Abstract
Zoonotic infections are on the increase worldwide, but most research into the biological, environmental and life science aspects of these infections has been conducted in separation. In this review we bring together contemporary research in these areas to suggest a new, symbiotic framework which recognises the interaction of biological, economic, psychological, and natural and built environmental drivers in zoonotic infection and transmission. In doing so, we propose that some contemporary debates in zoonotic research could be resolved using an expanded framework which explicitly takes into account the combination of motivated and habitual human behaviour, environmental and biological constraints, and their interactions.
Key words: zoonotic infection, human factors, environment, multi-disciplinary research.
Introduction
Up to three-quarters of emerging infectious diseases are zoonotic in origin,1 with zoonotic disease posing a considerable and increasing threat to global health.2 Pathogen transmission from both domestic and wild animals can cause zoonotic outbreaks, and epizootic outbreaks in the wild can also spill (back) into domesticated animals resulting in human exposure.3 Two drivers of zoonotic disease transmission into and within the human population can be identified. The first source is the occurrence of disease in animals, which may change with variations in several factors, including population dynamics of hosts or vectors, and alterations in habitat. A second source of pressure emerges from variations in the human population's composition or behaviour, resulting in changing susceptibility. Despite an increased interest in the human aspects that underlie infectious disease transmission,4 relatively little is known about how these factors interact with the environment in the spread and management of disease risk.5 We bring together psychological, economic, environmental and social contact modelling research in order to better conceptualise the key factors and dynamics that underpin both initial zoonotic disease transmission and wider population spread. We then propose a new heuristic framework to help guide future research.
Zoonotic transmission, and the risk environment
The One health movement has stressed the close inter-relationship between animal and human health.6,7 Thus while zoonotic diseases often originate in animals, there exists a wide range of potential transmission pathways for human infection. Some of these pathogens can be sustained within human populations alone, others require other species or environments to survive or propagate, or are simply spread more effectively there. In addition, several significant zoonoses are not generally spread by human-to-human contact but caught (almost exclusively) from animals alone (e.g. West Nile fever, Japanese encephalitis and rabies).
There are a wide range of settings in which human zoonotic infection may occur. In much of the developing world, proximity to animals as well as hunting and consumption of wild bush meat are important disease risk factors.8 In many developed countries, most at risk of infection are those working with animals or animal products, such as livestock and poultry workers (e.g. Zoonotic influenza, Q-fever, Streptococcosis, Rift valley fever), workers processing food (e.g. Campylobacteriosis, Crimean-Congo haemorrhagic fever), or employees working outside where they might have contact with animals or animal matter, such as forestry workers (e.g. Dobrava-Belgrade virus infection, human granulocytotropic anaplasmosis, tularaemia). Other groups may also be susceptible. For example, Vero cytotoxin-producing Escherichia coli 0157 (VTEC) affected almost 100 children visiting an open farm in Surrey, England.9 Walkers and other countryside users throughout Europe face an increasing risk from Lyme Disease, and may have an increased probability of acquiring cryptosporidiosis.9 Tables 1 and 2 provide a list of animals that serve as zoonotic infection reservoirs, and major routes of transmission into humans. Transmission may be bidirectional,10 further complicating the route of infection and potentially exacerbating spread. In addition, further incursions may be possible. The One Health movement emphasises the significance of horizon scanning, and recognises that important diseases can be potentially established in a wide variety of locations, rather than where they are currently found. For example, West Nile Fever, has been shown to have competent vectors in the UK.11
Table 1. Potential disease reservoirs.
| Reservoir species | Zoonotic disease examples | |
|---|---|---|
| Companion animals | Cats | Toxoplasmosis, Q fever, variant Creutzfeldt-Jakob disease, Capnocytophaga canimorsus, Plague, Bartonellosis |
| Dogs | Q fever, Rabies, Leptospirosis, Capnocytophaga canimorsus | |
| Horses | Tuberculosis | |
| Livestock | Cattle | Q fever, Creutzfeldt-Jacob disease, Crimean-Congo haemorrhagic fever, Tuberculosis, Leptospirosis, Rift Valley fever, Tuberculosis, Brucellosis |
| Pigs | Toxoplasmosis, Japanese encephalitis, Campylobacteriosis, Tuberculosis, Streptococcosis, Tularaemia, Brucellosis, Leptospirosis, zoonotic influenza | |
| Sheep/goats | Toxoplasmosis, Q fever, Rift Valley fever, Tularaemia, Brucellosis | |
| Deer | Q fever, Tuberculosis, Human granulocytotropic anaplasmosis, Leptospirosis | |
| Poultry | Poultry/fowl | Campylobacteriosis, Chlamydiosis, Salmonellosis, influenza |
| Wild mammals | Badger, | Tuberculosis |
| Raccoons/skunks | Rabies | |
| Bats | Rabies, Ebola, SARS, Nipah virus | |
| Wild boar | Toxoplasmosis, Tuberculosis, Streptococcosis | |
| Wild deer | Q fever, Tuberculosis, Human granulocytotropic anaplasmosis | |
| Foxes | Q fever, Tularaemia, Echinococcosus, Rabies | |
| Rabbits/hares | Q fever, Tularaemia | |
| Rodents | Toxoplasmosis, Q fever, Leptospirosis, Dobrava-Belgrade virus, Tularaemia, Plague, Monkeypox | |
| Ground squirrels | Plague | |
| Wild birds | Birds including waterfowl | Influenza, Japanese encephalitis, Q fever, West Nile fever, Eastern equine encephalitis, Chlamydiosis |
| Aquatic | Fish | Leptospirosis |
| Arthropod | Insects and arachnids | Campylobacteriosis |
We exclude vector species which cannot sustain the pathogen in the absence of other hosts.
Table 2. Possible animal to human transmission routes for zoonotic diseases.
| Transmission route | Zoonotic disease examples |
|---|---|
| Airborne/respiratory | Influenza, Q fever, Tuberculosis |
| Physical contact | Influenza, Q fever |
| Bite | Rabies, Capnocytophaga canimorsus, Pasturellosis |
| Faecal matter | Influenza, Toxoplasmosis, Salmonellosis, E. coli |
| Infected carcases (handling) | Ebola, Crimean-Congo haemorrhagic fever, Streptococcosis |
| Food | Toxoplasmosis, Campylobacteriosis |
| Water | Leptospirosis, Tularaemia |
| Arthropod vector (flea, midge, mosquito, tick) | Q fever, Crimean-Congo haemorrhagic fever, Lyme disease, West Nile Virus |
Disease transmission occurs in a wide social and cultural environment.7 Major societal transitions that affect the relationship between the environment and social and behavioural factors can have important implications for disease spread.8 The term risk environment describes a combination of economic, cultural, and psychological factors that can contribute to disease risks.12 At the macro level, the risk environment includes large population movements, resulting from both external changes (e.g. floods, wars) and internal, often gradual, societal changes (e.g. industrialization).13,14 It also includes changes in land use, public health infrastructure, and local, national and global weather patterns.8 The risk environment also incorporates further micro-level factors, including local environmental conditions (e.g. the use of different materials which affects the survival of pathogens on surfaces), psychological variables (e.g. perceptions of animals, individual understandings of risk), personal estimations of the economic costs and benefits associated with disease risk, and key characteristics of individual and community networks. We review each of these below.
Macro level features of the environment
Human migration
Changes in human demographics play an important role in the emergence of infectious diseases, either directly or indirectly by causing ecological changes. Human migration and trade, including the transportation of animals, has the potential to relocate pathogens long distances and thus introduce an emerging disease into a hitherto unexposed and therefore highly susceptible population.15 Large scale political transitions can also impact on zoonotic disease.14 The fall of communism in Eastern Europe, for example, and the consequent agricultural and economic reforms, have been linked to a complex series of factors that acted independently yet synergistically to increase tick-borne disease (e.g. decline of agriculture and regeneration of shrubs leading to increases in transmitting rodents, greater leisure time contributing to greater exposure to ticks in forests).16
Land use
As suggested above, land use change and agriculture have been related to the emergence of new pathogens,17 with the expansion of the human/economic system into previously pristine environments providing consequent exposure to a pool of known and unknown pathogens.3 Changes in farming practices, such as movement from rural to peri-urban and urban areas, are likely to affect pathogen ecology and its transmissibility to the urban population.13,17,18 Habitat destruction has played an important but often complex role in the spread/emergence of infectious diseases.8 Outbreaks of hantavirus have been linked to forest clearance in South America.8 The Nipah virus was transmitted from bats to pigs and then to humans as a consequence of the destruction of bat habitats coupled with intensive pig farming practices.19
Urban planning, infrastructure and design
The Built Environment - including public health infrastructure such as sanitation, water supply, wastewater and solid waste treatment - is a core defence against pathogens and disease transmission in most parts of the world. Many pathogenic microorganisms can contaminate water. Leptospirosis, for example, is caused by contact with the urine of infected animals or urine-polluted water contaminated by infected wild animals.20 New methods of sustainable waste treatment and management influence potential exposure to pathogens. Increases in community and industrial composting facilities can release a large quantity of aerosolised particles into the air.21 Several zoonotic pathogens such as Mycobacterium avium, E. coli 0157:H7, Listeria monocytogenes and Salmonella spp can be detected during the composting process.22 Moreover, land application of bio-solids from animal origins are known to create various pathways to increase human exposure to the pathogens.22
Urban planning, architectural layout, occupant density and usage also influences disease transmission, especially airborne transmission. A Q-fever outbreak reported in an urban school in Central Israel occurred far away from local farms,23 with the air-conditioning system suspected of contributing to the spread of the disease. Other urban-engineered environments associated with zoonotic disease transmission include ventilation and water and drainage systems. An outbreak of SARS in a housing campus in Hong Kong, which infected hundreds of occupants within a few weeks, was associated with a dysfunctional drainage system, as well as the proximity of living spaces.24
The natural environment
Climate, seasonality, and weather events such as pressure, humidity, and wind speed and direction, can also influence zoonotic spread.14 Climate change has increased the range and number of viruses and bacteria to which humans may be exposed, as well as competent vectors and reservoir species.25 In 1989, the largest outbreak of Q-fever (147 cases) was recorded in the UK (West Midlands),26 the spread of which was attributed to unusual gale activities. Influenza viruses may survive better in winter than in summer because of the preservation of the viral coat at low temperature, although the climatic link between time of year and respiratory disease outbreaks is often unclear due to seasonal changes in people's behaviour.27 Seasonal and weather factors (e.g. rainy seasons, flooding) and natural disasters interact with poor sanitation and housing environments, which can bring infected animals, contaminated water and air into the human environment. Increased rainfall will also lead to increased run-off from agricultural land, heightening the potential for environmental transmission of zoonotic pathogens from livestock (e.g. E. coli, hepatitis E virus, Salmonella etc.)
Micro and local level influences on zoonotic infection
Interactions between pathogen characteristics and the local environment
As outlined above (Table 2), transmission is likely to occur through different pathways, leading to varied levels of exposure. The respiratory route of exposure to pathogens can be divided into wind-borne, dust-borne, droplet-borne and true airborne transmission. Airborne particles are very small, less than 5 microns, which allow them to suspend in the air for a long time. Wind-borne, dust-born and droplet-borne transmission involve bigger size particles (>5 microns) and require adequate air current to keep airborne; otherwise, like droplet-borne transmission, aerosol spreading distance is limited to 3 feet.28 Air is generally considered a largely hostile environment for microbial life; it has low moisture content and is lacking in nutrients.28 However, as evident in recent zoonotic threats, such as SARS outbreaks and the Swine and Avian Flu pandemics, pathogens can successfully infect via the air.
Local environmental factors play an important part in determining the survival of pathogens, their physical characteristics and potential transmission pathways. For example, droplet release through coughing or sneezing is influenced by environmental factors such as temperature and relative humidity, which control the extent and rate of droplet evaporation.29 The longer the time a pathogen can survive on a surface, the greater the opportunity for transmission. Q- fever bacterium is resistant to desiccation, with the survival rate on environmental surfaces such as clay and wool shown to be potentially years.23 In a comparative analysis of the survival of influenza viruses A and B on hands, non-porous surfaces (stainless steel and plastic), and porous surfaces (e.g. handkerchiefs), viruses survived longer on non-porous than on porous surfaces (48 hours compared to 8–12 hours), and could survive for up to 5 minutes on hands that touched these surfaces.30
Psychological factors
At present, little work has examined cultural and normative understandings of human-animal interactions. Although some work has identified the human wellbeing and health that may result from interactions with animals,31 a number of bacterial, parasitic and viral zoonoses have been associated with sleeping with, sharing a bed with, kissing or being licked by pets.32 There is also growing evidence of disease risk associated with the keeping and feeding of non-traditional exotic or status animals:33 in the UK a new phage type of Salmonella Typhimurium (DT 191a) was detected in 2008, linked to the frozen mice used for reptile food. This means it is important to understand the psychological and cultural motivations underlying interactions with animals.
Many interactions with animals are a combination of mindless and ritualised (rather than consciously motivated) behaviours, alongside more motivated or long-term goals.34,35 Stroking a cat may be a habitual, routine behaviour that meets an affiliative need; grooming a horse may also express an affiliative need, but also be part of a longer-term economic investment. Differences in motivational behaviour will impact on the efficacy of interventions aimed at minimising pathogen spread. Apparently mindless behaviours can be cued by environment features,35 such as hand-washing facilities in a petting zoo: longer-term motivations require attention to specific disease risks (for example, those associated with threats to equine health). In some settings, such as farms, it is also important to consider broader social representations of particular animals. These are influenced by culture, the nature of the zoonotic threat, and the past history of related diseases. For example, pig farmers in Malaysia did not greatly change their everyday behaviours during the H1N1 pandemic, seeing this as less of a threat than the previous Nipah virus.36 Religious and cultural views on the animal concerned (e.g. pigs and their products), also tempered reactions to zoonotic threat.36 Traditional burying rituals, such as the touching and touching of the dead, have been implicated in the spread of Ebola.37 Psychological factors also influence reactions to specific zoonotic threats. Individuals sceptical about the source or trustworthiness of an official communication may be unwilling to take appropriate actions.38 A lack of trust in authorities, low involvement in control post reporting, and even a sense of guilt can act against disease reporting.39 Social media (e.g. Facebook, Twitter) is increasingly being used by official information providers and those concerned with co-ordinating crisis response.40 It is important to understand how credibility is afforded to such media, as well as how this information is combined with other information sources in order to make disease-relevant decisions. This needs to be fed into assessments of risk management activities and their implications for animals, humans and the environment.18
Economic factors
Health risks to animals can have significant economic implications for a society.18 In general, people value person-to-person, and person-to-animal contacts, and are willing to accept some disease risk to gain contact-related benefits. However, economic concerns about the costs of reporting or particular interventions can act as major factors in the emergence of zoonotic diseases.19 Changes in economic incentives can occur either directly as a consequence of coordinated collective action (e.g. policy changes) or indirectly as a result in the change of prevailing environmental conditions and/or disease dynamics/prevalence (e.g. changes in the pay-offs associated with a given course of action).41 Normative economic theory recognises that decision making about zoonotic threat is also influenced by group level phenomena.42 During an epidemic individuals may start to adopt defensive or precautionary practices that in turn effect the spread of the disease. As the state of the ecological system changes, the payoffs associated with each set of action will change, with individuals adapting by modifying their behaviour. The significant impact behaviours can have on these environment in an iterative way is an example of a Complex Adaptive System.43 Infectious diseases reflect the dynamics of an adaptive complex system, which changes its identity in time.44 Characterizing the adaptive element of human behaviour in an epidemiological context remains an important challenge that will enable us to better predict and understand future outbreaks.5
Social contacts and interactions
Diseases may be transmitted in a number of different ways, which require different levels of contact for infection.45 In addition, some individuals are more vulnerable due to both inherent immunities and social patterns (e.g. children), while others are potentially at risk due to their occupation (e.g. health care workers, animal handlers).
Three factors determine the rate at which new infections spread within a given population: the contact rate between individuals, the proportion of contacts between infected and susceptible individuals, and the probability that once an appropriate contact has occurred, the infection will be transmitted.46 The onward spread of a zoonotic will depend on whether the disease requires interaction with an animal at intermediate stages - pets are obviously more likely to be found in the family home. The clustering of an information network around particularly infected individuals can also constrain the spread of a pathogen.4 There is emerging evidence to suggest such clustering has implications for viable interventions: attitudes to vaccination are influenced by local community factors, such as schooling.47
Bringing this together: combining biology, environment and behaviour
It is well recognised that both the infectiousness of a disease, and its potential for wide-scale transmission, will depend on the biology of the infectious agent (e.g. the pathways through which it is spread) and of the infected humans/animals, the nature of the surrounding environment, and the social practices, and behaviours of both individuals and organisations.13,14 All of these interact in both initial individual infection and wider spread. However, the interactions described in many traditional models fail to allow for the full interplay between psychological, environmental and biological factors (e.g. through a recognition of the significance of risk perception). We suggest a heuristic framework we term the BEB framework (Biology - Environment - Behaviour) (Figure 1). This framework incorporates both macro and micro level contributors to infection and transmission, and allows explicitly for feedback between biological, environmental and behavioural influences on infection or transmission. A large number of possible examples could be used to illustrate this: in the text below we discuss indicative cases included in Figure 1. Our specific inclusion of psychological and economic variables can be seen as complementing the wider One Health approach to health methodologies, with its particular focus on interactions between the environment and human and veterinary medicine.7 It also belongs aside other initiatives calling for an interdisciplinary approach which specifically consider economic factors and the consequences of risk management when responding to zoonotic threats.18
Figure 1.
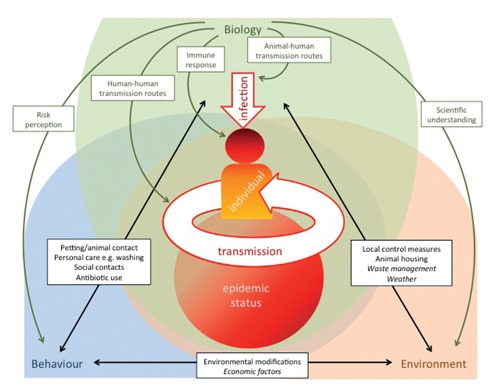
Biology, environment, behaviour and their interactions as predictors of zoonotic infection and transmission. Italicised text refers to macro-level examples of behaviour and environment; non-italic text to micro-level examples.
In our framework. the human seed case for individual infection depends partly on biological factors related to the pathogen (e.g. animal-human transmission routes, infectiousness of the pathogen), and an individual's level of immunity. During transmission individuals act iteratively, with each onwards transmission a newly infected individual with the potential to transmit. However, spread of a pathogen also includes wider aspects of the environment and society in which the infection occurs. Biological factors include other vectors for transmission (both animal and human), as well as human-to-human transmission rates and likely levels of resistance/ immunization in a given population. This may depend on factors such as population age profile, and the percentage of the population with compromised immunity.
In any environment there will be features that can intensify or attenuate infection. These include macro-level factors (e.g. weather, climate change), and service infrastructures (e.g. health, waste management services). Micro-level environmental determinants can include local infection control measures (such as cleaning and ventilation), the composition of potentially contaminable surfaces, and local animal housing arrangements. Risk perceptions can lead to modifications in the environment (e.g. cleaning or increasing ventilation), and introducing physical barriers (e.g. to prevent children touching animals). Changes in both micro and macro-level environmental features (e.g. cleaning regimes, climatic change) will also impact on infection risk.
Turning to behaviour, individuals have their own risk perceptions and modify their behaviour accordingly, for example by wearing protective clothing while interacting with a potentially infected animal. Some of these behaviours (e.g. hand washing) impact further on (biological) infection risks. Human behaviour (e.g. contact patterns) is adaptive and will respond to the perceived progression of a disease and risks of the infection. Changes in behaviour (for example, social distancing, either voluntary or enforced by governmental authorities, or normative mask wearing) are also likely to impact on the risks of infection. At the same time, some biological features of a pathogen (e.g. drug resistance) may be altered through public behaviours (e.g. widespread use, or misuse, of an antibiotic leading to drug resistance).
Finally, social behaviour is also likely to reflect other environmental factors: fear of unemployment, for example, may lead to greater risk taking in some communities when interacting with animals, or the adoption of short cuts during food preparation. Environmental modifications can lead to the mindless nudging of individual behaviour change (such as when moving washbasins leads to increased handwashing behaviour), while purposive behavioural interventions can have significant impacts on the environmental ecology of particular locations (e.g. in areas marked as off limits during a zoonotic outbreak).
Implications
While it is possible to control some zoonotic diseases by directly targeting reservoir hosts or vectors (e.g. culling mountain hares, use of acaricides), changes in a habitat (for example through vegetation management), may also induce changes in populations, with potential impacts on the interface between carriers and humans. In our framework, macro level factors include reciprocal interactions between external factors (such as climate change) and biological risk, although these pathways may occur over some time. Risk representations and governmental policies are likely to have a more immediate impact on key behaviour and environmental modifications, although these are often underplayed in models of microbial threat. Our model underlines the significance of the social construction of biological risk, including non-rational biases and prejudices that might influence such perceptions. At the same time, optimal behavioural interventions depend partly on environmental conditions, such as the physical space in which animals are housed, and an economic environment that encourages appropriate (but potentially costly) precautionary measures.
Employing such an approach can shed light on enduring issues in the biology of zoonotic threats. One continuing debate concerns the efficacy of wearing facemasks to prevent influenza infection in community settings.48 An environmental engineering assessment can clarify the optimal situations for effective mask wearing as a barrier against a particular pathogen (e.g. through aerosol studies of pathogen spread), but psychological variables are likely to influence adherence behaviours (risks might be underestimated, mask wearers might feel stigmatised leading to poor adherence). Changes in an environmental factor (for example, temperature) can influence transmission risk through (non-) adherence to mask wearing. Similar opportunities arise for the study of contact patterns and infection. Such work often provides culturally thin descriptions of likely transmission patterns. Research which includes cultural understandings of risk perception, and identifies environmental conditions which affect zoonotic exposure (e.g. indoor vs. outdoor location, in large vs. confined spaces) can provide new data on pathogen survival and transmission in different interactional settings. In our framework, changes in one factor (e.g. modifications in the social facilities shared by farm workers) can influence behaviour (e.g. hand washing) and the potential onward transmission of a pathogen.
Such work will involve a broad team of researchers, from different disciplinary approaches. It is likely to involve the building of new, more complex models of zoonotic disease spread, identifying new proxies for behaviour where necessary. Building an effective bridge between expert advisers and lay individuals is often a delicate process, requiring socially sensitive natural scientists and medically-informed social researchers. Zoonotic diseases have significant potential to be a major drain on national resources as well as posing a genuine threat to public health for both current and future generations. As their threat increases, researchers need to develop bold and elaborate models acknowledging the interplay between biology, ecological and environmental phenomena, and individual and group perceptions and behaviours. National, and ideally international, funding resources will be required to support the development of such models and their use in effective responses to the threat of zoonotic disease transmission.
Acknowledgements:
this paper was the product of a series of multi-disciplinary exchanges promoted through an interdisciplinary catalyst grant awarded to the first author under the ESEI initiative to encourage multidisciplinary approaches to zoonoses. Funding was jointly from the MRC, NERC, ESRC and BBSRC (Grant G0902431).
References
- 1.Kruse H, Kirkemo A, Handeland K. Wildlife as source of zoonotic infections. Emerg Infect Dis. 2004;10:2067–72. doi: 10.3201/eid1012.040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–4. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daszak P, Cunningham AA, Hyatt DD. Emerging infectious diseases of wildlife. Threats to biodiversity and human health. Science. 2000;287:443–9. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 4.Funk S, Salathe M, Jansen VAA. Modelling the influence of human behaviour on the spread of infectious diseases: a review. J R Soc Interface. 2010;7:1247–56. doi: 10.1098/rsif.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson N. Capturing human behaviour. Nature. 2007;446:733. doi: 10.1038/446733a. [DOI] [PubMed] [Google Scholar]
- 6.Madoff L. Cooperation between animal and human health sectors is key to the detection, surveillance, and control of emerging disease: IMED 2007 meeting in Vienna, February 2007. Euro Surveill. 2006;11 doi: 10.2807/esw.11.51.03101-en. pii 3101. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3101. [DOI] [PubMed] [Google Scholar]
- 7.King LJ, Blackwell MJ, Meyer TE, et al. Executive summary of the AVMA One Health Initiative Task Force report. JAVMA. 2008;233:259–61. doi: 10.2460/javma.233.2.259. [DOI] [PubMed] [Google Scholar]
- 8.Weiss RA, McMichael A. Social and environmental risk factors in the emergence of infectious diseases. Nat Med. 2004;10:570–6. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DEFRA. [Accessed on May 2012];2009 Zoonoses report. 2011 Available from: http://archive.defra.gov.uk.
- 10.Bengis RG, Kock RA, Fischer J. Infectious animal diseases: the wildlife/livestock interface. Rev Sci Tech. 2002;21:53–65. doi: 10.20506/rst.21.1.1322. [DOI] [PubMed] [Google Scholar]
- 11.Higgs S, Snow K, Gould E. The potential for West Nile virus to establish outside of its natural range: a consideration of potential mosquito vectors in the United Kingdom. Trans R Soc Trop Med Hyg. 2004;98:82–7. doi: 10.1016/s0035-9203(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes T, Stimson GV, Crofts N, et al. Drug injecting, rapid HIV spread, and the risk environment: implications for assessment and response. AIDS. 1999;13(Suppl A):S259–S269. [PubMed] [Google Scholar]
- 13.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2010;430:242–9. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine. [Accessed on June 2012];Microbial threats to health: emergence, detection, and response. Available from: http://iom.edu/Activities/PublicHealth/MicrobHealthThreats.aspx.
- 15.Pfeffer M, Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit Vectors. 2010;3:35. doi: 10.1186/1756-3305-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph SE. Tick-borne encephalitis incidence in Central and Eastern Europe: consequences of political transition. Microbes Infect. 2008;10:209–16. doi: 10.1016/j.micinf.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Patz JA, Daszak P, Tabor GM, et al. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–8. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Council of Canadian Academies. [Accessed on August 2012];Healthy animals, healthy Canada. 2011 Available from: http://www.scienceadvice.ca/en/assessments/completed/animal-health.aspx.
- 19.Chua KB, Lam SK, Koh CL, et al. Reservoir of Nipah virus identified. Proc Int Conf Emerge Infect Dis Atlanta. 2000 [Google Scholar]
- 20.World Health Organisation. [Accessed on June 2012];Human Leptospirosis: guidance for diagnosis, surveillance and control. Available from: http://whqlibdoc.who.int/hq/2003/WHO_CDS_CSR_EPH_2002.23.pdf.
- 21.Lai KL, Emberlin J, Colbeck I. Outdoor environments and human pathogens in air. Enviro Health. 2009;8(Suppl 1):S15. doi: 10.1186/1476-069X-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grewal SK, Rajeev S, Sreevatsan S, Michel FC. Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl Environ Microbiol. 2006;72:565–74. doi: 10.1128/AEM.72.1.565-574.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amitai Z, Bromberg M, Bernstein M, et al. A large Q fever outbreak in an urban school in central Israel. Clin Infect Dis. 2010;50:1433–8. doi: 10.1086/652442. [DOI] [PubMed] [Google Scholar]
- 24.Gao NP, Niu JL, Perino M, Heiselberg P. The airborne transmission of infection between flats in high-rise residential buildings: tracer gas simulation. Build Environ. 2008;43:1805–17. doi: 10.1016/j.buildenv.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissenböck H, Hubálek Z, Bakonyi T, Nowotny N. Zoonotic mosquito-borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet Microbiol. 2010;140:271–80. doi: 10.1016/j.vetmic.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Hawker JI, Ayres JG, Blair I, et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–7. [PubMed] [Google Scholar]
- 27.Ayres JG, Forsberg B, Annesi-Maesano I, et al. Climate change and respiratory European Respiratory Society position statement. Eur Respir J. 2009;34:295–302. doi: 10.1183/09031936.00003409. [DOI] [PubMed] [Google Scholar]
- 28.Cox CS. The aerobiological pathway of microorganisms. Chichester: John Wiley & Sons; 1987. [Google Scholar]
- 29.Yang W, Marr LC. Dynamics of airborne influenza A viruses indoors and dependence on humidity. PLoS One. 2011;6:e21481. doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bean B, Moore BM, Sterner B, et al. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 31.Barker SB, Wolen AR. The benefits of human-companion animal interaction: a review. J Vet Med Educ. 2008;35:487–95. doi: 10.3138/jvme.35.4.487. [DOI] [PubMed] [Google Scholar]
- 32.Chomel BB, Sun B. Zoonoses in the bedroom. Emerg Infect Dis. 2011;17:167–72. doi: 10.3201/eid1702.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reaser JK, Clark EE, Meyers NM. All creatures great and minute: A public policy primer for companion animal zoonoses. Zoonoses Public Health. 2008;55:385–401. doi: 10.1111/j.1863-2378.2008.01123.x. [DOI] [PubMed] [Google Scholar]
- 34.Curtis V, Danquah L, Aunger R. Planned, motivated and habitual hygiene behaviour: an eleven-country review. Health Educ Res. 2009;24:655–73. doi: 10.1093/her/cyp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Pers Soc Psychol Rev. 2004;8:220–47. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin R, Haque S, Hassan S, Dhanoa A. Representations of swine flu: perspectives from a Malaysian pig farm. Public Underst Sci. 2011;20:477–90. doi: 10.1177/0963662510392484. [DOI] [PubMed] [Google Scholar]
- 37.Legrand J, Grais RF, Boelle PY, et al. Understanding the dynamics of Ebola epidemics. Epidemiol Infect. 2007;135:610–21. doi: 10.1017/S0950268806007217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quine CP, Barnett J, Dobson ADM, et al. Frameworks for risk communication and disease management: the case of Lyme disease and countryside users. Philos Trans R Soc Biol Sci. 2011;366:2010–22. doi: 10.1098/rstb.2010.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbers ARW, Gorgievski-Duijvesteijn MJ, van der Velden W, et al. A socio-psychological investigation into limitations and incentives concerning reporting a clinically suspect situation aimed at improving early detection of classical swine fever outbreaks. Vet Microbiol. 2010;142:108–18. doi: 10.1016/j.vetmic.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 40.The Red Cross. [Accessed on June 2012];The Case for Integrating Crisis Response with Social Media. 2010 Available from http://www.scribd.com/doc/35737608/White-Paper-The-Case-for-Integrating-Crisis-Response-With-Social-Media.
- 41.Fenichel EP, Castillo-Chavez C, Ceddia MG, et al. Adaptive human behaviour in epidemiological models. Proc Natl Acad Sci. 2011;108:6306–11. doi: 10.1073/pnas.1011250108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condon BJ, Sinha T. Who is that masked person: the use of face masks on Mexico City public transportation during the Influenza A (H1N1) outbreak. Health Policy. 2010;95:50–6. doi: 10.1016/j.healthpol.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Levin SA. Ecosystems and the biosphere as complex adaptive systems. Ecosystems. 1998;1:431–6. [Google Scholar]
- 44.Prigogine I. From being to becoming. San Francisco: WH Freeman and Co.; 1978. [Google Scholar]
- 45.Read JM, Eames KTD, Edmunds WJ. Dynamic social networks and the implications for the spread of infectious disease. J R Soc Interface. 2008;5:1001–7. doi: 10.1098/rsif.2008.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCallum N, Barlow N, Hone J. How should pathogen transmission be modelled? Trends Ecol Evol. 2001;16:295–300. doi: 10.1016/s0169-5347(01)02144-9. [DOI] [PubMed] [Google Scholar]
- 47.Eames KTD. Networks of influence and infection: parental choices and childhood disease. J R Soc Interface. 2009;6:811–4. doi: 10.1098/rsif.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowling BJ, Zhou Y, Ip DKM, et al. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138:449–56. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]


