Abstract
Infections caused by Pseudomonas aeruginosa are becoming more common and increasingly more difficult to treat due to the continued development of drug resistance. While sensitivity to colistin (polymyxin E) is well known, it is frequently avoided due to concerns of nephrotoxicity. Reported here is a case of a multi-drug resistance pseudomonal typhlitis, bacteremia and pleural cavity infection that required significant intensive care, and serial abdominal washouts. Intra-peritoneal tobramycin in combination with broad-spectrum intravenous antibiotics including colistin were used. Several instillations of tobramycin into the abdominal cavity along with concomitant IV administration of colistin, ceftazidime and tobramycin and per os colistin, tobramycin and nystatin resulted in the clearance of the pseudomonal infection without any evidence of toxicity from the treatment. Intra-abdominal tobramycin with parenteral colistin therapy can be used in complicated clinical settings with appropriate nephroprotection.
Key words: Pseudomonas aeruginosa, typhlitis, pediatric, Burkitt's leukemia, intra-abdominal tobramycin, colistin (polymyxin E).
Introduction
The management of infections with Pseudomonas aeruginosa is becoming increasingly more difficult because of the development of multi-drug resistance (MDR) and the ability of P. aeruginosa to develop resistance to monotherapies.1–3 Historically, Pseudomonas aeruginosa has been implicated in a wide range of community and hospital acquired infections including pneumonia, necrotizing cellulitis, orbital cellulitis, chronic otitis media and externa, and in immunocompromised patients with neutropenic enterocolitis.3–5 Particularly, in the pediatric immunocompromised patient population, Pseudomonas infections are a cause of increasing morbidity and mortality, and frequently are multi-drug resistant.4,5 Reviewed here is a case of a neutropenic seven year-old girl who was transferred from an out-side hospital's (OSH) pediatric intensive care unit (PICU) in septic shock secondary to typhlitis. She was found to have MDR P. aeruginosa originating from her gastrointestinal tract. The extent of the infection and the severity of the illness ultimately required intra-abdominal administration of tobramycin in conjunction with parenteral colistin and tobramycin to eradicate the infection. A review of the current literature regarding pediatric abdominal P. aeruginosa infections in children found a relative paucity of articles regarding pediatric pseudomonal treatment with intra-abdominal administration of antimicrobials to eradicate infection.
Case Report
A seven year-old girl diagnosed with Burkitt's Leukemia one month prior to admission was transferred to a tertiary care center from an OSH intensive care unit following a rapid clinical deterioration. On transfer the patient was febrile (38°C), hypotensive, tachycardic and complained of progressive abdominal pain, distention and guarding. The patient had been febrile and neutropenic for the week prior to transfer.
One month prior to the current admission the patient developed a febrile illness. A hematologic evaluation suggested an initial diagnosis of pre B-cell acute lymphoblastic leukemia. The initial chemotherapy included vincristine, pegaspargase, dexamethasone and intrathecal cytarabine but then cytogenic analysis revealed the diagnosis of Burkitt's leukemia and a course of cyclophosphamide, doxorubicin, vincristine and dexamethasone was administered prior to discharge. Eleven days following discharge, the patient was re-admitted to the OSH because of increasing abdominal pain, non-bloody diarrhea, fever and neutropenia.
The patient received meropenem (20 mg/kg/dose Q 8 hours) and vancomycin for presumed sepsis with fever and neutropenia. Liposomal amphotericin B was added to provide antifungal coverage and pneumocystis pneumonia prophylaxis with trimethoprim/sulfamethoxazole (TMP/SMX) was continued. Blood cultures showed no growth. An ultrasonography and computerized tomography (CT) of the abdomen revealed no abnormalities. Intrathecal methotrexate and intravenous vincristine were administered per chemotherapy protocol. However 10 days after chemotherapy, the patient's respiratory status worsened and she became hemodynamically unstable. The patient was transferred to our hospital for extracorporeal membrane oxygenation (ECMO).
On arrival the patient was in septic shock requiring inotropic support, intubation and mechanical ventilation. The abdomen was distended, diffusely tender to palpation with rebound tenderness and guarding. Bowel sounds were hypoactive. Upper extremities were warm and relatively well perfused but lower extremities were cool with distant pulses and a two-second capillary refill. The patient was admitted to PICU. A right thoracostomy tube was placed for evacuation of a right-sided pleural effusion and the patient was placed on ECMO.
The patient's initial work-up revealed leukopenia [white blood cell (WBC) = 0.3 K/mm3], and rare quantities of P. aeruginosa from the pleural effusion. The pleural fluid appeared clear with a WBC count of 103/mm3, red blood cell of 2/mm3, protein of less than 2 g/dL, Lactate dehydrogenase of 172 IU/L and cholesterol of 14 mg/dL. An abdominal CT showed mild ascites and non-specific bowel wall thickening with no gross evidence of perforation. During the first 24 hours after transfer the abdominal distention progressed and the urine output decreased acutely. Abdominal compartment syndrome was suspected as the etiology of her acute renal failure and an exploratory laparotomy was urgently performed on day four in the PICU. Intraoperative findings demonstrated multiple small areas of focal necrosis along the mesenteric border of the small bowel without visible perforation. Ascitic fluid grew a rare quantity of P. aeruginosa, with a different susceptibility pattern than the isolate from the pleural fluid (Table 1). Blood cultures drawn from the central intravenous line on day three grew two isolates of P. aeruginosa which displayed two different susceptibility patterns, and were also different from the isolates from the peritoneal and pleural fluid. At this point, there were 4 different pseudomonas aeruginosa isolates growing from pleural fluid, peritoneal fluid and blood and continuous renal replacement therapy (CRRT) was initiated for anuric renal failure. The patient remained neutropenic during the first 4 days in the PICU but then the WBC count reached 36 K/mm3 on day five with an absolute neutrophil count of 4.0 K/mm3 and remained elevated until the patient recovered from the infection. Additionally, during a trial off CRRT on day five, the patient remained febrile (39.8°C).
Table 1. Minimal inhibitory concentrations (MIC's) of the different isolates of Pseudomonas aeruginosa from the different sites.
| Pleural fluid Day 1 | Blood Day 3 | Peritoneal fluid Day 4 | Pleural fluid Day 7 | Peritoneal fluid Day 14 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day after admission Susceptibility | MIC | MIC | MIC | MIC | MIC | MIC | MIC | |||||||
| Cefepime | 8 | S | 16 | I | 8 | S | ≥64 | R | 16 | I | 8 | S | 32 | R |
| Piperacillin/Tazobactam | 32 | S | >64 | R | >64 | R | 128 | R | 32 | S | 32 | S | ≥256 | R |
| Ceftazadime | ---- | - | >16 | R | ---- | - | 16 | I | 8 | S | ---- | - | 16 | I |
| Aztreonam | ---- | - | >16 | R | >16 | R | ≥16 | R | >16 | R | ---- | - | ---- | - |
| Imipenem | ≥16 | R | >8 | R | >8 | R | ≥16 | R | >8 | R | ≥16 | R | ≥16 | R |
| Meropenem | ≥16 | R | >8 | R | >8 | R | ≥16 | R | >8 | R | ≥16 | R | ≥16 | R |
| Doripenem | ---- | - | ---- | - | ---- | - | >2 | ---- | - | ---- | - | ---- | - | |
| Gentamicin | 8 | I | 2 | S | 2 | S | 8 | I | ≤1 | S | 2 | S | 8 | I |
| Tobramycin | ≤1 | S | ≤1 | S | ≤1 | S | 2 | S | ≤1 | S | ≤1 | S | ≤1 | S |
| Amikacin | 16 | S | 8 | S | 8 | S | 32 | I | ≤4 | S | 4 | S | 16 | S |
| Colistimethate | ---- | - | ---- | - | ---- | - | 2 | S | ---- | - | ---- | - | 2 | S |
| Ciprofloxacin | 0.5 | S | 2 | I | 1 | S | 2 | I | 0.5 | S | 0.5 | I | 2 | I |
| Levofloxacin | 4 I | I | 8 | R | 4 | I | ≥8 | R | 2 | S | 4 | I | ≥8 | R |
MIC, minimal inhibitory concentrations; I, intermediare; R, resistant; S, susceptible.
Following the use of meropenem, levofloxacin (10 mg/kg/dose Q 24 hours), ciprofloxacin (15 mg/kg/dose Q 12 hours), cefepime (50 mg/kg/dose Q 12 hours), ceftazidime and tobramycin in different combinations and based on the susceptibility profiles of the different pseudomonal isolates (Figure 1) a final change of the regimen to intravenous colistin (2.5 mg/kg/dose Q 12 hours), ceftazidime (50 mg/kg/dose Q 12 hours) and tobramycin (8 mg/kg/dose Q 24 hours) was made. Ceftazidime was continued despite intermediate resistance because of the known synergy between beta-lactams and aminoglycosides. However formal synergy testing was not performed. Intravenous vancomycin and metronidazole were continued for empiric enterococcal and anaerobic coverage because of suspected intestinal perforation. This regimen was initiated on hospital day eleven. The peritoneal fluid culture obtained during an abdominal washout on day 14 still grew Pseudomonas aeruginosa with variable susceptibilities raising the concern of evolving resistance. Since the patient's clinical status showed no improvement despite parenteral administration of antibiotics with evidence of in vitro activity against the microorganism, an attempt to control the source of the infection by concomitant administration of a selective digestive tract decontamination regimen along with an intraperitoneal antibiotic was made. Therefore, the patient received a regimen of oral colistin (2 mg/kg/dose Q 8 hours), tobramycin (1.5 mg/kg/dose) Q 8 hours and nystatin (500,000 units oral suspension Q 8 hours) through her nasogastric tube. To achieve adequate intraperitoneal concentrations of the aminoglycoside, the patient received an abdominal irrigation with 450 mL of 0.48 mg/mL tobramycin during an abdominal washout procedure. Two Jackson-Pratt drains were placed in each side of her abdomen to provide a route for intraperitoneal administration of tobramycin. Intraperitoneal tobramycin was administered with an average of 70 mL of 0.48 mg/mL in each drain for a dwelling time of 30 min, after which it was removed by suction through her wound vacuum-associated closure system. The treatment was repeated every 2–3 hours.
Figure 1.
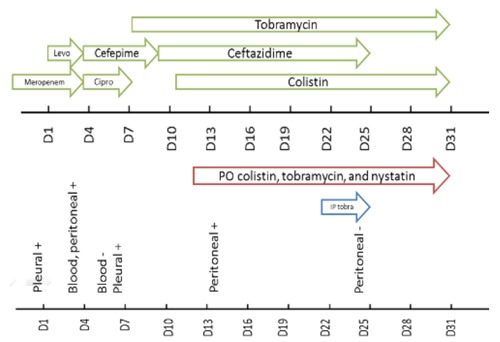
Timeline of the patient's course in our hospital demonstrating the parenteral drug combinations (green arrows), selective digestive tract decontamination (red arrow) and intraperitoneal (IP) tobramycin (blue arrow). In the lower part of the figure, we plotted the patient's positive cultures (+) and the first negative from blood and peritoneal sites (−).
Two days following the administration of the intraperitoneal tobramycin, peritoneal cultures showed no growth and the patient's WBC count started to normalize. The patient's creatinine remained within the range of 0.5 and 0.6 mg/dL during the course of the treatment and CRRT was discontinued the following week. Her creatinine showed a brief increase to 1.2 mg/dL when the CRRT was discontinued but rapidly normalized. Tobramycin trough levels were between 1.1-2.3 ug/mL during the intraperitoneal administration. The abdomen was closed few days after the sterilization of her cultures. Antibiotic treatment with IV colistin, tobramycin, and ceftazidime and oral colistin, tobramycin and nystatin was continued for additional fourteen days and blood cultures remained negative. At the end of her treatment course, chemotherapy was resumed with no additional infectious complications to our knowledge.
Discussion
This case represents an unorthodox management of an immunocompromised patient with a life-threatening infection, caused by a multi-drug resistant organism in a location where antibiotics have limited bio-availability. The primary infectious process is believed to be typhlitis. Micro-perforation of the bowel and subsequent contamination of the peritoneal cavity is most likely the underlying cause of the sepsis even though the first positive culture that led to the identification of MDR Pseudomonas was retrieved from the pleural cavity. The initial organism was resistant to meropenem. Based on the available susceptibility profiles, the antipseudomonal coverage was modified several times to different combinations of cefepime, ceftazidime, ciprofloxacin and tobramycin. However, isolates rapidly developed resistance to all these antibiotics except the aminoglycoside. The patient remained in a critical condition with significant abdominal tenderness.
Blood, peritoneal and pleural cultures demonstrated the growth of Pseudomonas aeruginosa isolates with variable resistance patterns. It is unclear whether the variable resistance patterns represent different pseudomonal isolates or the emergence of localized resistance of a single invasive organism. The patient's clinical condition and abdominal symptoms were suggestive of an ongoing infectious process. Isolation of pseudomonas from fresh peritoneal samples several days after IV antibiotic administration raised two concerns; first, that the source of infection, the GI tract, continued to seed bacteria into the peritoneal cavity, and second, that the parenteral route did not provide an effective distribution of the antibiotics into the peritoneum. In light of the different susceptibility patterns and the absence of additional treatment options if resistance continued to evolve an unorthodox but comprehensive approach was taken with the antibiotic regimen. The organism was susceptible to colistin and tobramycin so both antibiotics were given parenterally hoping that they would limit further development of resistance. Due to tobramycin's poor distribution into the peritoneal cavity, intraperitoneal instillations of tobramycin were performed. Because of the concern of a potential persistent intestinal leak secondary to a perforation, a regimen of selective digestive tract decontamination using oral colistin, tobramycin and nystatin was administered. Since some sensitivity to ceftazidime existed, it was maintained for potential synergy between the beta-lactam and the aminoglycoside. Sterilization of the peritoneal cavity was achieved using this combination in few days. The patient's clinical condition continued to improve. Her renal function remained within normal during the treatment.
Polymyxins are bactericidal antibiotics that have been used in clinical practice since 1959. However, as the aminoglycosides became more available in the 1970's, their use declined due to their toxicity profiles. The major adverse effects of the polymyxins are nephrotoxicity and neurotoxicity.6–8 Nephrotoxicity is dose-dependent and reversible upon discontinuation of the antibiotic. It appears to be less common than previously reported with the newer preparations of polymyxins. Neurotoxicity is rare and results in a variety of manifestations ranging from focal neurological deficits to neuromuscular blockade resulting in respiratory failure. The recent emergence of MDR pathogens has led to the re-introduction of polymyxins in clinical practice.8–10 Intravenous colistin (polymyxin E) is now more commonly used in the management of MDR nosocomial infections with gram-negative bacilli.9,11 It has been increasingly used in treatment of MDR gram-negative infections in patients without cystic fibrosis, specifically in critically-ill and burn patients. It also appears to be well-tolerated in this age-group.12,13 Oral colistin is safely used in digestive tract decontamination regimens as pre-operative prophylaxis or in infection control in ICU's.14,15 It is not systemically absorbed when administered orally. In this specific case, we believe that the digestive tract decontamination regimen had an important role in controlling the source of the infection as evidenced by the improvement of the patient's clinical status and the sterilization of her cultures a few days following its initiaition. Intraventricular and intrathecal administration of colistin for treatment of MDR Pseudomonas aeruginosa and Acinetobacter baumannii in ventriculitis and meningitis has been reported.16,17 In addition, inhalational colistin is used in patients with cystic fibrosis.18,19
Conclusions
In the setting of a MDR Pseudomonas infection creative approaches to antimicrobial therapy may be warranted. The use of polymyxins and intraperitoneal administration of antibiotics can be used in complicated intra-abdominal infection with appropriate nephroprotection and close monitoring of renal and neurological function.
References
- 1.Livermore D. multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34:634–40. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 2.Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang MA, Lee J, Choi EH, Lee HJ. Pseudomonas aeruginosa bacteremia in children over ten consecutive years: analysis of clinical characteristics, risk factors of multi-drug resistance and clinical outcomes. J Korean Med Sci. 2011;26:612–8. doi: 10.3346/jkms.2011.26.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grisaru-Soen G, Lerner-Geva L, Keller N, et al. Pseudomonas aeruginosa bacteremia in children: analysis of trends in prevalence, antibiotic resistance and prognostic factors. Pediatr Infect Dis J. 2000;19:959–63. doi: 10.1097/00006454-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Caselli D, Cesaro S, Ziino O, et al. Multidrug resistant Pseudomonas aeruginosa infection in children undergoing chemotherapy and hematopoietic stem cell transplantation. Haematologica. 2010;95:1612–5. doi: 10.3324/haematol.2009.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care. 2011;2:30. doi: 10.1186/2110-5820-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balaji V, Jeremiah SS, Baliga PR. Polymyxins: antimicrobial susceptibility concerns and therapeutic options. Indian J Med Microbiol. 2011;29:230–42. doi: 10.4103/0255-0857.83905. [DOI] [PubMed] [Google Scholar]
- 8.Evans ME, Feola DJ, Rapp PP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–7. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 9.Levin AS, Barone AA, Penço J, et al. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28:1008–11. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–41. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 11.Kasiakou SK, Michalopoulos A, Soteriades ES, et al. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother. 2005;49:3136–46. doi: 10.1128/AAC.49.8.3136-3146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goverman J, Weber JM, Keaney TJ, Sheridan RL. Intravenous colistin for the treatment of multi-drug resistant, gram-negative infection in the pediatric burn population. J Burn Care Res. 2007;28:421–6. doi: 10.1097/BCR.0B013E318053D346. [DOI] [PubMed] [Google Scholar]
- 13.Iosifidis E, Antachopoulos C, Ioannidou M, et al. Colistin administration to pediatric and neonatal patients. Eur J Pediatr. 2010;196:867–74. doi: 10.1007/s00431-009-1137-3. [DOI] [PubMed] [Google Scholar]
- 14.Donelly P. Selective decontamination of the digestive tract and its role in antimicrobial prophylaxis. J Antimicrob Chemother. 1993;31:813–29. doi: 10.1093/jac/31.6.813. [DOI] [PubMed] [Google Scholar]
- 15.Gastinne H. A controlled trial in intensive care units of selective decontamination of the digestive tract with nonabsorbable antibiotics. N Engl J Med. 1992;326:594–9. doi: 10.1056/NEJM199202273260903. [DOI] [PubMed] [Google Scholar]
- 16.Vasen W, Desmery P, Ilutovich S, Di Martino A. Intrathecal use of colistin. J Clin Microbiol. 2000;38:3523. doi: 10.1128/jcm.38.9.3523-3523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn AL, Parada JP, Belmares J, O'Keefe JP. Intrathecal colistin and sterilization of resistant Pseudomonas aeruginosa shunt infection. Ann Pharmacother. 2005;39:94. doi: 10.1345/aph.1E485. [DOI] [PubMed] [Google Scholar]
- 18.Jensen T, Pedersen SS, Garne S, et al. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother. 1987;19:831–8. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 19.Hamer DH. Treatment of nosocomial pneumonia and tracheobronchitis caused by multidrug-resistant Pseudomonas aeruginosa with aerosolized colistin. Am J Respir Crit Care Med. 2000;162:328–33015. doi: 10.1164/ajrccm.162.1.9910071. [DOI] [PubMed] [Google Scholar]


