Abstract
Human metapneumovirus (hMPV) is a recently discovered virus that causes respiratory illness in children that can lead to hospitalization. Our study was undertaken to further understand hMPV-associated illness, compare clinical characteristics of hMPV and respiratory syncytial virus (RSV), and establish the utility of routine screening for hMPV. We retrospectively identified hMPV-associated illnesses described among children with respiratory symptoms admitted to a tertiary care center in southeast Michigan during the 2006–2007 respiratory viral season. A convenience sample of 256 nasopharyngeal specimens was subjected to nucleic acid extraction and amplification to identify those specimens positive for hMPV. A medical record review was undertaken to retrieve demographic and clinical data of patients with hMPV, comparing them to RSV-positive patients and patients evaluated for respiratory symptoms who were negative for hMPV and RSV. We found that hMPV was the second most commonly identified virus after RSV. hMPV-positive patients were older than RSV-positive patients. Among hMPV-positive patients, pneumonia was diagnosed in 37.5% and bronchiolitis in 31.2%, peribronchial cuffing was present on chest radiographs of 37.5%, antibiotic treatment was used in 81.2%, and admission to the ICU was seen in 37.5%. Finally, hMPV-positive patients were more likely to have fever than RSV-positive patients or patients negative for hMPV and RSV. We concluded that hMPV is a major pathogen associated with hospitalization of children and with the same severity of illness as RSV but in a slightly older population. Because of the apparent prevalence and severity of illness, routine screening should be implemented.
Key words: human metapneumovirus, respiratory syncytial virus, severity.
Introduction
Human metapneumovirus (hMPV) is an RNA virus in the Pneumovirinae subfamily of the Paramyxoviridae family that was first isolated in the Netherlands in 20011 and has subsequently been identified worldwide.2–14 It has been implicated as a significant cause of hospitalization for young children,1 second only to respiratory syncytial virus (RSV) in infants hospitalized with acute respiratory infections (ARIs).15 hMPV has been detected in 1.5–43.0% of patients with ARIs.5 While it circulates predominantly in the winter, infections have been reported year-round16–18 and the incidence varies yearly.5,18–21 Seroprevalence studies have shown that almost all children over five years of age have evidence of past infection.22 Past infection with hMPV is thought to confer only partial immunity to subsequent infections.2,13,14
Clinical syndromes associated with hMPV infection are similar to those of RSV infection,4,23 ranging from mild upper respiratory tract infections to wheezing and severe lower respiratory tract infections requiring mechanical ventilation.2,11,23–26 Rare cases of fatalities have been associated with hMPV11,27–30 and it has been implicated in a handful of cases of encephalitis.12,31,32 Although hMPV infections have been diagnosed in adults, their greatest impact occurs in children.33 A significant association with hMPV and wheezing is seen in young children,23,34,35 and hMPV has been linked to apparent life-threatening events in infants.25 hMPV has been associated with ARIs with super-infections as a result of Staphylococcus aureus and Streptococcus pneumoniae.2
To begin to understand the impact of hMPV on our institution, we analyzed children admitted to our tertiary care center in southeast Michigan with respiratory symptoms during the respiratory season of 2006–2007 through an observational, retrospective study. The primary purposes of our study were to establish the utility of testing for hMPV in children who were admitted to our hospital during the respiratory virus season and to compare the impact of hMPV and RSV on the healthcare system.
Materials and Methods
We identified a convenience sample of 256 nasopharyngeal (NP) specimens from children younger than 18 years of age admitted with respiratory symptoms between November 1, 2006 and May 31, 2007. The specimens were obtained by a NP wash or swab based on the admitting physician’s discretion. After routine testing by direct fluorescent antibody (DFA) and/or culture for RSV, parainfluenza viruses 1–3, influenza viruses A and B, adenovirus, and rhinovirus, the NP specimens were frozen at −70°C and later subjected to nucleic acid extraction using the EasyMag system (bioMérieux, Durham, NC, USA) and following the manufacturer’s protocol with slight modifications. Two hundred microliters of each specimen were pre-treated with 20 units of DNase (New England Biolabs, UK) at 37°C for 45 min before extraction. Extracts were used as the template for detection of hMPV using the NucliSense real-time analyte specific reagent (ASR) assay performed on the EasyQ instrument (bioMérieux). A proprietary internal control containing the same primer binding sites as the hMPV target with unique internal sequences targeted by a separate molecular beacon probe were spiked into each specimen before extraction to monitor amplification integrity.
Respiratory specimen testing data were linked to patient demographic and clinical data. Laboratory personnel were blinded to the clinical data and the clinical investigator was blinded to the laboratory results. Only the first specimen from which a virus was identified per admission was considered. The χ2-test was used for analysis of categorical variables, comparing hMPV-positive patients to RSV-positive patients as well as patients who were negative for both hMPV and RSV. The Student’s t-test was used for an analysis that compared continu ous variables. Data were analyzed using SPSS version 16.0 for Windows.
Results
Of the 256 specimens, RSV was identified in 52 (20.3%), hMPV in 18 (7.0%), influenza in 9 (3.5%), rhinovirus in 5 (2.0%), parainfluenza in 8 (3.1%), and adenovirus in 4 (1.6%). Three specimens had co-infections: hMPV and RSV in two and RSV and influenza A in one. hMPV was detected primarily in specimens collected between January and March (83.4%), while RSV was uniformly detected in those from November through February and then decreased into May. No hMPV was detected in specimens obtained in April and May (Figure 1). Because the primary goal of our study was to compare patients with hMPV to those with RSV, and because the number of patients with isolated viruses other than hMPV or RSV was low, cases with viruses other than hMPV or RSV and those with no specific virus isolated were combined as a separate group for the additional analyses detailed in the following section.
Figure 1.
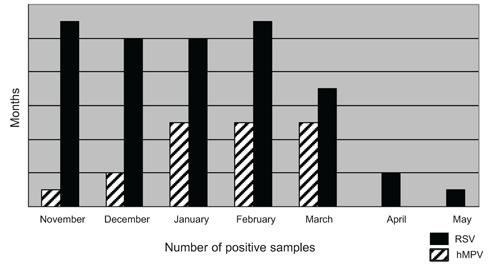
Distribution of human metapneumovirus and respiratory syncytial virus according to month.
While several studies have shown that hMPV occurs more in older children than does RSV, other reports showed no difference in age predilection, gender predominance, or presence of underlying medical disorders.23,36 In our study, the majority of hMPV-positive patients were aged 13–24 months (n=7, 43.8%), whereas most RSV-positive patients (n=35; 71.4%) were younger than 12 months of age (P<0.01). No statistical significance in gender predomin ance was found. The proportion of children with underlying medical disorders was similar across all three groups. Underlying medical disorders considered were prematurity, chronic lung disease, bronchopulmonary dysplasia, asthma, congenital heart disease, congestive heart failure, immunosuppression, immunodeficiencies, hematological and solid organ malignancies, diabetes, and renal failure. We also found that children attending daycare or school were not at increased risk of being positive for hMPV or RSV or for having a respiratory illness not associated with either of these viruses (Table 1).
Table 1. Demographic data.§.
| hMPV+(%) N=16 | RSV+(%) N=49 | Negative for hMPV and RSV (%) N=188 | |
|---|---|---|---|
| Age | |||
| 0–12 months | 4 (25.0)* | 35 (71.4) | 77 (41.0)* |
| 13–24 months | 7 (43.8) | 9 (18.4) | 38 (20.2) |
| ⩾25 months | 5 (31.2) | 5 (10.2) | 73 (38.8) |
| Male | 7 (43.8) | 26 (53.1) | 98 (52.1) |
| Daily activities | |||
| Home | 11 (68.8) | 37 (75.5) | 118 (62.8) |
| School/daycare | 5 (31.2) | 12 (24.5) | 70 (37.2) |
| Presence of underlying medical condition | 11 (68.8) | 32 (65.3) | 140 (74.5) |
P values not listed here were >0.05 and thus deemed not statistically significant;
P<0.01.
hMPV has previously been reported to be a rare cause of community-acquired pneumonia.37 We found that hMPV-positive patients were more likely to be diagnosed with pneumonia (37.5%) than were the other two groups (14%, P=0.04 for RSV-positive, P=0.02 for negative for both hMPV and RSV). In addition, hMPV-positive patients were equally likely to be diagnosed with bronchiolitis as were RSV-positive children (approximately 30%) but less likely than children with respiratory symptoms who were negative for hMPV and RSV (11%, P=0.02). In our study, the rate of abnormal chest radiographs was comparable in all three groups, but peribronchial cuffing was more likely to be present in hMPV-positive patients than in the other two groups (Table 2).
Table 2. Clinical features.§.
| Feature | hMPV+(%) N=16 | RSV+(%) N=49 | Negative for hMPV and RSV (%) N=188 |
|---|---|---|---|
| Pneumonia | 6 (37.5)*,** | 7 (14.3)* | 27 (14.4)** |
| Bronchiolitis | 5 (31.2)† | 14 (29.2) | 21 (11.2)† |
| Abnormal chest radiograph | 14 (87.5) | 35 (77.8) | 123 (71.9) |
| Peribronchial cuffing | 6 (37.5)‡,‡‡ | 5 (11.1)‡ | 18 (10.4)‡‡ |
| Oxygen supplementation | 12 (75.0) | 38 (77.6) | 104 (55.3) |
| Antibiotic use | 13 (81.2)∧ | 25 (51.0)∧ | 136 (72.3) |
| Antiviral use | 0 (0) | 1 (2.0) | 4 (2.1) |
| Steroid use | 8 (50.0) | 15 (30.6) | 66 (35.1) |
| Mechanical ventilation | 2 (12.5) | 2 (4.1) | 26 (13.8) |
| ICU admission | 6 (37.5)¶ | 6 (12.2)¶ | 50 (26.6) |
Pvalues not listed here were >0.05 and thus deemed not statistically significant;
P=0.04;
P=0.02;
P=0.02;
P<0.01;
P=0.03;
P=0.02.
In order to compare severity of illness across the three patient populations, we examined their hospital course. Mean duration of hospital stay was 6 days (range 1–37 d) for hMPV-positive patients, 6 days (range 1–112 d) for RSV-positive patients, and 12 days (range 1–117 d) for patients negative for hMPV and RSV (P=0.83 for hMPV vs. RSV; P=0.06 for hMPV vs. negative for hMPV and RSV). hMPV-positive patients were more likely to be treated with antibiotics than were RSV-positive patients (81.2% vs. 51.0%, P=0.03) and were more likely to be admitted to the intensive care unit (ICU) (37.5% vs. 12.2%, P=0.02). hMPV-positive patients were as likely to require oxygen supplementation, mechanical ventilation, and steroid use as were RSV-positive patients (Table 2).
Infections with hMPV and RSV have been reported to be clinically indistinguishable,23,36 but subtle differences were identified in our study. The most common presenting symptom for hMPV-positive patients was fever, which occurred more often in hMPV-positive patients than in the other two patient populations. hMPV-positive patients were also more likely than patients negative for hMPV and RSV to have decreased urine output (Table 3; 43.8% vs. 14.4%, P<0.01). On physical examination, hMPV-positive patients were more likely than RSV-positive patients to exhibit focal decreased breath sounds (18.8% vs. 2.0%, P=0.01) and were more likely than patients negative for hMPV and RSV to exhibit signs of otitis media (18.8% vs. 4.8%, P=0.02). Otherwise, there were no differences in the signs and symptoms noted in hMPV+ and RSV-positive patients (Table 4).
Table 3. Clinical symptoms.§.
| hMPV+(%) N=16 | RSV+(%) N=49 | Negative for hMPV and RSV (%) N=188 | |
|---|---|---|---|
| Fatigue | 6 (37.5) | 9 (18.4) | 153 (81.4) |
| Fever | 15 (93.8)*,** | 30 (61.2)* | 102 (54.3)** |
| Rash | 1 (6.2) | 1 (2.0) | 10 (5.3) |
| Vomiting | 5 (31.2) | 19 (38.8) | 67 (35.6) |
| Diarrhea | 4 (25.0) | 11 (22.4) | 31 (16.5) |
| Poor feeding | 9 (56.2) | 30 (61.2) | 80 (42.6) |
| Decreased urine output | 7 (43.8)† | 14 (28.6) | 27 (14.4)† |
| Watery eyes | 1 (6.2) | 1 (2.0) | 7 (3.7) |
| Red eyes | 0 (0) | 1 (2.0) | 4 (2.1) |
| Rhinorrhea | 6 (37.5) | 30 (61.2) | 74 (39.4) |
| Congestion | 8 (50.0) | 25 (51.0) | 61 (32.4) |
| Ear pain | 1 (6.2) | 2 (4.1) | 7 (3.7) |
| Sore throat | 1 (6.2) | 1 (2.0) | 15 (8.0) |
| Cough | 13 (81.2) | 44 (89.8) | 128 (68.1) |
| Rapid breathing | 4 (25.0) | 12 (24.5) | 30 (16.0) |
| Difficulty breathing | 9 (56.2) | 29 (59.2) | 80 (42.6) |
| Apnea | 2 (12.5) | 1 (2.0) | 12 (6.4) |
Pvalues not listed here were >0.05 and thus deemed not statistically significant;
P=0.01;
P=<0.01;
P<0.01.
Table 4. Clinical signs.§.
| hMPV+(%) N=16 | RSV+(%) N=49 | Negative for hMPV and RSV (%) N=188 | |
|---|---|---|---|
| Respiratory distress | 6 (37.5) | 24 (49.0) | 63 (33.5) |
| Respiratory failure | 1 (6.2) | 0 (0) | 15 (8.0) |
| Tachypnea | 5 (31.2) | 16 (32.7) | 40 (21.3) |
| Retractions | 4 (25.0)* | 30 (61.2)* | 48 (25.5) |
| Crackles | 4 (25.0) | 10 (20.4) | 28 (14.9) |
| Rhonchi | 2 (12.5) | 6 (12.2) | 17 (9.0) |
| Wheezing | 6 (37.5) | 23 (46.9) | 48 (25.5) |
| Rales | 0 (0) | 2 (4.1) | 7 (3.7) |
| Focal decreased breath sounds | 3 (18.8)† | 1 (2.0)† | 13 (6.9) |
| Tachycardia | 5 (31.2) | 18 (36.7) | 46 (24.5) |
| Poor perfusion | 1 (6.2) | 4 (8.2) | 13 (6.9) |
| Conjunctivitis | 1 (6.2) | 0 (0) | 5 (2.7) |
| Phayrngitis | 2 (12.5) | 1 (2.0) | 10 (5.3) |
| Signs of otitis media | 3 (18.8)‡ | 8 (16.3) | 9 (4.8)‡ |
| Lymphadenopathy of head/neck region | 1 (6.2) | 0 (0) | 11 (5.9) |
Pvalues not listed here were >0.05 and thus deemed not statistically significant;
P=0.01;
P=0.02;
P=0.02.
Discussion
hMPV was the second most commonly identified respiratory virus during the respiratory season of 2006–2007 in our study. Our results confirm the previous findings that children with symptomatic hMPV infection are older than those infected with RSV,2,6 possibly because of differences in the upper respiratory tract or lung anatomy of older children that allow for hMPV acquisition. In our study, school and/or daycare attendance was not identified as a risk factor for hMPV acquisition.
Although a previous report37 found that hMPV is a rare cause of community-acquired pneumonia among hospitalized patients (4.9%), 37.5% of our hMPV-positive patients were admitted with a diagnosis of pneumonia. This difference is likely because of a requirement of three independent radiologists’ interpretations of the radiographs in the earlier study compared to the diagnosis of one admitting physician in our study. hMPV-positive patients were more likely than the other two groups to have peribronchial cuffing on their chest radiographs, evidence suggesting that these patients have interstitial edema, likely a result of the disruption of the respiratory epithelial structure and inflammation that hMPV has been shown to cause in animal studies.38 hMPV may have a stronger predilection for the respiratory epithelial cells than RSV, corroborated by reports that hMPV-positive patients frequently exhibited signs of otitis media.33 Further research is needed on the pathogenesis of hMPV in humans, specifically regarding the ability of hMPV to infect human respiratory epithelial cells compared to RSV and other viruses. The present study demonstrates, as previously reported,23 that hMPV-positive patients were as likely to be severely ill as were RSV-positive patients. In fact, in our study, hMPV-positive patients were more likely than RSV-positive patients to be admitted to the ICU even though they were not more likely to have an underlying medical illness. In addition, hMPV-positive patients were more likely than were RSV-positive patients to receive antibiotics. During the study time period, our institution did not routinely test specimens for hMPV. Physicians may have used antibiotics more often in patients only retrospectively shown to be hMPV-positive because they were not aware of a specific virus contributing to these patients’ illnesses. The finding of increased antibiotic use in children with other ARIs, most of whom had negative viral cultures, corroborates this suggestion. It is also possible that the increased diagnosis of pneumonia in the hMPV-positive patients contributed to the increased use of antibiotics. Routine diagnostic testing for hMPV therefore may reduce the use of unnecessary antibiotics.
Previous reports have suggested that hMPV and RSV are clinically indistinguishable.17,21 This study, however, shows that there may be subtle differences in the clinical features of hMPV and RSV infections. As previously reported,34 fever was the most common presenting symptom for hMPV-positive patients and was more common in hMPV-positive children than in the other two groups. hMPV-positive patients were also more likely to experience decreased urinary output than patients negative for hMPV and RSV. The raised fever may increase the likelihood of dehydration and decreased urinary output. More information is needed to compare the level of inflammation produced by hMPV with that of other viruses.
Our study was limited by extraction of the data from a convenience sample and by the fact that only one respiratory viral season was studied. In addition, the signs and symptoms associated with the studied viruses may have been exaggerated as only hospitalized patients were included. Lastly, we acknowledge the li mi tations associated with the use of different methods for detecting different viruses in our study. The real-time ASR assay that we used to detect hMPV was likely more sensitive than the DFA- and culture-based techniques used to detect RSV and other viruses. As such, it is possible that children infected with RSV were assigned to the group of patients not infected with hMPV or RSV. Even with this potential drawback, however, our data strongly suggest that hMPV was common in the patient population included in this study. Furthermore, as molecular methods gain more widespread use for the detection of many respiratory pathogens, continued studies assessing the correlation between laboratory and clinical information are warranted.
Our study confirms that hMPV is a significant pathogen particularly in young children and is frequently associated with respiratory symptoms resulting in hospitalization. In general, the clinical manifestations of hMPV- and RSV-associated infections in children are similar, although our data suggest subtle differences in illness presentation but not in severity. Our data also suggest that underidentification of children with hMPV may lead to inappropriate use of antibiotics. Therefore, considering the high prevalence of hMPV, the severity of hMPV illness, and the ease and accuracy of detection,35 routine diagnostic testing for hMPV should be implemented. Increasingly, hMPV is being recognized as a significant cause of disease in other populations such as elderly patients39 and immunocompromised cases,40 highlighting other groups that would likely benefit from routine testing for hMPV. Future studies with expanded patient populations will help to determine how identifying cases with hMPV-associated disease will allow clinicians to anticipate the patient’s clinical course, identify cohort patients appropriately, and decrease the use of unnecessary antibiotics.
Acknowledgements:
the study was approved by the University of Michigan Medical Center’s Institutional Review Board. bioMérieux provided the instruments and a portion of the reagents that were used in this study. The authors would like to thank Clarisse Starr and Dollie Jacosalem for their expert technical assistance in the hMPV testing.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 3.Jartti T, van den Hoogen B, Garofalo RP, et al. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–4. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esper F, Boucher D, Weibel C, et al. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111:1407–10. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- 5.Maggi F, Pifferi M, Vatteroni M, et al. Human metapneumovirus associated with respiratory tract infections in a 3-year study of nasal swabs from infants in Italy. J Clin Microbiol. 2003;41:2987–91. doi: 10.1128/JCM.41.7.2987-2991.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–33. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludewick HP, Abed Y, van Niekerk N, et al. Human metapneumovirus genetic variability, South Africa. Emerg Infect Dis. 2005;11:1074–8. doi: 10.3201/eid1107.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noyola DE, Alpuche-Solís AG, Herrera-Díaz A, et al. Human metapneumovirus infections in Mexico: epidemiological and clinical characteristics. J Med Microbiol. 2005;54:969–74. doi: 10.1099/jmm.0.46052-0. [DOI] [PubMed] [Google Scholar]
- 9.Sloots TP, Mackay IM, Bialasiewicz S, et al. Human metapneumovirus, Australia 2001–2004. Emerg Infect Dis. 2006;12:1263–6. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang SM, Liu CC, Wang HC, et al. Human metapneumovirus infection among children in Taiwan: a comparison of clinical manifestations with other virus-associated respiratory tract infections. Clin Microbiol Infect. 2006;12:1221–4. doi: 10.1111/j.1469-0691.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 11.Bao X, Liu T, Spetch L, et al. Airway epithelial cell response to human metapneumovirus infection. Virology. 2007;368:91–101. doi: 10.1016/j.virol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata M, Ito M, Kiyosawa S, et al. A fatal case of encephalopathy possibly associated with human metapneumovirus infection. Jpn J Infect Dis. 2007;60:328–9. [PubMed] [Google Scholar]
- 13.Lee N, Chan PK, Yu IT, et al. Co-circulation of human metapneumovirus and SARSassociated coronavirus during a major nosocomial SARS outbreak in Hong Kong. J Clin Virol. 2007;40:333–7. doi: 10.1016/j.jcv.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baer G, Schaad UB, Heininger U. Clinical findings and unusual epidemiologic characteristics of human metapneumovirus infections in children in the region of Basel, Switzerland. Eur J Pediatr. 2008;167:63–9. doi: 10.1007/s00431-007-0427-x. [DOI] [PubMed] [Google Scholar]
- 15.Osterhaus A, Fouchier R. Human metapneumovirus in the community. Lancet. 2003;361:890–1. doi: 10.1016/S0140-6736(03)12785-7. [DOI] [PubMed] [Google Scholar]
- 16.Smuts H, Workman L, Zar HJ. Role of human metapneumovirus, human coronavirus NL63 and human bocavirus in infants and young children with acute wheezing. J Med Virol. 2008;80:906–12. doi: 10.1002/jmv.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esper F, Martinello RA, Boucher D, et al. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–96. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–50. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAdam AJ, Hasenbein ME, Feldman HA, et al. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190:20–6. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- 20.Foulogne V, Guyon G, Rodière M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:354–9. doi: 10.1097/01.inf.0000207480.55201.f6. [DOI] [PubMed] [Google Scholar]
- 21.Caracciolo S, Minini C, Colombrita D, et al. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J. 2008;27:406–12. doi: 10.1097/INF.0b013e318162a164. [DOI] [PubMed] [Google Scholar]
- 22.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 23.Wilkesmann A, Schildgen O, Eis-Hübinger AM, et al. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr. 2006;165:467–75. doi: 10.1007/s00431-006-0105-4. [DOI] [PubMed] [Google Scholar]
- 24.Schildgen O, Simon A, Wilkesman J, et al. The human metapneumovirus: biology, epidemiological features and clinical characteristics of infection. Rev Med Microbiol. 2006;17:11–25. [Google Scholar]
- 25.Estrada B, Carter M, Barik S, et al. Severe human metapneumovirus infection in hospitalized children. Clin Pediatr (Phila) 2007;46:258–62. doi: 10.1177/0009922806293896. [DOI] [PubMed] [Google Scholar]
- 26.Heikkinen T, Osterback R, Peltola V, et al. Human metapneumovirus infections in children. Emerg Infect Dis. 2008;14:101–6. doi: 10.3201/eid1401.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelletier G, Déry P, Abed Y, Boivin G. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8:976–8. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donoso A, León JA, Camacho JF, et al. Fatal hemorrhagic pneumonia caused by human metapneumovirus in an immunocompetent child. Pediatr Int. 2008;50:589–91. doi: 10.1111/j.1442-200X.2008.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garbino J, Inoubli S, Mossdorf E, et al. Respiratory viruses in HIV-infected patients with suspected respiratory opportunistic infection. AIDS. 2008;22:701–5. doi: 10.1097/QAD.0b013e3282f470ac. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins P, McNeil K, Kermeen F, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178:876–81. doi: 10.1164/rccm.200711-1657OC. [DOI] [PubMed] [Google Scholar]
- 31.Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: Clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–77. doi: 10.1086/509330. [DOI] [PubMed] [Google Scholar]
- 32.Kaida A, Iritani N, Kubo H, et al. Seasonal distribution and phylogenetic analysis of human metapneumovirus among children in Osaka City, Japan. J Clin Virol. 2006;35:394–9. doi: 10.1016/j.jcv.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 33.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 34.Bosis S, Esposito S, Niesters HG, et al. Impact of human metapneumovirus in childhood: comparison with respiratory syncytial virus and influenza viruses. J Med Virol. 2005;75:101–4. doi: 10.1002/jmv.20243. [DOI] [PubMed] [Google Scholar]
- 35.Vinh DC, Newby D, Charest H, McDonald J. Evaluation of a commercial direct fluorescent-antibody assay for human metapneumovirus in respiratory specimens. J Clin Microbiol. 2008;46:1840–1. doi: 10.1128/JCM.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320–4. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 37.Don M, Korppi M, Valent F, et al. Human metapneumovirus pneumonia in children: Results of an Italian study and minireview. Scand J Infect Dis. 2008;40:821–6. doi: 10.1080/00365540802227110. [DOI] [PubMed] [Google Scholar]
- 38.Kuiken T, van den Hoogen BG, van Riel DA, et al. Experimental human metapneumovirus of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. Am J Pathol. 2004;164:1893–900. doi: 10.1016/S0002-9440(10)63750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Englung JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–9. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]


