profile of the fatty acids from Protaetia brevitarsis larvae
| No. | Components | Fatty acid | Retention time (min) | Amount (g/100 g) | Content (% of total fatty acids) |
|---|---|---|---|---|---|
| 1 | Myristic acid | C14:0 | 25.90 | 0.10 | 0.70 |
| 2 | Myristoleic acid | C14:1 | 27.73 | 0.02 | 0.15 |
| 3 | Pentadecanoic acid | C15:0 | 27.93 | 0.02 | 0.11 |
| 4 | Palmitic acid | C16:0 | 30.01 | 2.33 | 15.89 |
| 5 | Palmitoleic acid | C16:1 | 31.57 | 1.53 | 10.43 |
| 6 | Heptadecanoic acid | C17:0 | 31.99 | 0.01 | 0.09 |
| 7 | cis-10-heptadecanoic acid | C17:1 | 33.45 | 0.04 | 0.30 |
| 8 | Stearic acid | C18:0 | 34.00 | 0.27 | 1.81 |
| 9 | Oleic acid | C18:1 | 35.48 | 9.44 | 64.24 |
| 10 | Linoleic acid | C18:2 | 37.30 | 0.69 | 4.69 |
| 11 | Arachidic acid | C20:0 | 37.82 | 0.08 | 0.54 |
| 12 | γ-Linolenic acid | C18:3 | 38.75 | 0.01 | 0.05 |
| 13 | cis-11-Eicosenoic acid | C20:1 | 39.09 | 0.07 | 0.45 |
| 14 | Linolenic acid | C18:3 | 39.51 | 0.03 | 0.23 |
| 15 | Heneicosanoic acid | C21:0 | 39.70 | 0.01 | 0.09 |
| 16 | Tricosanoic acid | C23:0 | 43.64 | 0.03 | 0.23 |
The main characteristic of the fatty acid composition in P. brevitarsis larvae was the high proportion of unsaturated fatty acids (80.54% of total fatty acids) versus saturated fatty acids (19.46% of total fatty acids). The predominant fatty acid was monounsaturated fatty acids (MUFA, 75.57%), such as oleic acid (C18:1), myristoleic acid (C14:1), palmitoleic acid (C16:1) and eicosenoic acid (C20:1), followed by saturated fatty acids (SFA, 19.46%) and polyunsaturated fatty acids (PUFA, 4.97%). Overall, a total of 16 fatty acids were identified and oleic acid (C18:1) was the most predominant fatty acid in P. brevitarsis larvae, which accounted for 64.23% of total fatty acids. The other major fatty acids were palmitic acid (C16:1, 15.89% of total fatty acids), palmitoleic acid (C18:2, 10.43% of total fatty acids), linoleic acid (C18:0, 4.69% of total fatty acids) and stearic acid (C14:0, 1.81% of total fatty acids), comprising more than 95% of total fatty acids in P. brevitarsis larvae. Oleic acid was also reported to be the primary fatty acid in the larvae of Imbrasia belina, Oryctes rhinoceros and Rhynchophorus phoenicis(21,22) are well known for decreasing risk of cancer, heart attack, atherosclerosis and dementia (23,24).
Essential fatty acids such as linoleic acid (C18:1, ω6) and linolenic acid (C18:3, ω3) were found as 4.69% and 0.23% of total fatty acids, respectively, in P. brevitarsis larvae (Table 2). γ-Linolenic acid (C18:3, ω6), commonly derived from evening primrose oil, borage and black currant, was also identified in P. brevitarsis larvae (0.05% of total fatty acids). Small but significant amounts of linoleic, linolenic and γ-linolenic acid bestow P. brevitarsis larvae with considerable nutritional value. Several odd-chain fatty acids including pentadecanoic acid (C15:0), heptadecanoic acid (C17:0), heneicosanoic acid (C21:0), tricosanoic acid (C23:0) were identified from P. brevitarsis larvae, all of which are rarely detected in insect fatty acids.
Volatile oil composition of Protaetia brevitarsis larvae
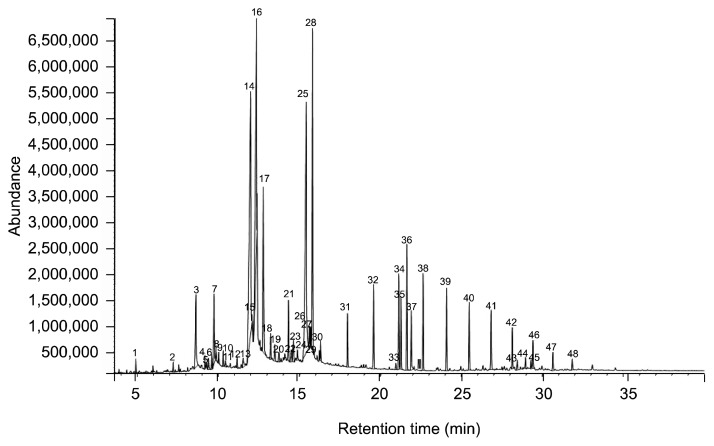
The identified volatile compounds in P. brevitarsis larvae analyzed by GC/MS are shown in Fig. 2. A total of 48 compounds were identified (in parenthesis, the number of chemical compounds identified), which included hydrocarbons (12), esters (11), acids (7), alcohols (6), terpenes (4), aldehydes (3) and miscellaneous including unknowns (5) (Table 3, 4). Acids (48.67%) were the largest group of volatile compounds in P. brevitarsis larvae, followed by esters (19.84%), hydrocarbons (18.90%), alcohols (8.37%), miscellaneous (1.71%), aldehydes (1.35%) and terpenes (1.16%). The major compounds belonging to acids of P. brevitarsis larvae ranged in chain-length of carbons from C12 to C18. The major acid compounds were 9-hexadecenoic acid (16.75%), 6-octadecenoic acid (14.88%) and n-hexadecanoic acid (11.06%).
Fig. 2.
GC/MS Chromatogram of volatile compounds in Protaetia brevitarsis larvae.
Table 3.
Relative content of functional groups in identified volatile compounds from Protaetia brevitarsis larvae
| Functional groups | Number | Relative area % |
|---|---|---|
| Acids | 7 | 48.67 |
| Alcohols | 6 | 8.37 |
| Aldehydes | 3 | 1.35 |
| Esters | 11 | 19.84 |
| Hydrocarbons | 12 | 18.90 |
| Miscellaneous (including unknowns) | 5 | 1.71 |
| Terpenes | 4 | 1.16 |
| Total | 48 | 100 |
Table 4.
Volatile compounds of Protaetia brevitarsis larvae
| No | Compound name | Retention time | Relative peak area % |
|---|---|---|---|
| 1 | Butylated hydroxytoluene | 4.913 | 0.28 |
| 2 | Cyclododecene, 1-methyl- | 7.221 | 0.25 |
| 3 | Tetradecanoic acid | 8.626 | 2.9 |
| 4 | Tetradecanoic acid, ethyl ester | 9.187 | 0.33 |
| 5 | cis-11-Hexadecenal | 9.405 | 0.34 |
| 6 | Tetradecanal | 9.596 | 0.33 |
| 7 | Dodecanoic acid | 9.741 | 2.39 |
| 8 | Pentadecanoic acid | 9.867 | 0.43 |
| 9 | 2,4-Diphenyl-4-methyl-2(E)-pentene | 10.038 | 0.34 |
| 10 | Pentadecanoic acid, ethyl ester | 10.308 | 0.37 |
| 11 | Undecanoic acid, 2,8-dimethyl-, methyl ester | 10.454 | 0.23 |
| 12 | 4-t-Butyl-2-(.alpha.,.alpha.dimethylbenzyl)phenol | 11.166 | 0.25 |
| 13 | Hexadecanoic acid, methyl ester | 11.542 | 0.37 |
| 14 | 9-Hexadecenoic acid | 12.004 | 16.75 |
| 15 | Hexadecenoic acid, Z-11- | 12.083 | 0.26 |
| 16 | n-Hexadecanoic acid | 12.36 | 11.06 |
| 17 | Hexadecanoic acid, ethyl ester | 12.776 | 5.76 |
| 18 | Octadecanal | 13.237 | 0.68 |
| 19 | Phenol, 2-(1,1-dimethylethyl)-4-(1-methyl-1-phenylethyl)- | 13.495 | 0.59 |
| 20 | 10,18-Bisnorabieta-8,11,13-triene | 13.745 | 0.37 |
| 21 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-(1-methyl-1-phenylethyl)- | 14.332 | 1.63 |
| 22 | Heptadecanoic acid, ethyl ester | 14.504 | 0.4 |
| 23 | 8-Octadecenoic acid, methyl ester, (E)- | 14.642 | 0.75 |
| 24 | Unknown | 14.893 | 0.35 |
| 25 | 6-Octadecenoic acid, (Z)- | 15.434 | 14.88 |
| 26 | Unknown | 15.632 | 0.55 |
| 27 | Linoleic acid ethyl ester | 15.704 | 0.45 |
| 28 | Ethyl oleate | 15.823 | 9.16 |
| 29 | Octadecanoic acid, ethyl ester | 16.252 | 0.22 |
| 30 | Heptadecane | 16.324 | 0.44 |
| 31 | Eicosane | 17.98 | 1.44 |
| 32 | Heneicosane | 19.596 | 2.45 |
| 33 | 2,4-Diphenyl-4-methyl-1-pentene | 20.968 | 0.2 |
| 34 | Tricosane | 21.146 | 2.68 |
| 35 | Phenol, 2,4-bis(1-methyl-1-phenylethyl)- | 21.285 | 2.05 |
| 36 | 2,4-Bis(dimethylbenzyl)-6-t-butylphenol | 21.648 | 3.51 |
| 37 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 21.918 | 1.8 |
| 38 | Hexacosane | 22.65 | 2.67 |
| 39 | Heptacosane | 24.095 | 2.35 |
| 40 | Tetracosane | 25.493 | 1.96 |
| 41 | Octadecane | 26.846 | 1.88 |
| 42 | Triacontane | 28.158 | 1.22 |
| 43 | Phenol, 2,4,6-tris(1-methyl-1-phenylethyl)- | 28.462 | 0.34 |
| 44 | Unknown | 28.963 | 0.3 |
| 45 | Unknown | 29.306 | 0.23 |
| 46 | Heptadecane, 8-methyl- | 29.425 | 0.88 |
| 47 | Tetratriacontane | 30.658 | 0.61 |
| 48 | Tetratetracontane | 31.85 | 0.32 |
The ester group derived from the esterification of alcohols with fatty acids was characterized as the second major chemical group. The major constituents of the esters were ethyl oleate (9.16%), hexadecanoic acid, ethyl ester (5.76%) and 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester (1.80%). Ethyl oleate and hexadecanoic, ethyl ester were also identified from edible black ants (Polyrhachis vicina Roger) (25).
Among the hydrocarbons group, tricosane (2.68%), hexacosane (2.67%), heneicosane (2.45%), heptacosane (2.35%), tetracosane (1.96%), octadecane (1.88%) and eicosane (1.44%) were identified. Six alcohol compounds were determined (8.37% of the total volatile components) including 2,4-bis(dimethylbenzyl)-6-t-bu-tylphenol (3.51%), phenol, 2,4-bis(1-methyl-1-phenylethyl) (2.05%), phenol, 2,6-bis(1,1-dimethylethyl)-4-(1- methyl-1-phenylethyl)- (1.63%).
In the present study, we investigated the composition of fatty acids and volatile compounds of P. brevitarsis larvae. The results may provide a scientific basis for the comprehensive utilization of insects, as well as to give the possibility for application as a food source. The novel findings of the present study suggest that P. brevitarsis larvae could be used as a nutritionally promising food source in virtue of its high content of proteins and lipids and, furthermore, might be a starting point for encouraging insects as a useful food source. Studies in protein characterization of P. brevitarsis larvae will be needed to further understanding of the larvae, although the study of bioactive components in P. brevitarsis larvae is currently underway.
ACKNOWLEDGMENTS
This research was supported by Bio-industry Technology Development Program (2011-311006-3), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Bukkens SGF. The nutritional value of edible insects. Ecol Food Nutr. 1997;36:287–319. [Google Scholar]
- 2.Srivastava SK, Babu N, Pandey H. Traditional insect bioprospecting as human food and medicine. Indian J Tradit Know. 2009;8:485–494. [Google Scholar]
- 3.Ladrón de Guevara O, Padilla P, García L, Pino JM, Ramos-Elorduy J. Amino acid determination in some edible Mexican insects. Amino Acids. 1995;9:161–173. doi: 10.1007/BF00805837. [DOI] [PubMed] [Google Scholar]
- 4.Hyun SH, Kwon KH, Park KH, Jeong HC, Kwon O, Tindwa H, Han YS. Evaluation of nutritional status of an edible grasshopper, Oxya Chinensis Formosana. Entomol Res. 2012;42:284–290. [Google Scholar]
- 5.Oonincx DGAB, van Itterbeeck J, Heetkamp MJW, van den Brand H, van Loon JJA, van Huis A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS One. 2010;5:e14445. doi: 10.1371/journal.pone.0014445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFoliart GR. Insects as food: why the western attitude is important. Annu Rev Entomol. 1999;44:21–50. doi: 10.1146/annurev.ento.44.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Pemberton RW. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999;65:207–216. doi: 10.1016/s0378-8741(98)00209-8. [DOI] [PubMed] [Google Scholar]
- 8.DeFoliart GR. Edible insects as minilivestock. Biodivers Conserv. 1995;4:306–321. [Google Scholar]
- 9.Suh HJ, Kang SC. Antioxidant activity of aqueous methanol extracts of Protaetia brevitarsis Lewis (Coleoptera: Scarabaedia) at different growth stages. Nat Prod Res. 2012;26:510–517. doi: 10.1080/14786419.2010.530267. [DOI] [PubMed] [Google Scholar]
- 10.Yoo YC, Shin BH, Hong JH, Lee J, Chee HY, Song KS, Lee KB. Isolation of fatty acids with anticancer activity from Protaetia brevitarsis larva. Arch Pharmacal Res. 2007;30:361–365. doi: 10.1007/BF02977619. [DOI] [PubMed] [Google Scholar]
- 11.Kang M, Kang C, Lee H, Kim E, Kim J, Kwon O, Lee H, Kang H, Kim C, Jang H. Effects of fermented aloe vera mixed diet on larval growth of Protaetia brevitarsis seulensis (Kolbe) (Coleopteran: Cetoniidae) and protective effects of its extract against CCl4-induced hepatotoxicity in Sprague-Dawley rats. Entomol Res. 2012;42:111–121. [Google Scholar]
- 12.Yoon HS, Lee CS, Lee SY, Choi CS, Lee IH, Yeo SM, Kim HR. Purification and cDNA cloning of inducible antibacterial peptides from Protaetia brevitarsis (Coleoptera) Arch Insect Biochem Physiol. 2003;52:92–103. doi: 10.1002/arch.10072. [DOI] [PubMed] [Google Scholar]
- 13.Kim BY, Kim HJ, Lee KS, Seo SJ, Jin BR. Catalase from the white-spotted flower chafer, Protaetia brevitarsis: cDNA sequence, expression, and functional characterization. Comp Biochem Physiol Part B. 2008;149:183–190. doi: 10.1016/j.cbpb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim BY, Lee KS, Choo YM, Kim I, Je YH, Woo SD, Lee SM, Park HC, Sohn HD, Jin BR. Insect transferrin functions as an antioxidant protein in a beetle larva. Comp Biochem Physiol Part B. 2008;150:161–169. doi: 10.1016/j.cbpb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JS, Kang BR, Kim SR, Yun EY, Park KH, Jeon JP, Nam SH, Suh HJ, Hong MY, Kim I. Molecular characterization of a defensin-like peptide from larvae of a beetle, Protaetia brevitarsis. Int J Entomol. 2008;17:131–135. [Google Scholar]
- 16.Lee HC, Yoo CM. Characteristics of antifreeze protein-1 induced during low temperature acclimation in the Protaetia brevitarsis (Coleoptera; Cetonidae) larva. Korean J Biol Sci. 1999;3:47–52. [Google Scholar]
- 17.Kim EY, Lee CS, Kim HR. Purification and properties of ferritin from the last larval hemolymph of Protaetia brevitarsis (Coleoptera) Entomol Res. 2004;34:91–99. [Google Scholar]
- 18.AOAC. Official methods of analysis. 15th ed. Association of Official Analytical Chemists; Washington, DC, USA: 1990. p. 69.p. 70.p. 79. [Google Scholar]
- 19.Van Wijngaarden D. Modified rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1967;39:848–849. [Google Scholar]
- 20.Schultz TH, Flath RA, Mon TR, Eggling SB, Teranishi R. Isolation of volatile components from a model system. J Agric Food Chem. 1977;25:446–449. [Google Scholar]
- 21.Ekpo KE, Onigbinde AO, Asia IO. Pharmaceutical potentials of the oils of some popular insects consumed in southern Nigeria. Afr J Pharm Pharacol. 2009;3:51–57. [Google Scholar]
- 22.Raksakantong P, Meeso N, Kubola J, Siriamornpun S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res Int. 2010;43:350–355. [Google Scholar]
- 23.Win DT. Oleic acid: The anti-breast cancer component in olive oil. Au J T. 2005;9:75–78. [Google Scholar]
- 24.Amtul Z, Uhrig M, Beyreuther K. Additive effects of fatty acid mixtures on the levels and ratio of amyloid β40/42 peptides differ from the effects of individual fatty acids. J Neurosci Res. 2011;89:1795–1801. doi: 10.1002/jnr.22706. [DOI] [PubMed] [Google Scholar]
- 25.Shen L, Li D, Feng F, Ren Y. Nutritional composition of Polyrhachis vicina Roger (edible Chinese black ant) Songklanakarin J Sci Technol. 2006;28:107–114. [Google Scholar]