Abstract
Viral load generally rises in HIV-infected individuals with a concomitant infection, but falls markedly in some individuals with scrub typhus (ST), a common Asian rickettsial infection. ST infection appears to shift the viral population from CXCR4-using (X4) to CCR5-utilizing (R5) strains, and there is evidence of cross-reactivity between ST-specific antibodies and HIV-1. We examined the mechanism of ST suppression of HIV by measuring the effects of ST infection on X4 and R5 viruses in vivo and in vitro, and assessing the relative contributions of antibodies and chemokines to the inhibitory effect. In vivo, a single scrub typhus plasma infusion markedly reduced the subpopulation of HIV-1 viruses using the X4 co-receptor in all 8 recipients, and eliminated X4 viruses 6 patients. In vitro, the 14 ST sera tested all inhibited the replication of an X4 but not an R5 virus. This inhibitory effect was maintained if ST sera were depleted of chemokines but was lost upon removal of antibodies. Sera from STinfected mice recognized a target that co-localized with X4 HIV gp120 in immunofluorescent experiments. These in vivo and in vitro data suggest that acute ST infection generates cross-reactive antibodies that produce potent suppression of CXCR4- but not CCR5-using HIV-1 viruses. ST suppression of HIV replication could reveal novel mechanisms that could be exploited for vaccination strategies, as well as aid in the development of fusion inhibitors and other new therapeutic regimens. This also appears to be the first instance where one pathogen is neutralized by antibody produced in response to infection by a completely unrelated organism.
Key words: HIV-1, scrub typhus, antibody, Orientia tsutsugamushi, AIDS
Introduction
Coinfection generally raises HIV-1 viral load. One prospective study found that average plasma HIV-1 RNA loads increase approximately 8-fold following coinfection by an assortment of opportunistic pathogens,1 and rises in viral load have been observed with a variety of concomitant bacterial, parasitic, and viral diseases. 2,3 Surprisingly, a few coinfections have the opposite effect. Transient declines in HIV-1 RNA load have been reported for acute dengue and measles infections, both of which induce HIV-suppressive chemokines that bind to the CCR5 HIV coreceptor.4-6 The first infectious agent found to exhibit an HIV-1 suppressive effect was Orientia tsutsugamushi, the causative agent of scrub typhus.7 A mite-borne rickettsial disease generally unfamiliar to Western scientists because its distribution is limited to Asia, scrub typhus (ST) affects an estimated one million people annually and one billion people are thought to be at risk of infection. 8 Earlier studies demonstrated that acute O. tsutsugamushi infection was associated with a substantial decrease in HIV-1 RNA levels in some ST-infected patients in northern Thailand and viral load sometimes fell below the limits of detection.7 ST also appeared to shift the viral population from CXCR4-using (X4) to CCR5-utilizing (R5).7 To explore the mechanism by which ST coinfection suppresses HIV, we tested in vivo and in vitro the hypothesis that the reduction in HIV-viral load associated with ST infection was attributable to effects on X4 viruses.
There is evidence of cross-reactivity between ST-specific antibodies and HIV-1. Immune sera from mice experimentally inoculated with O. tsutsugamushi have been shown to selectively stain with HIV-1 infected lymphocytes in an in vitro immunofluorescence assay.7 Several studies have proposed a protective role for chemokines in HIV-1 infection, demonstrating an inverse relationship between chemokine production and plasma viral load.9,10 We therefore assessed the relative contributions of antibodies and chemokines to ST-associated HIV inhibition.
Materials and Methods
HIV-1 coreceptor usage was determined in longitudinal plasma samples from antiretroviral naïve HIV-1 infected individuals being treated by passive transfer of ST plasma.11 Individual units of plasma from donors of one unit of whole blood with mild, acute scrub typhus were safety-tested for HIV, HBV, and HCV viral markers, subjected to virucidal heat treatment, and administered to HIV-1-infected recipients.11 Plasma processing exceeded the safety requirements of both the Thai Red Cross and US FDA at the time of the study. Plasma recipients were all late-stage AIDS patients for whom antiretroviral drugs were not an option under Thai Ministry of Public Health HIV Treatment guidelines at the time. Informed consent was obtained under a protocol approved by both the Thai Ministry of Public Health and the Walter Reed Army Institute of Research. Samples collected from three individuals who received placebo infusions of saline were included as controls.
We used a method developed previously to compute the change in proportion of HIV virus using each coreceptor and to make numerical comparisons of coreceptor use over time and in different individuals.12,13 Briefly, HIV-1 virions were isolated from plasma samples and subjected to RT-PCR amplification, and 920bp amplicons spanning the V3 region of the env gene were sequenced. Envelope sequences were used to predict coreceptor usage on the basis of the overall charge of the V3 loop and the presence of basic or acidic residues at positions 275 and 287 of the env gene.12,13 In this model, λ is a variable that represents the fraction of virus in a specimen using the R5 coreceptor. If λ = 1, almost all of the viruses in a population use R5; if λ=0, almost all use X4. If λ=0.50, half of the HIV-1 viruses in a blood specimen use the R5 and half use the X4 coreceptor. We calculated the proportion of X4- specific virus for each plasma specimen according to the formula: X4 viral load =1-λ/total viral load. We analyzed total, R5-specific, and X4-specific HIV-1 RNA levels in these patients immediately prior to plasma infusion and 3, 14 and 28 days following plasma transfer. Virus production from triplicate cultures of infected peripheral blood mononuclear cells (PBMCs) was assayed at day 14 by measuring p24 antigen production. Virus was cultured with admission sera from 14 HIV-uninfected ST patients and with fetal bovine serum controls. Experiments were conducted on an exclusively X4-coreceptor-using virus (LAV) and on a solely R5-coreceptor-using virus (SF162).
We assessed possible mechanisms by which ST could inhibit HIV by depleting selected chemokines or antibodies from the serum of an HIV-uninfected scrub typhus patient. The chemokine ligands of the HIV-1 coreceptors CCR5 and CXCR4 (MIP-1α, MIP-1β, RANTES, and SDF-1α) were removed by adsorption of the sera using monoclonal antibodies immobilized on a plastic microtiter plate. Following overnight incubation, the concentration of these four chemokines was below the level of detection, as measured by using commercial ELISA kits (Invitrogen, Carlsbad, California, USA). Serum antibodies were depleted by using a protein A column.
Immune sera from mice experimentally inoculated with O. tsutsugamushi have been shown previously to selectively stain with HIV-1 infected lymphocytes in an immunofluorescence assay.7 We therefore attempted to identify the HIV-1 target of potentially cross-reactive ST-induced antibodies by performing co-localization experiments to see if ST-sera targeted with HIV-1 gp120 envelope protein in LAVinfected GHOST-CXCR4 cells. We used i) DAPI nuclear stain; ii) convalescent sera from STinfected mice, conjugated with a FITC-labeled secondary antibody; iii) red fluorescence of Texas Red-labeled secondary antibodies marking gp120; iv) co-localization mask of blue (i), green (ii), and red (iii) fluorescence. GHOSTCXCR4 cells were infected with the X4 LAV virus and GHOST-CCR5 cells were infected with an X4-using virus (SF162).
Results
Previous work showed that after a single infusion of scrub typhus plasma, the total HIV-1 RNA copy number (R5+X4 viruses) fell modestly in 7 of 10 plasma recipients.11 The mean age of the 5 male and 5 female plasma recipients was 29 years (SD±5 years). The median HIV-1 RNA copy number prior to plasma infusion was 114,438 (range 43,150-193,597 copies/μL). Median CD4+ cell count prior to plasma transfer was 63 cells/μL (range 15-130) and rose to 72 cells/μL (range, 10-147) one month after plasma infusion (P<0.05, Wilcoxon signed rank test). We made viral isolations at least twice from every patient and all viruses were subtype E.
Mixed-effects analysis showed that this reduction in HIV RNA level was accompanied by a significant shift in the viral population from X4 to R5 strains (P=0.0008).
Here, we computed the proportion of HIV virus using each coreceptor before and after scrub typhus plasma infusion and made numerical comparisons of coreceptor use over time and in different individuals. We found that 2 patients who showed no drop in total HIV viral load after receiving ST plasma had only R5 virus circulating preceeding plasma infusion. Viral load and coreceptor usage did not change in 3 patients who received placebo infusions of saline. However, ST infusion to the to the eight HIV-1-infected individuals in whom X4-viruses were present at the time ST plasma was infused markedly reduced the proportion of X4-using viruses (Figure 1). The X4-specific viral load decreased to zero in 6 of 8 patients (Figure 1; patients A,B,C,D,F,H). The inhibitory effect was prolonged, and X4-using viruses remained undetectable one month after a single infusion of ST plasma in 5 of 8 patients (Figure 1; patients B, C, D, F, H).
Figure 1.
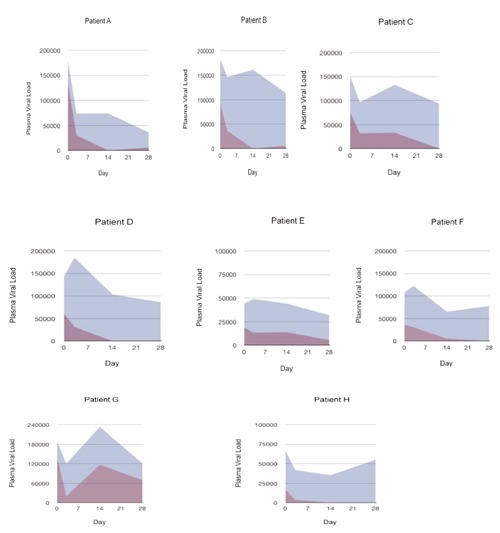
The effects of a single infusion of scrub typhus plasma administered on Day 0 to 8 HIV-infected recipients are shown as the area under the curve (AUC) for the total HIV-1 RNA copy number (shaded in blue) and for the X4-specific viral load (shaded in purple) immediately prior to and then 3, 14 and 28 days after infusion.
In vitro, we tested sera collected from 14 HIV-negative patients with acute ST for its ability to inhibit HIV replication. All sera markedly inhibited the replication of an X4 virus (Figure 2). However, R5 viral replication was virtually unaffected by these same 14 ST sera (Figure 2).
Figure 2.
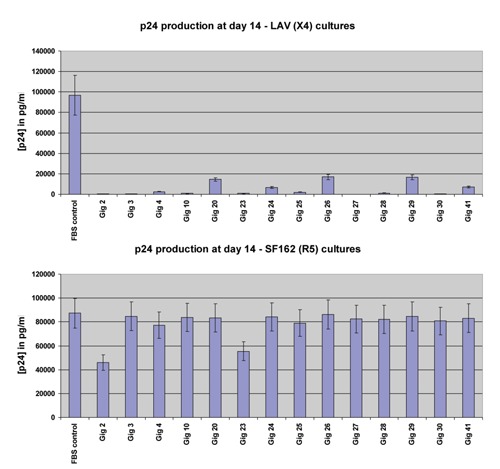
Effects of sera from 14 scrub typhus-infected patients on HIV replication within X4-using lymphocytes (above) and R5-using lymphocytes (below).
Compared with control serum, whole sera from a patient with acute scrub typhus markedly inhibited the replication of an X4-using virus in culture (Figure 3, blue column). When this serum was depleted of the chemokine ligands of the HIV-1 coreceptors CCR5 and CXCR4 (MIP-1α, MIP-1β, RANTES, and SDF-1β), its inhibitory effect on HIV replication persisted (Figure 3, red column). However, the inhibitory effect was lost when antibodies were removed (Figure 3, green column).
Figure 3.
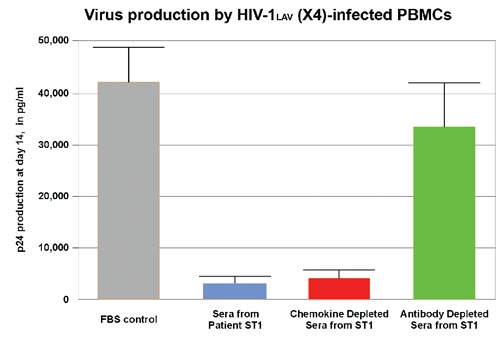
The effects of chemokine and antibody depletion on the inhibitory effects of scrub typhus serum on X4 virus replication. Selected chemokines or antibodies were depleted from the serum of an HIV-uninfected scrub typhus patient. Using p24 antigen as a marker, we measured HIV-1 viral replication in peripheral blood mononuclear cells.
Sera from ST-infected mice recognized a target that co-localized with gp120 in GHOSTCXCR4 cells infected with the X4 LAV virus (Figure 4). This staining pattern was not seen with pre-immune sera or with uninfected target cells. No co-localization was seen after GHOST-CCR5 cells were infected with the R5 virus SF162.
Figure 4.
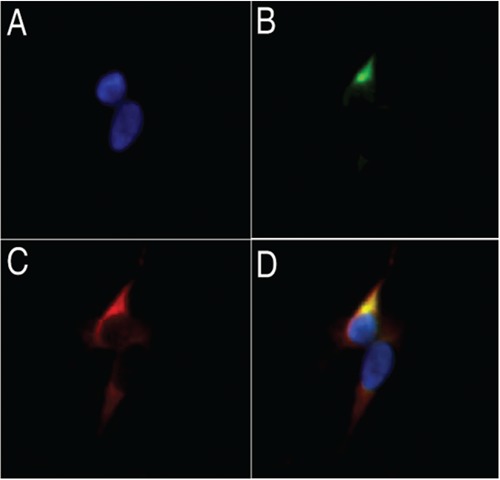
Co-localization of scrub typhus sera targets with HIV-1 gp120 envelope protein in LAV-infected GHOST-CXCR4 cells. a) DAPI nuclear stain; b) convalescent sera from ST-infected mice, conjugated with a FITC-labeled secondary antibody; c) red fluorescence of TexasRed-labeled secondary antibodies marking gp120; d) co-localization mask of blue (a), green (b), and red (c) fluorescence. Co-localization is revealed by yellow.
Discussion
The infusion of plasma from HIV-1 seronegative patients with acute ST completely eliminated circulating X4-using viruses in 6 of 8 HIV-infected recipients, and markedly reduced the proportion of X4 viruses in the remaining two individuals (Figure 1). In vitro, all 14 ST sera tested markedly inhibited the replication of an X4-using HIV virus but had no inhibitory effect on an R5 virus (Figure 2). This inhibitory effect was maintained if ST sera were depleted of chemokines, but was lost upon removal of antibodies (Figure 3). These data suggest that the inhibitory effect of ST coinfection on HIV is attributable to the induction of cross-reactive antibodies that neutralize X4 strains of the HIV-1 virus. Infectious agents sometimes share antigenic sites with each other or with host cell components, a phenomenon known as molecular mimicry. Several studies have demonstrated mimicry between HIV-1 and other infectious organisms. Rabies vaccination can induce the production of anti-HIV-1 gp120 antibodies,14 and active measles virus infection often results in the production of antibodies that are cross-reactive with HIV-1 proteins.15 Similar results have been reported using sera obtained from patients with malaria, leishmaniasis, and leprosy.16-19 This is, however, the first time that suppression of HIV-1 by cross-reactive antibodies has been seen. Indeed, to date this appears to be the first case where natural antibodies made in response to infection with one organism – a rickettsial intracellular bacteria, both crossreact with and suppress a completely unrelated infectious agent – HIV X4-using viruses.
Sera from ST-infected mice recognize a target that co-localizes with X4 HIV gp120 in immunofluorescent co-localization experiments (Figure 4). Short amino acid matches between O. tsutsugamushi surface antigens and HIV-1 have been found by searching the NCBI database,11 although the relevance of these sequence similarities remains to be determined. For example, protein sequence comparisons have revealed a striking similarity between an immunodominant 47kDa ST antigen (positions 433 to 442) and HIV gp120 (codons 214 to 223):
ST 47kDa sAg: T L R E I V T N I K
HIVgp120: T L R Q I V T N L K
This potential mimiotope is intriguing in that it is a relatively conserved portion of the HIV envelope protein that is just C-terminal of the V3 loop, and is both a principal neutralizing determinant and a key determinant of viral coreceptor specificity. Crystallographic data suggests that this region should be visible on the native envelope.20 The scrub typhus 47kDa is also a surface antigen. The interaction between surface ST and HIV antigens could explain both the suppression and its X4 specificity.
The ST suppressive effect is potent. In two HIV-infected patients not receiving antiretroviral treatment, viral load fell below the limits of detection during naturally acquired scrub typhus infection.7 Just one infusion of ST plasma eliminated circulating X4 viruses from 6 of 8 plasma recipients (Figure 1). ST suppressor factors appear to be produced commonly during scrub typhus infection. All ST plasma tested inhibited X4 viruses in vivo (Figure 1) and all ST sera tested inhibited X4 viruses in vitro (Figure 2).
Unfortunately, we did not have any opportunity to evaluate the effect of ST co-infection in primary HIV-1 infection. However, the R5 coreceptor is used by almost all primary HIV-1 isolates regardless of viral genetic subtype. Since ST neutralizes X4 but not R5 viruses, it is unlikely that ST co-infection would have a significant impact on either HIV-1 transmission or on the course of early infection, which are mediated almost entirely by R5-using viruses. However, by elucidating the mechanism of viral suppression by ST and characterizing potential cross reactive epitopes, our understanding of the natural correlates of protection and control of HIV-1 infection may be expanded. The search for antibodies that potently neutralize different HIV strains is a key element of rational vaccine design.21,22
Understanding how ST antibodies achieve such potent inhibition of X4-viruses could be of help in this search and identify additional targets for drug development. Preferential suppression of X4 viruses, associated with disease progression and pathogenicity, suggests the potential for a therapeutic vaccine aimed at slowing or halting HIV-1 disease progression. New weapons against HIV remain urgently needed. An effective HIV-1 vaccine is not on the horizon and there are approximately three times as many people who are estimated to need antiretroviral therapy than are estimated to be receiving it.23
Conclusions
In vivo and in vitro data suggest that acute ST infection generates cross-reactive antibodies that produce potent and long lasting suppression of CXCR4- HIV-1 viruses. Further study of the mechanism of suppression of HIV replication could reveal new mechanisms applicable to HIV-1 vaccine development and to therapies such as fusion inhibitors.
References
- 1.Sulkowski MS, Chaisson RE, Karp CL, et al. The effect of acute infectious illnesses on plasma human immunodeficiency virus (HIV) type 1 load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis 1998;178:1642-8 [DOI] [PubMed] [Google Scholar]
- 2.Kublin J, Jere C, Miller W, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet 2005;365:233-40 [DOI] [PubMed] [Google Scholar]
- 3.Duffus WA, Mermin J, Bunnell R, et al. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS 2005;16:733-5 [DOI] [PubMed] [Google Scholar]
- 4.Kannangara S, DeSimone JA, Pomerantz RJ. Attenuation of HIV-1 infection by other microbial agents. J Infect Dis 2005;192: 1003-9 [DOI] [PubMed] [Google Scholar]
- 5.Moss WJ, Ryon JJ, Monze M, et al. Suppression of human immunodeficiency virus replication during acute measles. J Infect Dis 2002;185:1035-42 [DOI] [PubMed] [Google Scholar]
- 6.Watt G, Kantipong P, Jongsakul K. Decrease in human immunodeficiency virus type 1 load during acute dengue fever. Clin Infect Dis 2003;36:1067-9 [DOI] [PubMed] [Google Scholar]
- 7.Watt G, Kantipong P, de Souza M, et al. HIV-1 suppression during acute scrub-typhus infection. Lancet 2000;356:475-9 [DOI] [PubMed] [Google Scholar]
- 8.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis 2003;16: 429-36 [DOI] [PubMed] [Google Scholar]
- 9.Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple highrisk sexual exposure. Nat Med 1996;4:412-7 [DOI] [PubMed] [Google Scholar]
- 10.Saha K, Bentsman G, Chess L, Volsky DJ. Endogenous production of betachemokines by CD4+ but not CD8+, T-cell clones correlates with the clinical state of human immunodeficiency virus type 1 (HIV-1) infected individuals and may be responsible for blocking infection with non-syncytium-inducing HIV in vitro. J Virol 1998;72:876-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watt G, Kantipong P, Jongsakul K, et al. Passive transfer of scrub typhus plasma to patients with AIDS: a descriptive clinical study. QJM 2001;94:599-607 [DOI] [PubMed] [Google Scholar]
- 12.Philpott SB, Weiser K, Anastos CMR, et al. Preferential suppression of CXCR4-specific strains of HIV-1 by antiviral therapy. J Clin Invest 2001;107:431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitchen CM, Philpott S, Burger H, et al. Evolution of human immunodeficiency virus type 1 coreceptor usage during antiretroviral therapy: a Bayesian approach. J Virol 2004;78:11296-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bracci L, Ballas SK, Spreafico A, Neri P. Molecular mimicry between the rabies virus glycoprotein and human immunodeficiency virus-1 GP120: cross-reacting antibodies induced by rabies vaccination. Blood 1997;90:3623-8 [PubMed] [Google Scholar]
- 15.Baskar PV, Collins GD, Dorsey-Cooper BA, et al. Serum antibodies to HIV-1 are produced post-measles virus infection: evidence for cross-reactivity with HLA. Clin Exper Immunol 1998;111:251-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biggar RJ, Gigase PL, Melbye M. ELISA HTLV retrovirus antibody reactivity associated with malaria and immune complexes in healthy Africans. Lancet 1985;i:520-3 [DOI] [PubMed] [Google Scholar]
- 17.Christie IL, Palmer SJ, Voller A, Banatvala JE. False-positive serology and HIV infection. Lancet 1993;341:441-2 [DOI] [PubMed] [Google Scholar]
- 18.Kashala O, Marlink OR, Ilunga M, et al. Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J Infect Dis 1994;169:296-304 [DOI] [PubMed] [Google Scholar]
- 19.Parry JV, Richmond J, Edwards N, Noone A. Spurious malarial antibodies in HIV infection. Lancet 1992;340:1412-3 [DOI] [PubMed] [Google Scholar]
- 20.Huang CC, Tang M, Zhang MY, et al. Structure of a V3-containing HIV-1 gp120 core. Science 2005;310:1025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trkola A, Kuster H, Rusert P, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 2005;11:615-22 [DOI] [PubMed] [Google Scholar]
- 22.Burton DR, Weiss RA. A boost for HIV vaccine design. Science 2010;329:770-3 [DOI] [PubMed] [Google Scholar]
- 23.WHO Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241500395_eng.pdf.


