Abstract
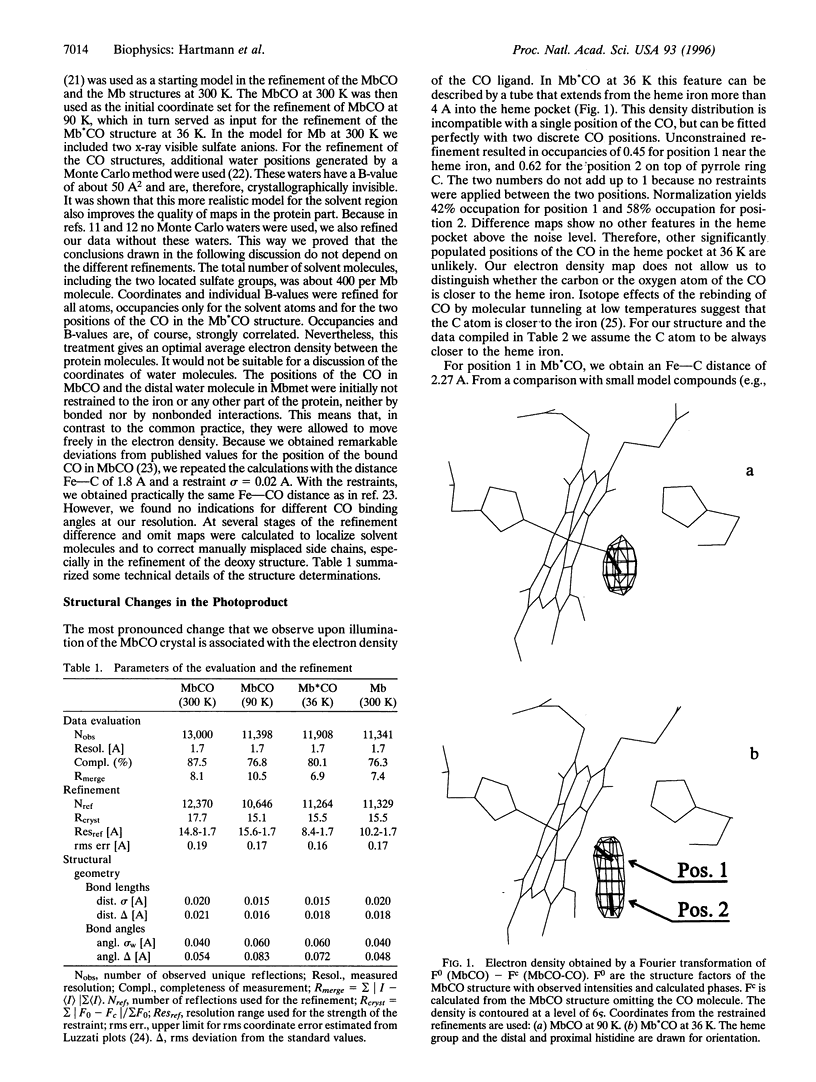
The x-ray structure of carbon monoxide (CO)-ligated myoglobin illuminated during data collection by a laser diode at the wavelength lambda = 690 nm has been determined to a resolution of 1.7 A at T = 36 K. For comparison, we also measured data sets of deoxymyoglobin and CO-ligated myoglobin. In the photon-induced structure the electron density associated with the CO ligand can be described by a tube extending from the iron into the heme pocket over more than 4 A. This density can be interpreted by two discrete positions of the CO molecule. One is close to the heme iron and can be identified to be bound CO. In the second, the CO is dissociated from the heme iron and lies on top of pyrrole ring C. At our experimental conditions the overall structure of myoglobin in the metastable state is close to the structure of a CO-ligated molecule. However, the iron has essentially relaxed into the position of deoxymyoglobin. We compare our results with those of Schlichting el al. [Schlichting, I., Berendzen, J., Phillips, G. N., Jr., & Sweet, R. M. (1994) Nature 317, 808-812], who worked with the myoglobin mutant (D122N) that crystallizes in the space group P6 and Teng et al. [Teng, T. Y., Srajer, V. & Moffat, K. (1994) Nat. Struct. Biol. 1, 701-705], who used native myoglobin crystals of the space group P2(1). Possible reasons for the structural differences are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Chance B., Fischetti R., Powers L. Structure and kinetics of the photoproduct of carboxymyoglobin at low temperatures: an X-ray absorption study. Biochemistry. 1983 Aug 2;22(16):3820–3829. doi: 10.1021/bi00285a017. [DOI] [PubMed] [Google Scholar]
- Chu K, Ernst RM, Frauenfelder H, Mourant JR, Nienhaus GU, Philipp R. Light-induced and thermal relaxation in a protein. Phys Rev Lett. 1995 Mar 27;74(13):2607–2610. doi: 10.1103/PhysRevLett.74.2607. [DOI] [PubMed] [Google Scholar]
- Della Longa S D., Ascone I., Fontaine A., Congiu Castellano A., Bianconi A. Intermediate states in ligand photodissociation of carboxymyoglobin studies by dispersive X-ray absorption. Eur Biophys J. 1994;23(5):361–368. doi: 10.1007/BF00188660. [DOI] [PubMed] [Google Scholar]
- Fiamingo F. G., Alben J. O. Structures of photolyzed carboxymyoglobin. Biochemistry. 1985 Dec 31;24(27):7964–7970. doi: 10.1021/bi00348a019. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Steigemann W., Reuscher H., Parak F. Structural disorder in proteins. A comparison of myoglobin and erythrocruorin. Eur Biophys J. 1987;14(6):337–348. doi: 10.1007/BF00262319. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Mourant J. R., Braunstein D. P., Chu K., Frauenfelder H., Nienhaus G. U., Ormos P., Young R. D. Ligand binding to heme proteins: II. Transitions in the heme pocket of myoglobin. Biophys J. 1993 Oct;65(4):1496–1507. doi: 10.1016/S0006-3495(93)81218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Chu K., Frauenfelder H. Ligand binding to heme proteins: the effect of light on ligand binding in myoglobin. Biochemistry. 1994 Nov 15;33(45):13413–13430. doi: 10.1021/bi00249a030. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F., Hartmann H., Aumann K. D., Reuscher H., Rennekamp G., Bartunik H., Steigemann W. Low temperature X-ray investigation of structural distributions in myoglobin. Eur Biophys J. 1987;15(4):237–249. doi: 10.1007/BF00577072. [DOI] [PubMed] [Google Scholar]
- Parak F., Hartmann H., Schmidt M., Corongiu G., Clementi E. The hydration shell of myoglobin. Eur Biophys J. 1992;21(5):313–320. doi: 10.1007/BF00188343. [DOI] [PubMed] [Google Scholar]
- Parak F., Thomanek U. F., Bade D., Wintergerst B. The orientation of the electric field gradient tensor in CO-liganded myoglobin. Z Naturforsch C. 1977 Jul-Aug;32(7-8):507–512. doi: 10.1515/znc-1977-7-805. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Powers L., Chance B., Chance M., Campbell B., Friedman J., Khalid S., Kumar C., Naqui A., Reddy K. S., Zhou Y. Kinetic, structural, and spectroscopic identification of geminate states of myoglobin: a ligand binding site on the reaction pathway. Biochemistry. 1987 Jul 28;26(15):4785–4796. doi: 10.1021/bi00389a028. [DOI] [PubMed] [Google Scholar]
- Powers L., Sessler J. L., Woolery G. L., Chance B. CO bond angle changes in photolysis of carboxymyoglobin. Biochemistry. 1984 Nov 6;23(23):5519–5523. doi: 10.1021/bi00318a021. [DOI] [PubMed] [Google Scholar]
- Schlichting I., Berendzen J., Phillips G. N., Jr, Sweet R. M. Crystal structure of photolysed carbonmonoxy-myoglobin. Nature. 1994 Oct 27;371(6500):808–812. doi: 10.1038/371808a0. [DOI] [PubMed] [Google Scholar]
- Steigemann W., Weber E. Structure of erythrocruorin in different ligand states refined at 1.4 A resolution. J Mol Biol. 1979 Jan 25;127(3):309–338. doi: 10.1016/0022-2836(79)90332-2. [DOI] [PubMed] [Google Scholar]
- Teng T. Y., Huang H. W., Olah G. A. 5 K extended X-ray absorption fine structure and 40 K 10-s resolved extended X-ray absorption fine structure studies of photolyzed carboxymyoglobin. Biochemistry. 1987 Dec 15;26(25):8066–8072. doi: 10.1021/bi00399a007. [DOI] [PubMed] [Google Scholar]
- Teng T. Y., Srajer V., Moffat K. Photolysis-induced structural changes in single crystals of carbonmonoxy myoglobin at 40 K. Nat Struct Biol. 1994 Oct;1(10):701–705. doi: 10.1038/nsb1094-701. [DOI] [PubMed] [Google Scholar]



