Abstract
This communication presents an update on the current role of positron emission tomography-computed tomography (PET-CT) in the various clinical decision-making steps in lung carcinoma. The modality has been reported to be useful in characterizing solitary pulmonary nodules, improving lung cancer staging, especially for the detection of nodal and metastatic site involvement, guiding therapy, monitoring treatment response, and predicting outcome in non-small cell lung carcinoma (NSCLC). Its role has been more extensively evaluated in NSCLC than small cell lung carcinoma (SCLC). Limitations in FDG PET-CT are encountered in cases of tumor histotypes characterized by low glucose uptake (mucinous forms, bronchioalveolar carcinoma, neuroendocrine tumors), in the assessment of brain metastases (high physiologic 18F-FDG uptake in the brain) and in cases presenting with associated inflammation. The future potentials of newer PET tracers beyond FDG are enumerated. An evolving area is PET-guided assessment of targeted therapy (e.g., EGFR and EGFR tyrosine kinase overexpression) in tumors which have significant potential for drug development.
Keywords: Lung cancer, PET-CT, staging, restaging, solitary pulmonary nodule
Introduction
The steadily increasing incidence of lung carcinoma makes it an important cause of cancer mortality worldwide in both genders.[1] The second most common cancer (as per statistics in the developed world), it accounts for 12.7% of all new cancer cases and 18.2% of cancer deaths annually and poses a major economic burden on healthcare systems (annually approximately 1,095,000 new cancer cases and 951,000 cancer-related deaths in men and 514,000 new cases and 427,000 deaths in women).[2] The estimated risk is 10-times higher for smokers than non-smokers.[3] Non-small cell lung cancer (NSCLC), the predominant histology with 85-90% of all lung cancers, encompasses three subtypes: Squamous cell carcinoma, adenocarcinoma, and large cell carcinoma.[4] The imaging assessment includes morphological imaging such as chest roentgenogram (CXR), CT and the nuclear medicine procedures including PET using 18F-fluorodeoxglucose (FDG), bone scintigraphy and in case of neuroendocrine tumor (NET), somatostatin receptor scintigraphy (SRS). Over the past decade, PET has become a routinely performed procedure for the assessment of lung cancer[5] and can detect abnormalities before they become evident on anatomical imaging.[6] A brief overview of the utility of PET-CT in patients with lung cancer is presented below.
Non-small cell lung cancer
Diagnosis-Solitary pulmonary nodule
A solitary pulmonary nodule (SPN) is defined as a single spherical lesion of 3 cm or less in diameter completely surrounded by lung parenchyma without any associated atelectasis or lymphadenopathy.[7] The probability of lung cancer increases with tumor size, those larger than 3 cm in diameter are frequently malignant.[8] The incidence of malignancy in SPN varies widely (5-70% in the literature), depending upon the patient population studied, geographic location and the prevalence of inflammatory lung disease. Although certain radiological features indicate a benign (calcification) or malignant (spiculated margins) etiology, a reliable characterization is frequently not possible and invasive procedures (e.g., fiberoptic bronchoscopy, transthoracic needle-aspiration biopsy, video assisted thoracoscopy, video-assisted thoracoscopic surgery, or thoracotomy) are employed, all of which are associated with considerable costs and morbidity.
Among the various non-invasive modalities,[9,10] CT is considered an excellent tool for detection and localization of SPNs with good sensitivity (96%, range 91-98%) but poor specificity (50%, range 41-58%).[11] FDG-PET is cost effective for evaluation of SPNs in various countries.[12,13,14] In a meta-analysis, sensitivity and specificity of FDG-PET for SPN diagnosis were 96.8% and 77.8%, respectively.[15] False-negative results occur mostly in association with bronchioalveolar carcinoma, carcinoids, and tumors less than 1 cm in diameter, whereas false-positive findings are frequent because of infectious and inflammatory processes (tuberculosis, sarcoidosis, histoplasmosis, and Wegener's granulomatosis). Integrated FDG PET-CT has been found to be more useful in characterizing SPN with better sensitivity, specificity, and accuracy [Figure 1].[16]
Figure 1.
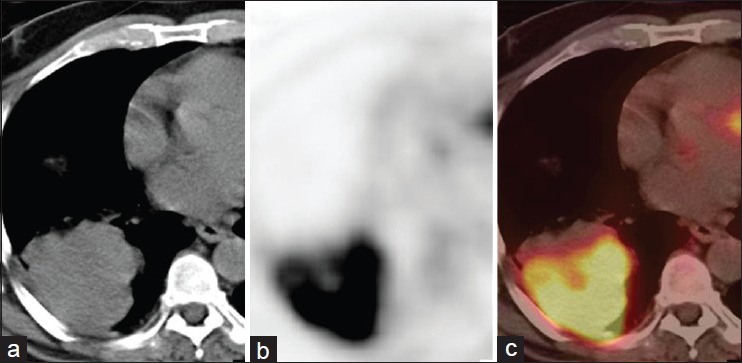
A 43-year-old male with right lung mass on CT (a). PET (b) and PET-CT (c) images showed intense FDG uptake in the mass (SUVmax-12) along with an area of necrosis. A diagnosis of primary malignant lung mass was made. It was confirmed to be NSCLC on histopathology
Semiquantitative analysis of glucose metabolism (SUVmax) is also frequently performed, in addition to visual assessment, because of observer-independence and reproducibility.[17,18] There have been endeavours to estimate the risk of malignancy by SUVmax of a given nodule and clinically relevant information,[15] with reports that mean SUVmax of malignant SPNs is higher than the benign counterparts (9.7 ± 5.5 vs. 2.6 ± 2.5; P < 0.01). Moreover, all SPNs with SUVmax <1.25 were associated with benign histology. The usual notion is that in patients with an increased surgical risk and a lesion with a low SUVmax, omission of diagnostic thoracotomy may be warranted and the lesion monitored over time. On the contrary, SPNs with a high SUVmax have a high risk of malignancy and therefore require pathological evaluation.
Staging
Initial disease staging is important in patients with newly diagnosed NSCLC, to select the most appropriate therapeutic strategy and determine prognosis. It is crucial to correctly differentiate patients with potentially curable disease (who may benefit from radical surgery or chemoradiotherapy) from those who cannot be treated with curative intent and are therefore candidates for palliative therapy. CT, though employed as the imaging modality of choice for NSCLC staging, is being increasingly replaced by FDG PET-CT.
T-staging
For the assessment of T stage, the combined PET-CT has increased the accuracy of tumor detection, chest wall, and mediastinal infiltration as compared to Pet alone.[19,20] In one meta-analysis, PET-CT accurately predicted the T stage in 82% of cases compared with 55% and 68% with Pet alone and CT alone, respectively.[21] Since diagnostic CT can accurately detect tumor size and infiltration of adjacent structures,[19,20] use of contrast-enhanced PET-CT is more appropriate in this setting. One potential advantage of PET over conventional imaging is the evaluation of extension of the primary tumor to involve the pleura with a high positive and negative predictive value for the evaluation of malignant pleural effusions.[22] Also FDG-PET is more accurate than CT in determining the size of primary tumor (T1 and T2) when there is adjacent collapse or consolidation.[23]
N-staging
Clinical staging of the nodal involvement in NSCLC is classified into four categories: N0, N1, N2, or N3. The identification of nodal involvement is vital to select candidates for curative surgery. Patients with N0–N1 disease (no metastatic lymph nodes or only intrapulmonary/hilar nodes) are generally candidates for surgical resection. On the contrary, patients with N2 disease (ipsilateral mediastinal lymph nodes metastases) could gain benefit from a combination of local and systemic treatment. Patients with N3 disease (contralateral mediastinal lymph nodes metastases) are presently considered unresectable.[24]
Conventional imaging modalities (CT/MRI), using only dimensional criteria (>1 cm) to detect nodal involvement, have poor accuracy in differentiating benign from malignant nodal disease (sensitivity: 60-83%; specificity: 77-82%).[25] In one study, 44% of metastatic lymph nodes in NSCLC measured <1 cm whereas 77% without metastatic lymph nodes had a lymph node measuring >1 cm in the short-axis diameter.[26] FDG PET-CT is reported to have a higher diagnostic accuracy than either CT or Pet alone.[27] A recent multicenter study has shown that FDG PET-CT has very high negative predictive value (91%) and specificity (83%), but limited positive predictive value (29%);[28] similar results were seen in a recent meta-analysis.[29] With respect to nodal size, the sensitivity of FDG PET-CT to detect malignant involvement was 32.4% in nodes <10 mm, and 85.3% in nodes ≥10 mm. It has been suggested that dual-time point imaging can improve the sensitivity of FDG PET-CT for mediastinal nodal staging.[30]
Although FDG-PET/CT appears more useful than other imaging modalities for the assessment of nodal metastatic involvement, PET findings cannot replace histological confirmation of FDG-positive lesions by mediastinoscopy.[31] False-negative rate for micrometastasis detection has been reported to be as high as 8%[32] and false-positive results has been reported in the setting of endemic granulomatous diseases [Figure 2]. Thus, FDG PET-CT cannot obviate the need for invasive procedures.[33] However, FDG PET-CT provides valuable information about inaccessible nodal stations that may be missed by conventional imaging. Lymph nodes in the aorto-pulmonary window, anterior mediastinum, and in the posterior subcarinal region are difficult to reach without modifying the mediastinoscopic approach and are not routinely sampled. FDG-PET detection of hypermetabolic lymphnodes at these stations suggests the need for other methods of lymph node evaluation like anterior mediastinotomy/transbronchial or percutaneous biopsy or endoscopic-guided fine needle aspiration. In one prospective study of 61 patients with stage IIIA disease who were candidates for neoadjuvant chemotherapy before being planned for surgical resection, FDG-PET resulted in tumor upstaging in 30% causing a switch to palliative treatment in 19% of patients.[34] PET-CT virtual mediastinoscopy has also been found to be a useful adjunct.[35]
Figure 2.
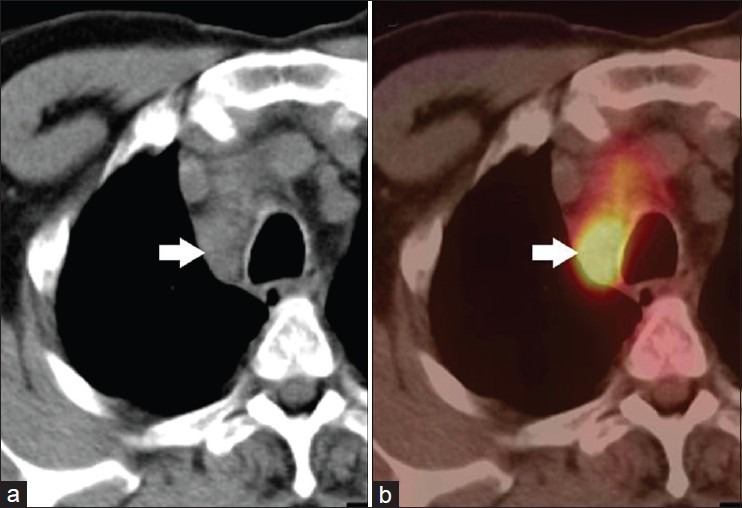
A 52-year-old male with NSCLC of right lung. FDG PET-CT was done for staging. CT (a) showed enlarged right paratracheal node (arrow) which was FDG avid (SUVmax-9.2) on PET-CT (b). A diagnosis of nodal metastasis was made on PET-CT. However, this turned out to be tuberculosis at histopathology. Hence, results of FDG PET-CT for nodal staging should be confirmed with FNAC/biopsy to avoid false positives
M-staging
Approximately 18-36% of patients with newly diagnosed NSCLC have distant metastases at presentation, which has major implications on management and prognosis.[36] The commonest sites for metastatic disease in NSCLC are the brain, bone, liver, and adrenals (in decreasing order) at presentation. Furthermore, among the patients apparently radically treated for NSCLC, around 20% relapse due to the presence of undetected micrometastasis at initial staging. Conventional staging for distant metastasis includes a CT scan of the chest including the upper abdomen for assessment of adrenal glands and liver, while bone scintigraphy and brain imaging are performed only for stage IIIA or IIIB.[37]
Being a whole-body non-invasive technique, FDG PET-CT provides valuable information regarding metastatic spread [Figure 3]. FDG-PET detects clinically unsuspected distant metastases in upto 28% of patients with NSCLC and impacts clinical management in as high as 53% of cases.[38,39] In one randomized study, FDG-PET reduced futile thoracotomies to 25% (from 46% with conventional work-up alone) in patients with clinical stages I-II tumors and to 11% (from 29% with conventional work-up alone) in patients with clinical stage III tumors.[40] In the ACOSOG Z0050 trial, 6.3% of patients were found to have extracranial distant metastasis not seen on previous CT staging at the time of FDG-PET.[41]
Figure 3.
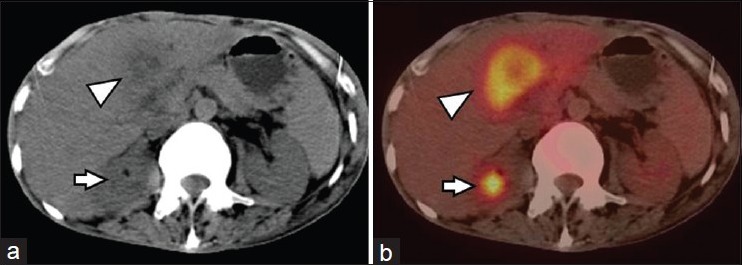
A 61-year-old male with NSCLC of left lung. FDG PET-CT was done for staging. CT (a) and PET-CT (b) images showed a large liver metastasis (arrowhead). Also noted was a right adrenal nodule on CT which showed FDG avidity on PET-CT (arrow), suggesting adrenal metastasis
Adrenal masses are detected in up to 20% of patients with NSCLC at initial presentation but approximately two-thirds of those actually represent adenomas, rather than metastases. FDG-PET has shown promising results in differentiating benign from metastatic adrenal masses in patients with known or suspected malignancies. In the study with the largest patient population, Kumar et al.[42] studied the usefulness of FDG-PET in the evaluation of adrenal masses detected on CT/MRI in NSCLC. One hundred thirteen adrenal masses were evaluated in 94 patients and interpreted as positive if FDG uptake of the adrenal mass was greater than or equal to that of the liver. The sensitivity, specificity, and accuracy for detecting metastatic disease were 93%, 90%, and 92%, respectively.
Metastases to the CNS are common and detected in 18% of patients with M1 disease at presentation. FDG-PET is not very useful due to increased FDG activity in normal brain. Bones are a common site of metastasis with an overall prevalence of 20% (range, 8-34%).[43] FDG PET-CT is highly accurate for detection of bone metastasis [Figure 4]. In a recent meta-analysis, it was shown that the pooled sensitivity and specificity for the detection of bone metastasis in lung cancer using FDG PET-CT, FDG-PET, MRI and bone scan were 92%, 87%, 77%, and 86%; and 98%, 94%, 92%, and 88%, respectively.[44] FDG-PET appears more accurate than CT in detecting liver metastases because of its better specificity. In addition to its clinical utility, FDG PET-CT has also been found to be a cost effective staging modality by avoiding futile thoracotomies.[45]
Figure 4.
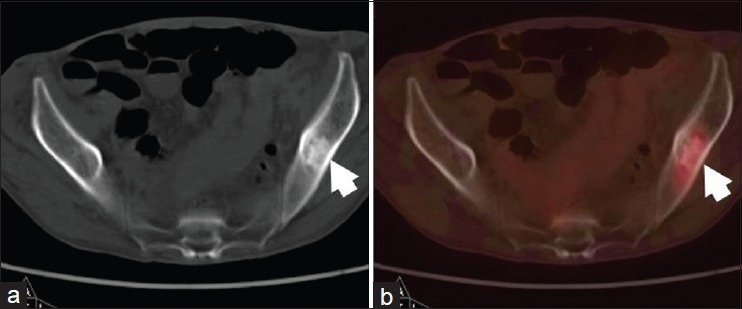
A 70-year-old male with adenocarcinoma of right lung, postpneumonectomy and adjuvant radiotherapy. He presented with bony pains. FDG PET-CT was done to rule out distant metastasis. On CT (a) images a sclerotic lesion was seen in left ilium (arrow). It showed mild FDG uptake on PET-CT (b) images, suggesting skeletal metastasis
Treatment planning
Radiation therapy (RT) is the attempted curative treatment in early stage (I–II) NSCLC patients who are not candidates for surgery. The use of FDG PET has important implications for the radiation oncologist, since PET provides valuable information influencing radiotherapy techniques, target volumes definition and radiation exposure.
Although the definition of volumes on PET images alone might be problematic due to the poorer resolution and higher noise levels; when combined with structural imaging, such as CT, FDG-PET provides the best available information on tumor extent. PET-CT should be used for RT planning in NSCLC because it more accurately images tumor extent than CT alone.[46] The impact of PET on RT planning can be summarized in both a reduction of the dose delivered to normal surrounding tissue (when PET tumor area is smaller than that defined on CT) and in the inclusion of adjacent areas with viable tumor cells outside the radiation fields (when PET detects more extensive tumor area than CT). FDG-PET has been reported to significantly change nodal staging in the thorax, usually by showing more positive nodes than CT,[47] and PET-CT imaging can improve the accuracy of target volume delineation using anatomic biological contour (ABC), determined directly on PET-CT images.[48] In a modeling study, van Der Wel et al.[49] reported that for 21 patients with N2 or N3 NSCLC, the use of PET-CT in radiotherapy planning resulted in a lower level of radiation exposure to the esophagus and lungs, allowing a significant increase in the dose delivered to the tumor. Finally, PET, especially PET-CT imaging has another positive effect on tumor volume delineation: Significantly reduced inter-observer and intra-observer variability for tumor volume delineation.[50,51]
Treatment response monitoring
Innovations in aggressive surgical techniques, neoadjuvant and adjuvant chemoradiotherapy and molecularly targeted therapies have led to spiraling costs and, in some cases, increased morbidity while yielding only modest improvements in survival for patients with NSCLC, particularly early-stage disease.[52,53,54] Thus, there is pressing need to validate the effectiveness of treatment in individual as well as in specific groups of NSCLC patients for the purpose of developing appropriate treatment guidelines that would allow the termination of ineffective agents and a change to alternatives that may be more effective.
Molecular imaging offers the potential to characterize the nature of tissues on the basis of their biochemical and biological features [Figure 5]. One of the major theoretical advantages of FDG-PET compared with structural imaging techniques is that there is usually a more rapid change in cellular metabolism than in tumor size [Figure 6].[55] A prospective study by MacManus et al.[56] suggested a much more powerful correlation of outcome to PET metabolic response versus CT response. In another study, quantitative dynamic FDG PET performed 2 weeks after chemoradiotherapy in a cohort of 29 patients with 30 lesions demonstrated a correlation between the residual rate of glucose metabolism, as estimated from FDG kinetics, and the pathological tumor response.[57] A larger retrospective study involving 56 patients, 33 of whom received neoadjuvant chemotherapy and 23 of whom received chemoradiation, revealed a nearly linear correlation between the change in the SUVmax and the percentage of nonviable tumor in the resected material (r2 50.75; P < 0.001).[58] Similarly, a study evaluating the utility of FDG PET-CT in assessing the response to neoadjuvant chemotherapy/chemoradiotherapy found a significantly greater percentage decrease in the SUVmax in patients showing an excellent pathological response in the primary tumor than in those with greater than 10% residual viable cells (P = 0.005).[59] In another study involving patients treated with neoadjuvant chemotherapy, Dooms et al.[60] found that patients with persistent major mediastinal nodal involvement on FDG PET had a 5-year overall survival rate of 0%. Apart from response to chemotherapy and radiotherapy, FDG PET-CT can be used to monitor response to biological therapy. It is superior to CT for this purpose because of the fact that metabolic changes appear earlier than anatomical changes. FDG PET-CT has been shown to be useful in monitoring response to the EGFR kinase inhibitor, erlotinib in few studies.[61,62]
Figure 5.
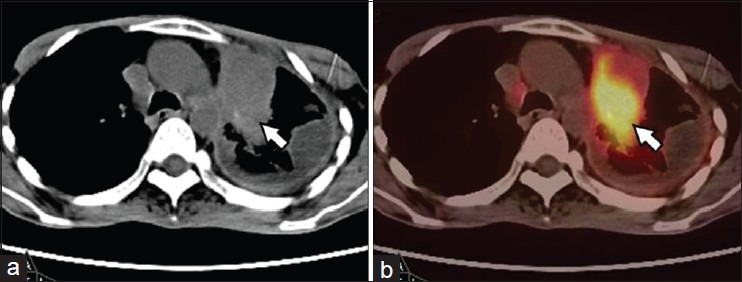
A 49-year-old male, postsurgery and radiotherapy for left lung NSCLC. PET-CT was done 9 months later for restaging. CT (a) images showed mass lesion in the thorax with fibrotic changes in pleura. PET-CT (b) images showed intense FDG uptake (SUVmax-13) in the mass suggesting recurrent disease (arrow). No uptake was noted in the pleura suggesting post therapy changes
Figure 6.
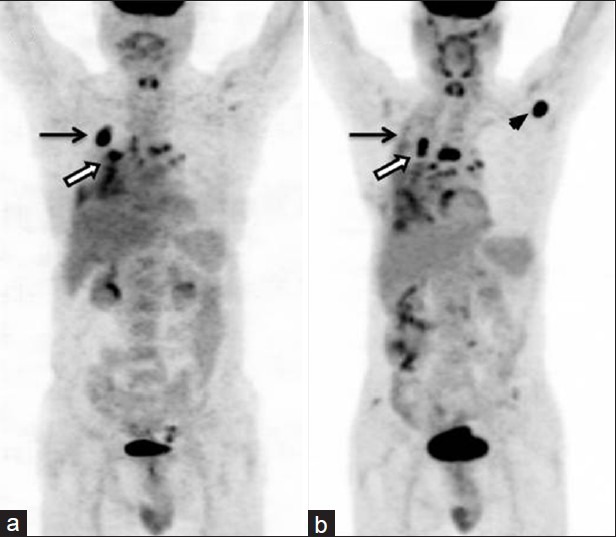
A 51-year-old male with right lung NSCLC with nodal metastasis. FDG PET-CT (a) showed primary lung lesions (arrow) with mediastinal nodal metastasis (bold arrow). He underwent three cycles of chemotherapy. Post therapy PET-CT (b) showed almost complete regression of primary lesion (arrow) but increase in size and uptake of mediastinal nodes (bold arrow). Also noted was appearance of new axillary nodal metastasis (arrowhead), suggesting progression of disease
However, FDG uptake in inflammatory tissues must be considered when FDG-PET is used for response assessment after radiotherapy. Serial imaging during and after radiotherapy suggests that inflammatory 18F-FDG uptake in normal tissues increases in the first few months after treatment rather than occurring early during radiotherapy.[63] However, these delayed changes need not prevent an experienced observer from correctly assessing a treatment response visually. Accurate region-of-interest assignment is critical when the SUV is used to assess the response after radiotherapy because uptake in the uninvolved lung may be in the range considered to be malignant (SUV-2.5).
Prognosis
As relatively few patients with locally advanced NSCLC are currently cured, the ability of diagnostic tests to predict the duration of survival is an important measure of therapeutic efficacy and may help in better selection of patients for salvage or palliative therapies. The ability of FDG-PET to provide prognostic information was demonstrated in a pilot study involving 15 patients receiving induction chemotherapy (n = 9) or radiotherapy (n = 6).[64] It was observed that patients with PET down-staging had significantly longer cumulative survival than patients with a persistent mediastinal nodal abnormality (P = 0.014), whereas a partial response on CT was not predictive of outcome. In a larger prospective study, a metabolic response to chemoradiation, as assessed by visual analysis of FDG-PET, was also much more powerfully correlated with survival than the response on CT determined from WHO criteria.[56] Another study involving 70 patients undergoing neoadjuvant chemoradiotherapy found that patients with either a complete metabolic response (CMR) had significantly longer survival than patients with a partial metabolic response (PMR) (P < 0.0001)[65] while progressive disease was associated with unfavorable outcome. Similar promising results of PET response in prognostification of disease have been reported by other investigators as well.[66,67]
In a systemic review of 13 studies comprising 1474 patients with NSCLC, increasing SUV on FDG PET was found to be prognostic as a continuous variable for lower survival though no clear cut-off was identified.[68] In a recent prospective study with 282 stage I lung cancer patients, it was demonstrated that SUVmax of primary tumor was an independent prognostic factor for survival.[69] Patients with an SUVmax more than 4.7 had a significantly higher risk of recurrence. Similar results were also seen for stage III and IV tumors.[70] There have been important outliers[71,72,73] amid all this encouraging data,[68,69] though, overall it is thought that tumors with high pre-treatment SUVmax on FDG-PET have inferior prognosis. However, there may be differences among disease stages and treatment modalities.
Small cell lung cancer
Clinically, SCLC is more aggressive than NSCLC, presenting with a rapid doubling time and higher propensity for widespread metastatic disease. Overall prognosis is dismal. In fact, despite initial chemosensitivity, most patients with SCLC relapse and die from recurrent disease.[74] At presentation, about 60-70% of patients with SCLC have extensive disease while 30-40% have limited disease (limited disease is defined as disease confined to one hemithorax, the mediastinum, and the supraclavicular lymph nodes).[75] Diagnostic procedures commonly used to stage the disease include chest and abdomen CT, brain CT or MRI, radionuclide bone scans, and bone marrow aspiration.
In comparison to NSCLC, the data on SCLC with PET-CT is limited. The impact of PET on stage classification of newly diagnosed SCLC has been investigated by several authors that reported how Pet allowed a modification of stage and clinical management in 10-33% of cases. In a population of 120 SCLC patients studied for staging by PET and conventional imaging, PET upstaged 10 patients and downstaged 3 patients.[76] In another recent study, among the 26 patients with limited disease on conventional imaging, 4/26 (15%) were upstaged to extensive disease after PET while among the 20 patients with extensive disease on conventional imaging, 8/20 (40%) were down staged to limited disease.[77] Because of the high physiological accumulation of FDG in brain, in patients who are found to have limited disease with PET, if brain metastases need to be excluded, a brain MRI is necessary.[78] The potential role of FDG-PET to assess early therapeutic response and disease prognostification have also been demonstrated in a limited number of studies.[79]
Newer directions: Tracers beyond FDG
FDG PET-CT is now an established modality in management of lung cancer. A host of newer radiopharmaceuticals which target different aspects of tumor biology are being explored in lung cancers. These include the proliferation tracer 18F-fluorothymidine which has been evaluated in few studies and found to be useful.[80] Other tracers which provide information regarding hypoxia (18F-FMISO, 64Cu-ATSM), angiogenesis (RGD peptides), amino acid metabolism (11C-Methionine), and choline metabolism (11C-choline, 18F-fluorocholine) have also been evaluated. An evolving area is the non-invasive assessment of epidermal growth factor receptor (EGFR) and EGFR tyrosine kinase overexpression in tumors by PET imaging that has the potential for in vivo a priori determination of EGFR-targeted drug efficacy.[81,82] These agents might give better insight into tumor behavior, aggressiveness, and therapy-related toxicity, thereby helping in formulation of individualised treatment strategies with targeted agents.[81] However, substantial prospective assessment is needed before these agents come into routine use.
Conclusions
PET-CT has established itself as an important step in the management of patients with lung cancer. FDG PET-CT is useful for characterising solitary pulmonary nodules. In addition, it has definite role in staging, radiotherapy planning, response monitoring and prognostication of NSCLC. While data for SCLC is limited, still FDG PET-CT appears to be useful in this subgroup. Further evaluation of newer PET tracers in lung cancer will better our understanding of tumor biology and may pave the path for personalised medicine.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–91. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15:5626–45. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan P, Hainaut P, Boffetta P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011;12:399–408. doi: 10.1016/S1470-2045(10)70126-1. [DOI] [PubMed] [Google Scholar]
- 5.Saif MW, Tzannou I, Makrilia N, Syrigos K. Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med. 2010;83:53–65. [PMC free article] [PubMed] [Google Scholar]
- 6.Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: Molecular biology correlates and disease characterization. J Nucl Med. 2009;50:1820–7. doi: 10.2967/jnumed.108.054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang AW, Moss HA, Robertson RJ. The solitary pulmonary nodule. Eur J Radiol. 2003;45:69–77. doi: 10.1016/s0720-048x(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 8.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348:2535–42. doi: 10.1056/NEJMcp012290. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin DR, Eaton T, Kolbe J, Christmas T, Milne D, Mercer J, et al. Management of solitary pulmonary nodules: How do thoracic computed tomography and guided fine needle biopsy influence clinical decisions? Thorax. 2002;57:817–22. doi: 10.1136/thorax.57.9.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman TE. Radiologic evaluation of the solitary pulmonary nodule. Radiol Clin North Am. 2005;43:459–65. doi: 10.1016/j.rcl.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sloan JA, Sykes AM, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology. 2003;226:756–61. doi: 10.1148/radiol.2263020036. [DOI] [PubMed] [Google Scholar]
- 12.Dietlein M, Weber K, Gandjour A, Moka D, Theissen P, Lauterbach KW, et al. Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: Priority for a PET-based strategy after nodal negative CT results. Eur J Nucl Med. 2000;27:1598–609. doi: 10.1007/s002590000376. [DOI] [PubMed] [Google Scholar]
- 13.Keith CJ, Miles KA, Griffiths MR, Wong D, Pitman AG, Hicks RJ. Solitary pulmonary nodules: Accuracy and cost-effectiveness of sodium iodide FDG-PET using Australian data. Eur J Nucl Med Mol Imaging. 2002;29:1016–23. doi: 10.1007/s00259-002-0833-2. [DOI] [PubMed] [Google Scholar]
- 14.Kosuda S, Ichihara K, Watanabe M, Kobayashi H, Kusano S. Decision-tree sensitivity analysis for cost-effectiveness of chest 2-fluoro-2-D-[(18) F] fluorodeoxyglucose positron emission tomography in patients with pulmonary nodules (non-small cell lung carcinoma) in Japan. Chest. 2000;117:346–53. doi: 10.1378/chest.117.2.346. [DOI] [PubMed] [Google Scholar]
- 15.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: A meta-analysis. JAMA. 2001;285:914–24. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Allen-Auerbach M, Goldin J, Fueger BJ, Dahlbom M, Brown M, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med. 2007;48:214–20. [PubMed] [Google Scholar]
- 17.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 18.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–8. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 19.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 20.De Wever W, Ceyssens S, Mortelmans L, Stroobants S, Marchal G, Bogaert J, et al. Additional value of PET-CT in the staging of lung cancer: Comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol. 2007;17:23–32. doi: 10.1007/s00330-006-0284-4. [DOI] [PubMed] [Google Scholar]
- 21.De Wever W, Stroobants S, Coolen J, Verschakelen JA. Integrated PET/CT in the staging of non-small cell lung cancer: Technical aspects and clinical integration. Eur Respir J. 2009;33:201–12. doi: 10.1183/09031936.00035108. [DOI] [PubMed] [Google Scholar]
- 22.Gupta NC, Rogers JS, Graeber GM, Gregory JL, Waheed U, Mullet D, et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest. 2002;122:1918–24. doi: 10.1378/chest.122.6.1918. [DOI] [PubMed] [Google Scholar]
- 23.Pawaroo D, Cummings NM, Musonda P, Rintoul RC, Rassl D, Beadsmoore C. Non-small cell lung carcinoma: Accuracy of PET/CT in determining the size of T1 and T2 primary tumors. AJR Am J Roentgenol. 2011;196:1176–81. doi: 10.2214/AJR.10.4980. [DOI] [PubMed] [Google Scholar]
- 24.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 25.Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: Mediastinal staging in the 1990s meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- 26.Prenzel KL, Monig SP, Sinning JM, Baldus SE, Brochhagen HG, Schneider PM, et al. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463–7. doi: 10.1378/chest.123.2.463. [DOI] [PubMed] [Google Scholar]
- 27.Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, et al. Non-small cell lung cancer: Dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526–33. doi: 10.1148/radiol.2292021598. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Zhang H, Xing L, Ma H, Xie P, Zhang L, et al. Mediastinal lymph nodes staging by (18) F-FDG PET/CT for early stage non-small cell lung cancer: A multicenter study. Radiother Oncol. 2012;102:246–50. doi: 10.1016/j.radonc.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Lv YL, Yuan DM, Wang K, Miao XH, Qian Q, Wei SZ, et al. Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: A bivariate systematic review and meta-analysis. J Thorac Oncol. 2011;6:1350–8. doi: 10.1097/JTO.0b013e31821d4384. [DOI] [PubMed] [Google Scholar]
- 30.Hu M, Han A, Xing L, Yang W, Fu Z, Huang C, et al. Value of dual-time-point FDG PET/CT for mediastinal nodal staging in non-small-cell lung cancer patients with lung comorbidity. Clin Nucl Med. 2011;36:429–33. doi: 10.1097/RLU.0b013e3182173810. [DOI] [PubMed] [Google Scholar]
- 31.Detterbeck FC, DeCamp MM, Jr, Kohman LJ, Silvestri GA. American College of Chest Physicians. Lung cancer. Invasive staging: The guidelines. Chest. 2003;123:167S–75S. doi: 10.1378/chest.123.1_suppl.167s. [DOI] [PubMed] [Google Scholar]
- 32.Dietlein M, Weber K, Gandjour A, Moka D, Theissen P, Lauterbach KW, et al. Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: Priority for a PET-based strategy after nodal negative CT results. Eur J Nucl Med. 2000;27:1598–609. doi: 10.1007/s002590000376. [DOI] [PubMed] [Google Scholar]
- 33.Metin M, Citak N, Sayar A, Pekcolaklar A, Melek H, Kök A, et al. The role of extended cervical mediastinoscopy in staging of non-small cell lung cancer of the left lung and a comparison with integrated positron emission tomography and computed tomography: Does integrated positron emission tomography and computed tomography reduce the need for invasive procedures? J Thorac Oncol. 2011;6:1713–9. doi: 10.1097/JTO.0b013e318225914e. [DOI] [PubMed] [Google Scholar]
- 34.Hoekstra CJ, Stroobants SG, Hoekstra OS, Vansteenkiste J, Biesma B, Schramel FJ, et al. The value of [18F] fluoro-2-deoxy-D-glucose positron emission tomography in the selection of patients with stage IIIA-N2 non-small cell lung cancer for combined modality treatment. Lung Cancer. 2003;39:151–7. doi: 10.1016/s0169-5002(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 35.Itano H, Hirokawa Y, Takauchi K. Clinical utility of three-dimensional integrated 18F-fluorodeoxyglucose positron-emission tomography/computed tomography virtual mediastinoscopy. Interact Cardiovasc Thorac Surg. 2010;10:981–5. doi: 10.1510/icvts.2009.217794. [DOI] [PubMed] [Google Scholar]
- 36.Quint LE. Staging non-small cell lung cancer. Cancer Imaging. 2007;7:148–59. doi: 10.1102/1470-7330.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruzzi JF, Munden RF. PET/CT imaging of lung cancer. J Thorac Imaging. 2006;21:123–36. doi: 10.1097/00005382-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Eschmann SM, Friedel G, Paulsen F, et al. For staging of advanced non-small cell lung cancer prior to neoadjuvant radiochemotherapy. Eur J Nucl Med Mol Imaging. 2002;29:804–8. doi: 10.1007/s00259-002-0801-x. [DOI] [PubMed] [Google Scholar]
- 39.Seltzer MA, Yap CS, Silverman DH, Meta J, Schiepers C, Phelps ME, et al. The impact of PET on the management of lung cancer: The referring physician's perspective. J Nucl Med. 2002;43:752–6. [PubMed] [Google Scholar]
- 40.van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: The PLUS multicentre randomised trial. Lancet. 2002;359:1388–93. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 41.Reed CE, Harpole DH, Posther KE, Woolson SL, Downey RJ, Meyers BF, et al. American College of Surgeons Oncology Group Z0050 trial. Results of the American College of Surgeons Oncology Group Z0050 trial: The utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1943–51. doi: 10.1016/j.jtcvs.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058–62. [PubMed] [Google Scholar]
- 43.Toloza EM, Harpole L, McCrory DC. Noninvasive Staging of Non-small Cell Lung Cancer: A Review of the Current Evidence. Chest. 2003;123:137S–146S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 44.Qua X, Huangc X, Yand W, Wue L, Daia K. A meta-analysis of 18FDG-PET-CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.01.126. [DOI] [PubMed] [Google Scholar]
- 45.Buck AK, Herrmann K, Schreyögg J. PET/CT for staging lung cancer: Costly or cost-saving? Eur J Nucl Med Mol Imaging. 2011;38:799–801. doi: 10.1007/s00259-011-1803-3. [DOI] [PubMed] [Google Scholar]
- 46.Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:78–86. doi: 10.1016/j.ijrobp.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 47.Ashamalla H, Rafla S, Parikh K, Mokhtar B, Goswami G, Kambam S, et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:1016–23. doi: 10.1016/j.ijrobp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Mac Manus MP, Hicks RJ. Impact of PET on radiation therapy planning in lung cancer. Radiol Clin North Am. 2007;45:627–38. doi: 10.1016/j.rcl.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 49.van Der Wel A, Nijsten S, Hochstenbag M, Lamers R, Boersma L, Wanders R, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small cell lung cancer: A modeling study. Int J Radiat Oncol Biol Phys. 2005;61:649–55. doi: 10.1016/j.ijrobp.2004.06.205. [DOI] [PubMed] [Google Scholar]
- 50.Mah K, Caldwell CB, Ung YC, Danjoux CE, Balogh JM, Ganguli SN, et al. The impact of (18) FDGPET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small cell lung carcinoma: A prospective study. Int J Radiat Oncol Biol Phys. 2002;52:339–50. doi: 10.1016/s0360-3016(01)01824-7. [DOI] [PubMed] [Google Scholar]
- 51.Fox JL, Rengan R, O’Meara W, Yorke E, Erdi Y, Nehmeh S, et al. Does registration of PET and planning CT images decrease interobserver and intraobserver variation in delineating tumor volumes for non-small-cell lung cancer? Int J Radiat Oncol Biol Phys. 2005;62:70–5. doi: 10.1016/j.ijrobp.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Wright G, Manser RL, Byrnes G, Hart D, Campbell DA. Surgery for non-small cell lung cancer: Systematic review and meta-analysis of randomised controlled trials. Thorax. 2006;61:597–603. doi: 10.1136/thx.2005.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Socinski MA. Adjuvant therapy of resected non-small-cell lung cancer. Clin Lung Cancer. 2004;6:162–9. doi: 10.3816/CLC.2004.n.029. [DOI] [PubMed] [Google Scholar]
- 54.Le Chevalier T, Lynch T. Adjuvant treatment of lung cancer: Current status and potential applications of new regimens. Lung Cancer. 2004;46(Suppl 2):S33–9. doi: 10.1016/s0169-5002(04)80039-4. [DOI] [PubMed] [Google Scholar]
- 55.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 56.MacManus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 57.Choi NC, Fischman AJ, Niemierko A, Ryu JS, Lynch T, Wain J, et al. Dose-response relationship between probability of pathologic tumor control and glucose metabolic rate measured with FDG PET after preoperative chemoradiotherapy in locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:1024–35. doi: 10.1016/s0360-3016(02)03038-9. [DOI] [PubMed] [Google Scholar]
- 58.Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78:1903–9. doi: 10.1016/j.athoracsur.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 59.Pottgen C, Levegrun S, Theegarten D, Marnitz S, Grehl S, Pink R, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res. 2006;12:97–106. doi: 10.1158/1078-0432.CCR-05-0510. [DOI] [PubMed] [Google Scholar]
- 60.Dooms C, Verbeken E, Stroobants S, Nackaerts K, De Leyn P, Vansteenkiste J. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: A model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–34. doi: 10.1200/JCO.2007.13.9550. [DOI] [PubMed] [Google Scholar]
- 61.Aukema TS, Kappers I, Olmos RA, Codrington HE, van Tinteren H, van Pel R, et al. Is 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer? J Nucl Med. 2010;51:1344–8. doi: 10.2967/jnumed.110.076224. [DOI] [PubMed] [Google Scholar]
- 62.Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, et al. 18F-FDG PET/CT for Monitoring Treatment Responses to the Epidermal Growth Factor Receptor Inhibitor Erlotinib. J Nucl Med. 2011;52:1684–9. doi: 10.2967/jnumed.111.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong FM, Frey KA, Quint LE, Ten Haken RK, Hayman JA, Kessler M, et al. A pilot study of [18F] fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–23. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- 64.Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Verbeken EK The Leuven Lung Cancer Group. Potential use of FDG-PET scan after induction chemotherapy in surgically staged IIIa-N2 non-small-cell lung cancer: A prospective pilot study. Ann Oncol. 1998;9:1193–8. doi: 10.1023/a:1008437915860. [DOI] [PubMed] [Google Scholar]
- 65.Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:463–71. doi: 10.1007/s00259-006-0273-5. [DOI] [PubMed] [Google Scholar]
- 66.de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non–small cell lung cancer. J Nucl Med. 2007;48:1592–8. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 67.Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362–70. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 68.Hellwig D, Ukena D, Paulsen F, Bamberg M, Kirsch CM. Onko-PET der Deutschen Gesellschaft fur Nuklearmedizin. Meta-analysis of the efficacy of positron emission tomography with F-18-fluorodeoxyglucose in lung tumors. Basis for discussion of the German Consensus Conference on PET in Oncology 2000. Pneumologie. 2001;55:367–77. doi: 10.1055/s-2001-16201. [DOI] [PubMed] [Google Scholar]
- 69.Shiono S, Abiko M, Sato T. Positron emission tomography/computed tomography and lymphovascular invasion predict recurrence in stage I lung cancers. J Thorac Oncol. 2011;6:43–7. doi: 10.1097/JTO.0b013e3181f9abca. [DOI] [PubMed] [Google Scholar]
- 70.Kim YS, Lee MK, Kim SJ, Kim IJ, Kim YK, Jo WS, et al. Prognostic stratification using F-18 FDG PET/CT in patients with advanced stage (stage III and IV) non-small cell lung cancer. Neoplasma. 2010;57:241–6. doi: 10.4149/neo_2010_03_241. [DOI] [PubMed] [Google Scholar]
- 71.Tanvetyanon T, Eikman EA, Sommers E, Robinson L, Boulware D, Bepler G. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol. 2008;26:4610–6. doi: 10.1200/JCO.2008.16.9383. [DOI] [PubMed] [Google Scholar]
- 72.Burdick MJ, Stephans KL, Reddy CA, Djemil T, Srinivas SM, Videtic GM. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1033–9. doi: 10.1016/j.ijrobp.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 73.Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK. Limited prognostic value of dual time point F-18 FDG PET/CT in patients with early stage (stage I and II) non-small cell lung cancer (NSCLC) Radiother Oncol. 2011;98:105–8. doi: 10.1016/j.radonc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Cheng S, Evans WK, Stys-Norman D, Shepherd FA. Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care. Chemotherapy for relapsed small cell lung cancer: A systematic review and practice guideline. J Thorac Oncol. 2007;2:348–54. doi: 10.1097/01.JTO.0000263720.15062.51. [DOI] [PubMed] [Google Scholar]
- 75.Rosti G, Bevilacqua G, Bidoli P, Portalone L, Santo A, Genestreti G. Small cell lung cancer. Ann Oncol. 2006;17(Suppl 2):ii5–10. doi: 10.1093/annonc/mdj910. [DOI] [PubMed] [Google Scholar]
- 76.Brink I, Schumacher T, Mix M, Ruhland S, Stoelben E, Digel W, et al. Impact of [18F] FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1614–20. doi: 10.1007/s00259-004-1606-x. [DOI] [PubMed] [Google Scholar]
- 77.Azad A, Chionh F, Scott AM, Lee ST, Berlangieri SU, White S, et al. High impact of 18F-FDGPET on mamagement and prognostic stratification of newly diagnosed small cell lung cancer. Mol Imaging Biol. 2010;12:443–51. doi: 10.1007/s11307-009-0295-z. [DOI] [PubMed] [Google Scholar]
- 78.Rohren EM, Provenzale JM, Barboriak DP, Coleman RE. Screening for cerebral metastases with FDG PET in patients undergoing whole-body staging of non-central nervous system malignancy. Radiology. 2003;226:181–7. doi: 10.1148/radiol.2261010920. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto Y, Kameyama R, Murota M, Bandoh S, Ishii T, Nishiyama Y. Early assessment of therapeutic response using FDG PET in small cell lung cancer. Mol Imaging Biol. 2009;11:467–72. doi: 10.1007/s11307-009-0227-y. [DOI] [PubMed] [Google Scholar]
- 80.Zander T, Scheffler M, Nogova L, Kobe C, Engel-Riedel W, Hellmich M, et al. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18) F] fluorodeoxyglucose and [(18) F] fluorothymidine positron emission tomography. J Clin Oncol. 2011;29:1701–8. doi: 10.1200/JCO.2010.32.4939. [DOI] [PubMed] [Google Scholar]
- 81.Basu S. The scope and potentials of functional radionuclide imaging towards advancing personalized medicine in oncology: Emphasis on PET-CT. Discov Med. 2012;13:65–73. [PubMed] [Google Scholar]
- 82.Mishani E, Abourbeh G, Eiblmaier M, Anderson CJ. Imaging of EGFR and EGFR tyrosine kinase overexpression in tumors by nuclear medicine modalities. Curr Pharm Des. 2008;14:2983–98. doi: 10.2174/138161208786404326. [DOI] [PMC free article] [PubMed] [Google Scholar]


