Abstract
The oxidation of luciferin catalyzed by sea pansy luciferase results in the emission of light. Molecular oxygen is required and carbon dioxide is produced. When the reaction occurs in the presence of H218O, both of the oxygens of the carbon dioxide are labeled. One of the oxygens arises from the nonenzymic exchange of the ketone group of the substrate; the other oxygen is incorporated during the enzymic oxidation of the luciferin. When the reaction is carried out in the presence of 18O2, neither of the oxygens of the carbon dioxide is labeled. Thus the source of oxygen in the carbon dioxide is water. A mechanism for the oxidative reaction is proposed.
Keywords: sea pansy luciferin, firefly
Full text
PDF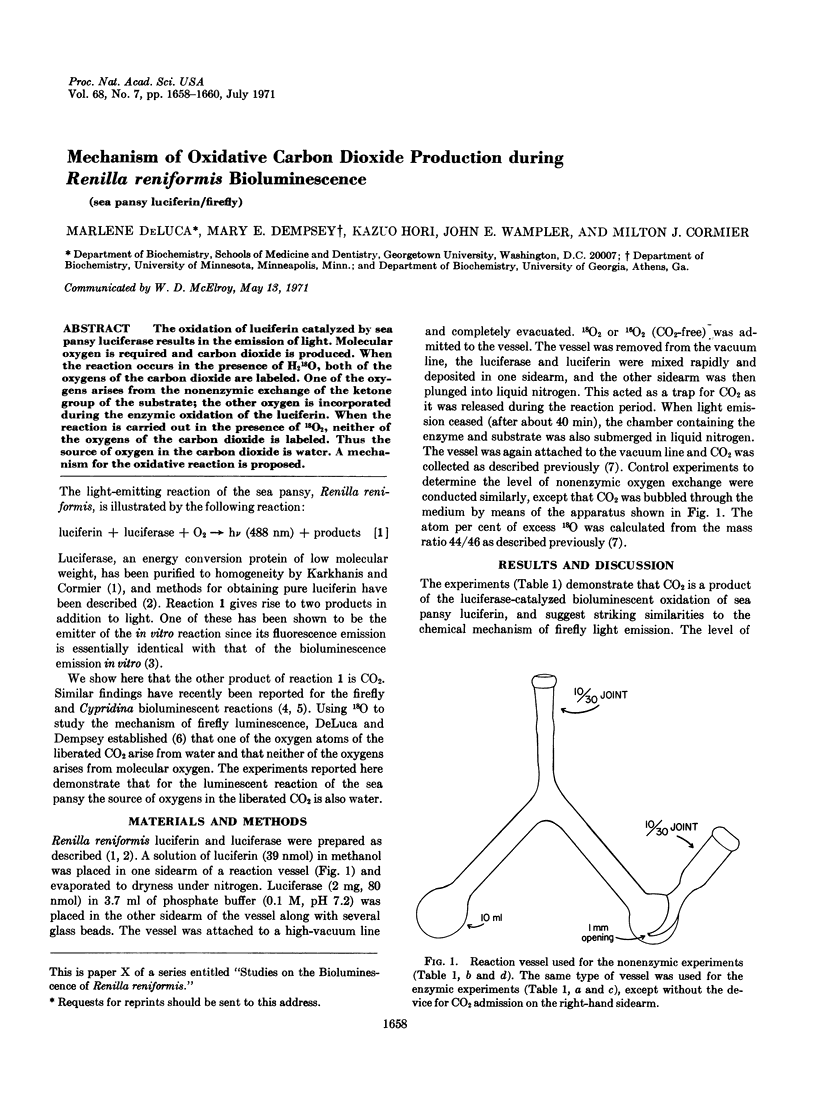
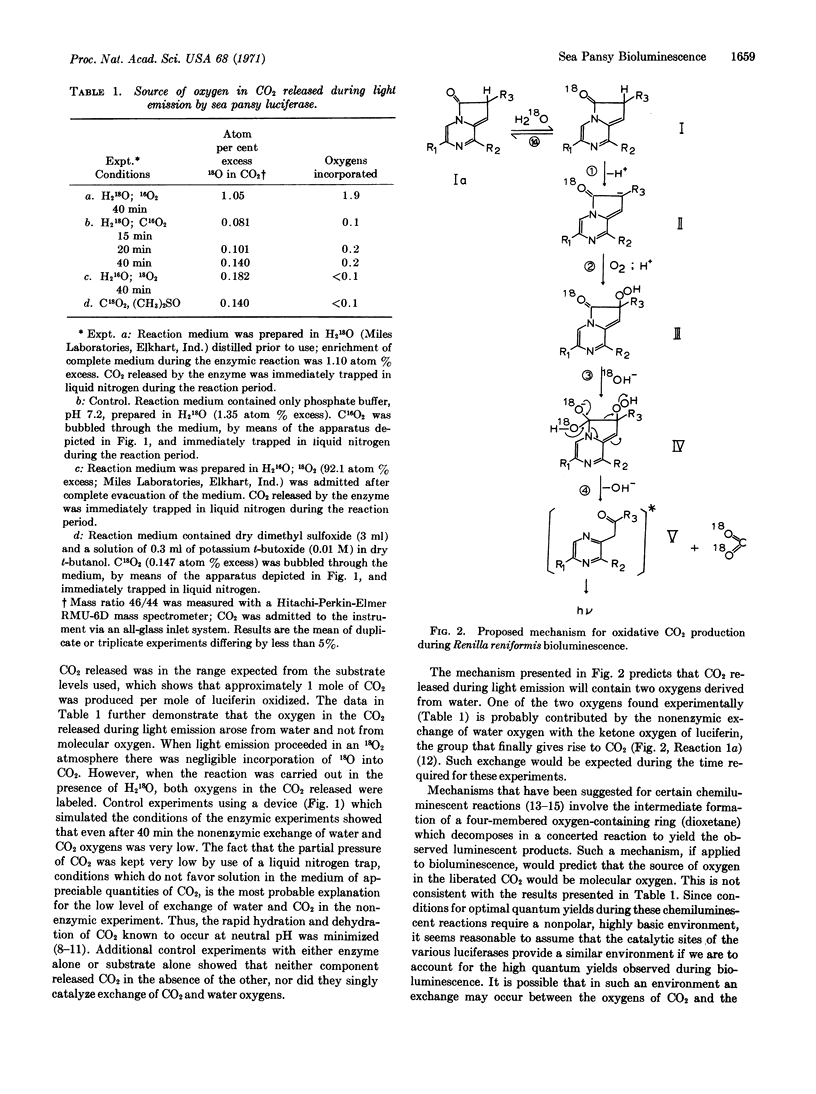

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper T. G., Tchen T. T., Wood H. G., Benedict C. R. The carboxylation of phosphoenolpyruvate and pyruvate. I. The active species of "CO2" utilized by phosphoenolpyruvate carboxykinase, carboxytransphosphorylase, and pyruvate carboxylase. J Biol Chem. 1968 Jul 25;243(14):3857–3863. [PubMed] [Google Scholar]
- Cormier M. J., Hori K., Karkhanis Y. D. Studies on the bioluminescence of Renilla reniformis. VII. Conversion of luciferin into luciferyl sulfate by luciferin sulfokinase. Biochemistry. 1970 Mar 3;9(5):1184–1189. doi: 10.1021/bi00807a019. [DOI] [PubMed] [Google Scholar]
- DeLuca M., Dempsey M. E. Mechanism of oxidation in firefly luminescence. Biochem Biophys Res Commun. 1970 Jul 13;40(1):117–122. doi: 10.1016/0006-291x(70)91054-5. [DOI] [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. RATE OF HYDRATION OF CARBON DIOXIDE AND DEHYDRATION OF CARBONIC ACID AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3502–3507. [PubMed] [Google Scholar]
- HO C., STURTEVANT J. M. THE KINETICS OF THE HYDRATION OF CARBON DIOXIDE AT 25 DEGREES. J Biol Chem. 1963 Oct;238:3499–3501. [PubMed] [Google Scholar]
- Hopkins T. A., Seliger H. H., White E. H., Cass M. H. The chemiluminescence of firefly luciferin. A model for the bioluminescent reaction and identification of the product excited state. J Am Chem Soc. 1967 Dec 20;89(26):7148–7150. doi: 10.1021/ja01002a076. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Cormier M. J. Isolation and properties of Renilla reniformis luciferase, a low molecular weight energy conversion enzyme. Biochemistry. 1971 Jan 19;10(2):317–326. doi: 10.1021/bi00778a019. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Roughton F. J. Carbonic anhydrase as a tool in studying the mechanism of reactions involving H(2)CO(3), CO(2) or HCO(3)'. Biochem J. 1948;43(4):550–555. doi: 10.1042/bj0430550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant P. J., White E. H., McElroy W. D. The decarboxylation of luciferin in firefly bioluminescence. Biochem Biophys Res Commun. 1968 Apr 5;31(1):98–103. doi: 10.1016/0006-291x(68)90036-3. [DOI] [PubMed] [Google Scholar]
- Stone H. The enzyme catalyzed oxidation of Cypridina luciferin. Biochem Biophys Res Commun. 1968 May 10;31(3):386–391. doi: 10.1016/0006-291x(68)90487-7. [DOI] [PubMed] [Google Scholar]


