Abstract
This paper provides a review of a recently published series of studies that give a detailed and comprehensive documentation of the severe acute respiratory syndrome (SARS) epidemic in mainland China, which severely struck the country in the spring of 2003. The epidemic spanned a large geographical extent but clustered in two areas: first in Guangdong Province, and about 3 months later in Beijing with its surrounding areas. Reanalysis of all available epidemiological data resulted in a total of 5327 probable cases of SARS, of whom 343 died. The resulting case fatality ratio (CFR) of 6.4% was less than half of that in other SARS-affected countries or areas, and this difference could only partly be explained by younger age of patients and higher number of community acquired infections. Analysis of the impact of interventions demonstrated that strong political commitment and a centrally coordinated response was the most important factor to control SARS in mainland China, whereas the most stringent control measures were all initiated when the epidemic was already dying down. The long-term economic consequence of the epidemic was limited, much consumption was merely postponed, but for Beijing irrecoverable losses to the tourist sector were considerable. An important finding from a cohort study was that many former SARS patients currently suffer from avascular osteonecrosis, as a consequence of the treatment with corticosteroids during their infection. The SARS epidemic provided valuable information and lessons relevant in controlling outbreaks of newly emerging infectious diseases, and has led to fundamental reforms of the Chinese health system. In particular, a comprehensive nationwide internet-based disease reporting system was established.
Key words: severe acute respiratory syndrome, China, case fatality ratio, avascular necrosis, epidemic preparedness.
Introduction
In the spring of 2003, an epidemic of severe acute respiratory syndrome (SARS) brought China virtually to a standstill, forcing the country to thoroughly review the infectious disease control policy. Since then, the Chinese government has implemented new and innovative strategies, such as a real-time monitoring system that is now serving as a model for worldwide surveillance and response to infectious disease threats.1 In order to understand the circumstances at the time, it was necessary to analyse the SARS epidemic in mainland China by dissecting it meticulously, performing a proper autopsy as it where. This was the focus of a multidisciplinary Chinese-European research collaboration of which the results were recently published in a special issue of Tropical Medicine & International Health.2 The world has moved on since the SARS epidemic, but the insights gained in mainland China remain valuable, with comparable infectious disease threats presenting continuously, such as avian influenza (H5N1) and novel influenza A (H1N1). In the current paper we provide a narrative review of the main findings of our series of papers and discuss these in the context of the broader literature.
SARS was caused by a novel coronavirus that was provisionally termed SARS-associated coronavirus (SARS-CoV).3,4 The earliest cases of SARS occurred in mid-November 2002 in Guangdong Province, China. SARS was first recognised in February 2003, when cases with an atypical pneumonia of unknown cause began appearing among hospital staff in Guangzhou, China. Within weeks, similar outbreaks occurred in Hanoi, Hong Kong, Toronto, Singapore and Taiwan. Soon thereafter, cases were reported from 32 countries and regions (later corrected to 29). After July 2003, SARS came under control thanks to enormous efforts made by national and international organisations. More than 8000 cases were reported worldwide, with over 5000 from mainland China, making the country the epicentre of the outbreak.5
During the SARS outbreak in China, epidemiological and clinical information was obtained in difficult circumstances. The available data were only to a limited extent analysed and reported, and mainly in Chinese literature. There was a need for good documentation, accessible to the international scientific community. The first step in the collaboration was to integrate all existing Chinese SARS data into one final database.6 With this comprehensive nationwide database it was possible to perform in-depth studies and dissect the SARS epidemic in mainland China, answering many questions that up to now were only studied in part or not at all.
Description of the epidemic
After extensive data cleaning and collection of additional information, the resulting database of SARS in mainland China contained 5327 probable cases, of whom 343 died, giving a case fatality ratio (CFR) of 6.4%. The epidemic spanned a large geographical extent (170 counties of 22 provinces) but clustered in two areas: first in Guangdong Province, and about 3 months later in Beijing with its surrounding areas Shanxi, Inner Mongolia Autonomic Region, Hebei and Tianjin. Figure 1 shows the temporal distribution of SARS in the six most seriously affected geographic areas of mainland China. Spatiotemporal analyses indicated that the spread of SARS occurred in two different patterns.7 In the early period of the epidemic, especially before strict control measures were taken, SARS spread to new areas incidentally through air travel of certain index cases. Thereafter, human travel along local transportation routes influenced the transmission of SARS. The epidemic period in middle-north China was shorter than in South China, but the geographic spread was wider. SARS not only spread locally, but also diffused quickly and resulted in several outbreaks close to Beijing city. In contrast, the SARS epidemic in South China was mainly limited to Guangdong Province. Transportation routes accelerated the spread of SARS in mainland China. However, especially national highways and inter-provincial freeways appeared to play a role, whereas railways seemed to be less important.7 For the definition of SARS cases a distinction was made between probable and suspected cases on the basis of contact history and number and severity of symptoms. Feng et al. provide a tabulated overview of the case criteria of SARS in the different time periods of the epidemic.6 In China, it was only possible late in the epidemic to confirm SARS through serological tests and serological confirmation was not included in the case definitions. In a study comparing clinical characteristics of probable and suspected cases it was found that although symptoms hardly differed, there were clearly different haematological profiles, justifying the distinction between probable and suspected cases and confirming that the suspected cases most likely did not have SARS.8
Figure 1.
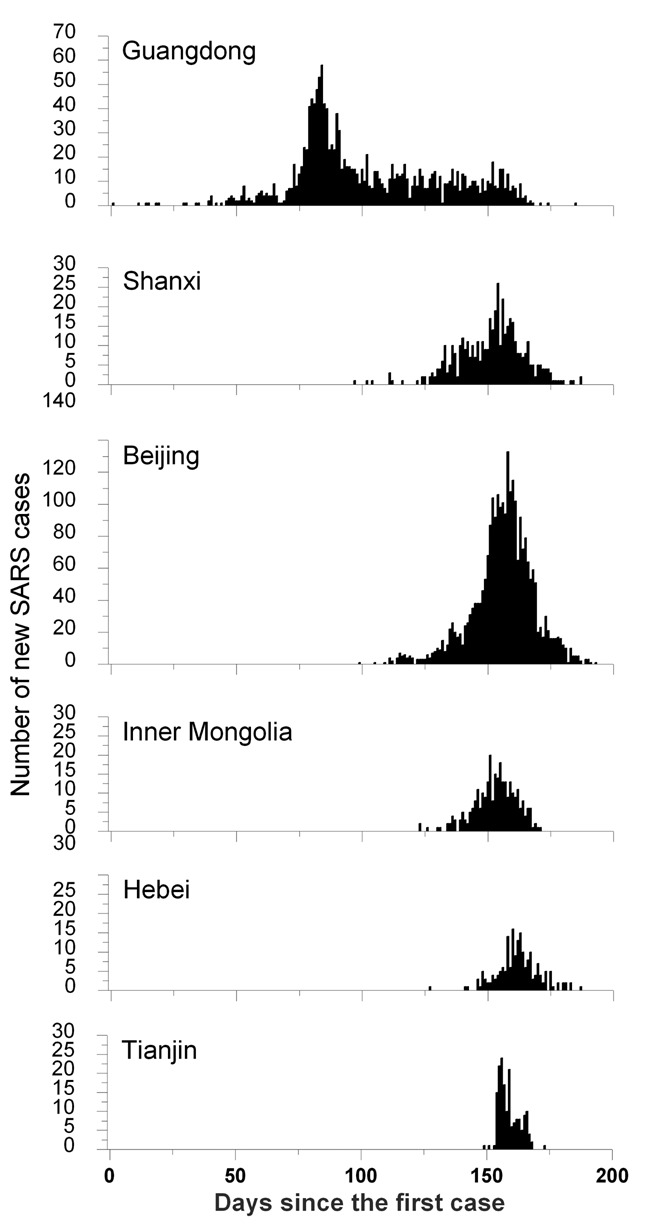
The temporal distribution of SARS outbreaks in the six most seriously affected geographic areas of mainland China by plotting the number of new cases per day of onset since the first SARS case on November 16, 2002, in Guangdong Province.
The average duration (3.8 days) and pattern (with time of epidemic and age) of onset of symptoms to hospital admission of SARS patients in mainland China were comparable to other affected areas.9 The duration of hospital admission to discharge for those who survived (29.7 days) was shorter than elsewhere in the world, possibly because of different hospitalisation policies. The duration of hospital admission to death in mainland China was 17.4 days, which is also shorter than in other areas. In the course of time, hospital epidemics in particular were rapidly brought under control, with increasing efficiency.10 This was due to increasing understanding of the disease and more effective preventive measures such as establishing isolation wards, training and monitoring hospital staff in infection control, screening of health care workers (HCWs), and compliance with the use of personal protection equipment.11
Case fatality
Figure 2 gives a comparison of the CFRs for SARS patients in Beijing, Guangdong and Tianjin with age. Because of their deteriorated health status and apparent complications, the risk of dying increased strongly in patients from the age of 50 years onwards. The Tianjin SARS outbreak happened mainly within hospitals, leading to a high impact of co-morbidity, which explains the relatively high CFR. Guangdong Province showed a considerably lower CFR than Beijing, the reason being unclear. The overall CFR in mainland China of 6.4% appeared much lower than reported in other areas and countries (Table 1), where CFRs varied from 9.7% in Vietnam to 17% in Hong Kong and Canada. This much lower case fatality in mainland China is also in contrast with the shorter duration of hospital admission to death compared to other countries or regions. Obvious reasons for this lower CFR are young age and a relatively higher number of community acquired infections as opposed to hospital acquired infections. However, the relatively young age of the cases (median age 33 years) only partly explains the low CFR in mainland China compared to other countries and areas affected by SARS, where median age varied from 37 years in Singapore to 49 years in Canada (Table 1).12 For example, using the age-related CFR values of mainland China for the age distributions of SARS cases in Hong Kong and Canada results in an expected overall CFR of about 9.2%, which is indeed higher than the overall value in mainland China (6.4%), but it only explains about 25% of the difference with the actual value in Hong Kong and Canada (17%). This finding was confirmed by a more direct comparative analysis of SARS in Hong Kong, Beijing and Taiwan.13 The relatively lower proportion of hospital acquired infections in mainland China – reflected in the lower proportion of infections among HCW compared to other areas (19% vs. 23–56%; Table 1) – is also most likely only partly responsible for the lower CFR in mainland China, especially since this factor is highly correlated with age. It has been suggested that mainland China had a substantial number of cases that were not really SARS, especially in Guangdong, where the epidemic started. A review study comparing seroprevalence rates in different SARS affected areas did show relatively lower proportions positive in mainland China, but the differences with other areas were only small and far from significant. Furthermore, even if the lower seroprevalence that was found in mainland China actually represented over-reporting, then this factor could only explain a modest 10% of the lower case fatality.14 The combined effect of these factors do not fully explain the lower death rates in mainland China, and therefore other (unknown) factors have probably also played a role. Options may be better treatment (see below) and use of Chinese traditional medicines, as was suggested by the well-known Chinese SARS expert Prof. Zhong.15
Figure 2.
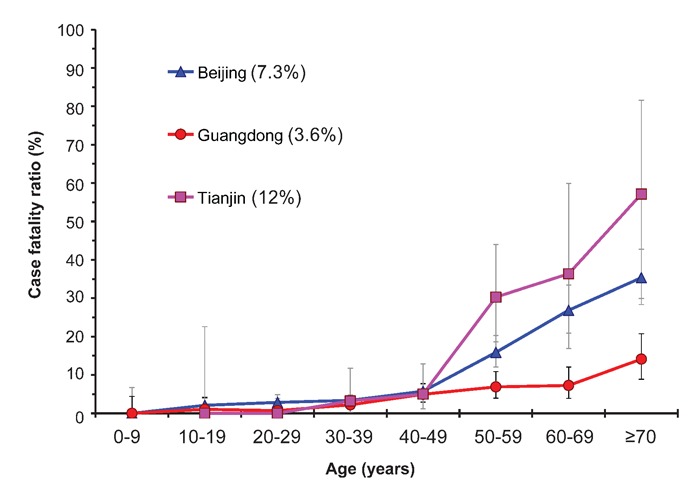
Comparison of the case fatality ratios for SARS patients in Beijing, Guangdong and Tianjin with age. Intervals indicate 90% binomially distributed confidence intervals. The values between parentheses represent the overall case fatality ratio for each of the three areas.
Table 1. The characteristics of the SARS outbreak in mainland China and other countries or regions in 2003. Data concern probable cases. See Feng et al. for data sources.6.
| Country or area | Mainland China | Hong Kong | Taiwan | Singapore | Vietnam | Canada |
|---|---|---|---|---|---|---|
| Number of cases | 5327 | 1755 | 674 | 238 | 62 | 251 |
| CFR: no. dead (%) | 343 (6.4) | 302 (17.2) | 87 (12.9) | 33 (13.9) | 6 (9.7) | 43 (17.1) |
| Age: median (yrs) | 33 | 40 | 46 | 37 | 43 | 49 |
| Occupation: HCW (%) | 1021 (19.2) | 405 (23.1) | 205 (30.3) | 97 (40.8) | 35 (56.5) | 101 (40.2) |
The effect of interventions
During the SARS epidemic in mainland China, various interventions were implemented to contain the outbreak.16 The overall set of measures taken was certainly effective, given the fact that the epidemic was fully controlled within 200 days after the first case emerged. The method of Wallinga and Teunis,17 with quantifications from Lipsitch et al.,18 was used to estimate Rt, the effective or net reproduction number, which helps to determine which interventions were most important. Rt is defined as the mean number of secondary cases generated by one primary case with symptom onset on day t. This number changes during the course of an epidemic, particularly as a result of effective control measures. If Rt is larger than the threshold value of one, a sustained chain of transmission will occur, eventually leading to a major epidemic. If this number is maintained below one, then transmission may still continue, but the number of secondary cases is not sufficient to replace the primary cases, leading to a gradual fade-out of the epidemic.
Figure 3 shows Rt over time for mainland China together with the timing of nine important events and public health control measures. The graph is characterised by a fluctuating pattern and wide confidence interval early in the epidemic, which can be explained by the initial low number of cases used in the calculations and the relatively more important impact of so-called super-spreading events. In Guangdong, where the epidemic started, standard control measures such as isolation and contact tracing (arrow 2) seem to already have helped to largely interrupt transmission in this province. However, during the period from day 80 to 140 the number of new SARS cases steadily increased, due to the spread to and within other parts of China, i.e. mainly Beijing and surrounding provinces. The first official report of an outbreak in Guangdong (arrow 3) and the WHO global alerts (arrow 4) were by no means reflected in a consistent reduction of Rt. Also, the first interventions in Beijing were not effective enough to cause any downward trend in the transmission (arrow 5). It was only around 11ߝ14 April 2003 that the Chinese authorities gained full control of all activities to combat SARS, with national, unambiguous, rational, widely followed guidelines and control measures, under central guidance (arrow 6). Immediately the reproduction number decreased dramatically and consistently. Within one week, Rt was below one. Strikingly, this marked decrease after the period 11–14 April was consistently present in the patterns of Rt for all heavily affected areas in mainland China.(19) The stringent control measures to prevent human contacts (arrow 7), including the decision to cancel the public holiday of May 1 (arrow 8), were all initiated after Rt was below one, i.e. when the epidemic was already dying down, again consistently for all areas in mainland China. Still, given the information available at the time, the most stringent interventions were rational since it was not clear to which extent the epidemic was under control. However, in retrospect, we can now conclude that these measures – which so much affected public life – contributed little to the factual containment of the SARS epidemic; the essential moment had occurred earlier. Obviously, the late interventions may still have played a role in speeding up the elimination of SARS. Also, cancelling the public holiday, where millions of people travel long distances to visit their family, may have prevented (smaller) outbreaks in yet unaffected locations. Nowadays methods are available to estimate Rt in real time, so that the course of the epidemic and the impact of control can be assessed more rapidly.20
Figure 3.
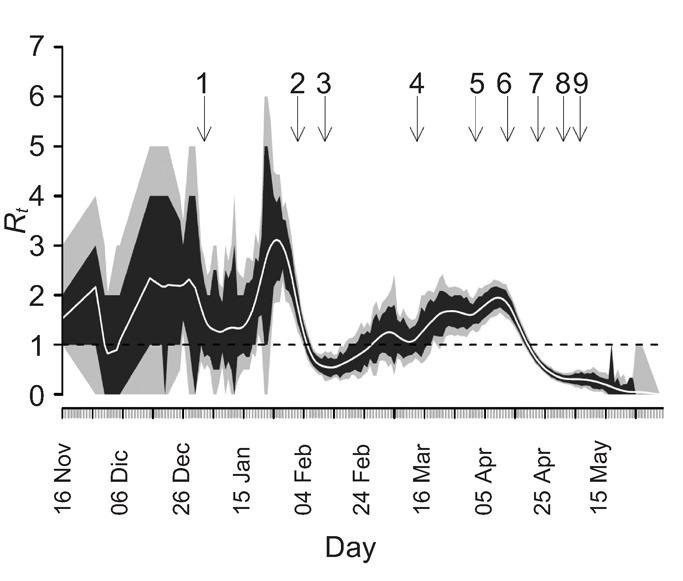
Estimated effective reproduction number (Rt) (number of secondary infections generated per primary case) during the SARS epidemic in China. Values represent average Rt (central white line) and associated 95% (grey) and 80% (black) confidence intervals, by date of symptom onset. The critical value of Rt=1, below which sustained transmission is impossible, is marked with a broken horizontal line. Arrows reflect the moment of important events and public health control measures: (1) Local newspaper report about outbreak of unknown infectious disease in Guangdong (2 January); (2) Start of control in Guangdong hospitals: e.g. isolation, contact tracing (1–3 February); (3) First official report of outbreak by Guangdong authorities (11 February); (4) WHO global alerts; first mentioning of SARS (12–15 March); (5) First protocol of SARS control; start isolation in Beijing hospitals (2 April); (6) Full control under central guidance by the Chinese authorities, including mandatory reporting of SARS; definition of diagnostic criteria and treatment (11–14 April); (7) Stringent control measures: quarantine in airports and stations; closure of schools, universities and public places; daily reporting by the national media (19–26 April); (8) Public holiday cancelled; new 1000-bed SARS hospital opened (1 May); (9) Further improvement of various guidelines and protocols (4–9 May).
We conclude that strong political commitment and a centrally coordinated response was the most important factor in the control of SARS in mainland China. With respect to future outbreaks of emerging infectious diseases, we emphasise that it is of first and foremost importance that effective control is based on clear (inter)national guidelines, communication and reporting structures, together with firm definition of responsibilities at all levels.
Consequences of the epidemic
There were many obvious immediate consequences of the epidemic, such as substantial morbidity and mortality, fear for becoming infected, panic in the public domain, stringent quarantine measures, travel restrictions, etc. Also two important middle and long-term consequences of the epidemic were identified. First, there was the economic impact. This was studied for Beijing by Beutels et al.21 through associating time series of daily and monthly SARS cases and deaths and volume of public train, airplane and cargo transport, tourism, household consumption patterns and gross domestic product growth in Beijing. It was concluded that especially leisure activities, local and international transport and tourism were affected by SARS, particularly in May 2003. Much of this consumption was merely postponed; but irrecoverable losses to the tourist sector alone were estimated at about US$ 1.4 billion, or 300 times the cost of treatment for SARS cases in Beijing.
Second, there were long-term health consequences for those former SARS patients who were treated with corticosteroids. Lv et al. investigated the relationship between avascular osteonecrosis (AVN) and corticosteroid treatment given to patients with SARS through a longitudinal study of 71 former SARS patients (mainly HCWs) who had been treated with corticosteroids, with an observation time of 36 months.22 Magnetic resonance images and X-rays of hips, knees, shoulders, ankles and wrists were taken as part of the post-SARS follow-up assessments. Thirty-nine per cent (39%) developed AVN of the hips within 3–4 months after starting treatment. Two more cases of hip necrosis were seen after 1 year and another 11 cases of AVN were diagnosed after 3 years, one with hip necrosis and 10 with necrosis in other joints. In total, a staggering 58% of the cohort had developed AVN after 3 years of observation. The sole factor explaining AVN in the hip was the total dose of corticosteroids received, which ranged from 1000 to 10 000 mg for the majority of cases, but was as high as 25 000 in some cases.22 The use of corticosteroids as well as other drugs (e.g. ribavirin) has been debated, with conflicting opinions about the effectiveness and possible harm of drugs used in the treatment of SARS.23,24 It has remained uncertain how the aggressive use of corticosteroids during the SARS epidemic has tipped the balance. Has it saved more lives by applying corticosteroids in high doses, and has it as such been responsible for the lower case fatality in mainland China? And, do immediate benefits in terms of saving lives weigh against the adverse effects, including AVN?
Lessons learnt and actions taken in China regarding epidemic preparedness
The SARS epidemic provided valuable information and lessons relevant in controlling outbreaks of newly emerging infectious diseases, which are surely due to come. Avian influenza and the novel A (H1N1) influenza threat are already knocking at our doors! Important lessons learnt in China included the need for more honesty and openness, improvement of surveillance, laboratory facilities and case management.25 Also, public health measures to control infectious diseases, reporting systems, and central command and coordination came under scrutiny. Another lesson was the need to inform and involve the public timely and adequately regarding control measures. There was a strong realisation that the best defence against any threat of newly emerging infectious diseases is a robust public health system in its science, capacity, practice, and through collaboration with clinical and veterinary medicine, academia, industry, and other public and private partners.
An important resolution of the Chinese government was to improve its disease surveillance system to rapidly identify newly emerging infectious diseases and to minimise their spread in China and the rest of the world. The traditional surveillance network using reporting cards filled out by hand and sent by mail or fax has been substituted by an automatic information system called the China Information System for Disease Control and Prevention (CISDCP), which is the world’s largest internet-based disease reporting system.1 The government has also increased their investment into enhancing the capabilities in detection, diagnosis, prevention and control of newly emerging infectious diseases at various levels. Education and training projects such as training courses for public health officials and health care workers have been initiated, and new training has been added to the education programs of universities. Funds for research projects on the development of vaccines, drugs and diagnostic techniques have been granted to develop new approaches in the prevention, diagnosis and treatment of emerging infectious diseases.
In conclusion, the epidemic of a new infectious disease, SARS, took firstly China and subsequently many other areas in the world completely by surprise. In the end, the consequences of this epidemic in terms of people afflicted and economic loss were not entirely catastrophic. Also, it turned out that SARS could be controlled relatively easily through standard interventions. However, the epidemic revealed some important weaknesses in the Chinese public health system, which has been dealt with efficiently by the Chinese government. At this point in time, China is better prepared than ever for epidemics, which may very well be worse than SARS in terms of speed of spread and number of deaths. As a matter of fact, SARS can justly be seen as a wake-up call.
Acknowledgements:
this study was supported by the Commission of the European Community under the Sixth Framework Program Specific Targeted Research Project, SARS Control “Effective and Acceptable Strategies for the Control of SARS and new emerging infections in China and Europe” (Contract No. SP22-CT-2004-003824), and Grants from National Science Fund for Distinguished Young Scholars (No. 30725032), and National Natural Science Foundation of China for International Cooperation (No. 30810103903). We thank Dr. Ben Cooper (HPA, London) for producing Figure 3.
References
- 1.Wang L, Wang Y, Jin S, et al. Emergence and control of infectious diseases in China. Lancet. 2008;372:1598–605. doi: 10.1016/S0140-6736(08)61365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vlas SJ, Cao WC, Richardus JH. Documenting the SARS epidemic in mainland China (Editorial) Trop Med Int Health. 2009;14:1–3. doi: 10.1111/j.1365-3156.2009.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Severe Acute Respiratory Syndrome (SARS) [cited 2009 12 June 2009];2004 Available from: http://www.who.int/csr/sars/en/
- 6.Feng D, de Vlas SJ, Fang LQ, et al. The SARS epidemic in mainland China: bringing together all epidemiological data. Trop Med Int Health. 2009;14:4–13. doi: 10.1111/j.1365-3156.2008.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang LQ, de Vlas SJ, Feng D, et al. Geographical spread of SARS in mainland China. Trop Med Int Health. 2009;14:14–20. doi: 10.1111/j.1365-3156.2008.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei MT, de Vlas SJ, Yang Z, et al. The SARS outbreak in a general hospital in Tianjin, China: clinical aspects and risk factors for disease outcome. Trop Med Int Health. 2009;14:60–70. doi: 10.1111/j.1365-3156.2009.02347.x. [DOI] [PubMed] [Google Scholar]
- 9.Feng D, Jia N, Fang LQ, et al. Duration of symptom onset to hospital admission and admission to discharge or death in SARS in mainland China: a descriptive study. Trop Med Int Health. 2009;14:28–35. doi: 10.1111/j.1365-3156.2008.02188.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper BS, Fang LQ, Zhou JP, et al. Transmission of SARS in three Chinese hospitals. Trop Med Int Health. 2009;14:71–8. doi: 10.1111/j.1365-3156.2009.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Tang F, Fang LQ, et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Health. 2009;141:52–9. [Google Scholar]
- 12.Jia N, Feng D, Fang LQ, et al. Case fatality of SARS in mainland China and associated risk factors. Trop Med Int Health. 2009;14:21–7. doi: 10.1111/j.1365-3156.2008.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau EH, Hsiung CA, Cowling BJ, et al. A comparative epidemiologic analysis of SARS in Hong Kong, Beijing and Taiwan. BMC infectious diseases. 2010;10:50–50. doi: 10.1186/1471-2334-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Han XN, Tang F, et al. No evidence of over-reporting of SARS in mainland China. Trop Med Int Health. 2009;14:46–51. doi: 10.1111/j.1365-3156.2009.02300.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhong N. Management and prevention of SARS in China. Philosophical transactions of the Royal Society of London. 2004;359:1115–6. doi: 10.1098/rstb.2004.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad A, Krumkamp R, Reintjes R. Controlling SARS: a review on China's response compared with other SARS-affected countries. Trop Med Int Health. 2009;14:36–45. doi: 10.1111/j.1365-3156.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallinga J, Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160:509–16. doi: 10.1093/aje/kwh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsitch M, Cohen T, Cooper B, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–70. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vlas SJ, Feng D, Cooper BS, et al. The impact of public health control measures during the SARS epidemic in mainland China. Trop Med Int Health. 2009;14:101–4. doi: 10.1111/j.1365-3156.2009.02348.x. [DOI] [PubMed] [Google Scholar]
- 20.Cauchemez S, Boelle PY, Thomas G, Valleron AJ. Estimating in real time the efficacy of measures to control emerging communicable diseases. Am J Epidemiol. 2006;164:591–7. doi: 10.1093/aje/kwj274. [DOI] [PubMed] [Google Scholar]
- 21.Beutels P, Jia N, Zhou QY, et al. The economic impact of SARS in Beijing, China. Trop Med Int Health. 2009;14:85–91. doi: 10.1111/j.1365-3156.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 22.Lv H, de Vlas SJ, Liu W, et al. Avascular osteonecrosis after treatment of SARS: a 3-year longitudinal study. Trop Med Int Health. 2009;14:79–84. doi: 10.1111/j.1365-3156.2008.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomersall CD. Pro/con clinical debate: steroids are a key component in the treatment of SARS. Pro: Yes, steroids are a key component of the treatment regimen for SARS. Crit Care. 2004;8:105–7. doi: 10.1186/cc2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e2. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong N, Zeng G. What we have learnt from SARS epidemics in China. Br Med J. 2006;333:389–91. doi: 10.1136/bmj.333.7564.389. [DOI] [PMC free article] [PubMed] [Google Scholar]


