Abstract
Despite significant therapeutic advances, the prognosis of patients with heart failure (HF) remains poor and current therapeutic approaches are palliative in the sense that they do not address the underlying problem – the loss of cardiac tissue. Stem cell-based therapies have the potential to fundamentally transform the treatment of HF by achieving what would have been unthinkable only a few years ago – myocardial regeneration. For the first time since cardiac transplantation, a therapy is being developed to eliminate the underlying cause of HF, not just to achieve damage control. Since the initial report of cell therapy (skeletal myoblasts) in HF in 1998, research has proceeded at lightning speed and numerous preclinical and clinical studies have been performed that support the ability of various stem cell populations to improve cardiac function and reduce infarct size in both ischemic and nonischemic cardiomyopathy. Nevertheless, we are still at the dawn of this therapeutic revolution. Many important issues (e.g., mechanism(s) of action of stem cells, long-term engraftment, optimal cell type(s), dose, route, and frequency of cell administration) remain to be resolved, and no cell therapy has been conclusively shown to be effective. The purpose of this article is to critically review the large body of work carried out with respect to the use of stem/progenitor cells in HF, both at the experimental and clinical level, and to discuss current controversies, unresolved issues, challenges, and future directions. The review focuses specifically on chronic HF; other settings (e.g., acute myocardial infarction, refractory angina) are not discussed.
Keywords: Stem cells, myocardial infarction, congestive heart failure, myocardial regeneration
Introduction
Heart failure (HF) is a common, lethal, disabling, and expensive disorder. Its prevalence in industrialized nations has reached epidemic proportions and continues to rise. Despite significant therapeutic advances, the prognosis for patients who are admitted to the hospital with HF remains poor, with a 5-year mortality of nearly 50% – worse than that for patients with breast or colon cancer 1. In the United States, HF affects nearly 6 million persons, kills over 300,000 people per year, and is directly responsible for more than $40 billion in healthcare expenditures 2.
Although current therapeutic approaches to HF improve symptoms and prolong life, they are palliative in the sense that they do not address the fundamental problem – the loss of cardiac tissue. It is for this reason that stem cells have sparked intense interest. Stem cell-based therapies have the potential to dramatically transform the treatment and prognosis of HF by achieving what would have been unthinkable only a few years ago – myocardial regeneration. For the first time since cardiac transplantation, the goal is not damage control, but damage elimination – i.e., removal of the underlying cause of HF. It is the curative potential of this new therapy that explains why translational efforts have proceeded at lightning speed (Fig. 1). The first study of bone marrow cells in experimental myocardial infarction (MI) was published in 2001 3; within a year, this therapy had been applied in patients 4. In the setting of HF, it took only three years from the first use of stem cells (skeletal myoblasts) in an animal model 5 to the first use of these cells in humans 6. Few ideas in medicine have been translated from the experimental laboratory to the clinical arena faster than the use of stem cells in heart disease.
Figure 1. Use of various types of stem cell therapies in patients with cardiovascular disease.
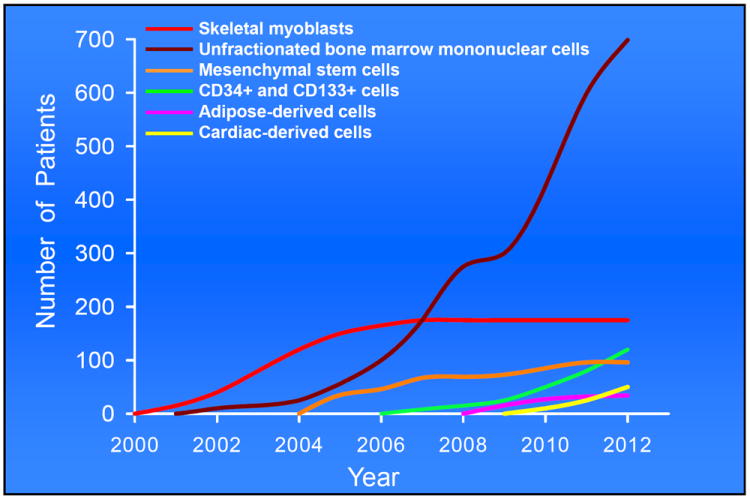
Illustrated is the number of patients treated with six major types of cells from 2000 (when the first cell therapy for heart disease was performed) to 2012.
Over the past 15 years, numerous preclinical and clinical studies have been performed that support the ability of various stem cell populations to improve cardiac function and attenuate adverse left ventricular (LV) remodeling in both ischemic and nonischemic cardiomyopathy. Despite this rapid progress, however, many fundamental issues remain to be resolved and, to date, no cell therapy has been conclusively shown to be effective in patients with HF. The purpose of this article is to critically review the large body of work carried out with respect to the use of stem/progenitor cells in HF, both at the experimental and clinical level, and to discuss current controversies, unresolved issues, challenges, and future directions. This review focuses specifically on chronic HF; studies of stem cells in acute MI, refractory angina, and other conditions not relevant to chronic HF are not discussed.
Stem cell types investigated heretofore in HF
Stem cells are undifferentiated, self-renewing cells that possess a multi-lineage differentiation potential. As illustrated in Fig. 2, various types of stem cells have been considered for the treatment of HF. The preclinical and clinical studies that have assessed the utility of stem cells in chronic HF are summarized in Tables 1 and 2, respectively.
Figure 2. Sources of stem cells used for cardiac repair.
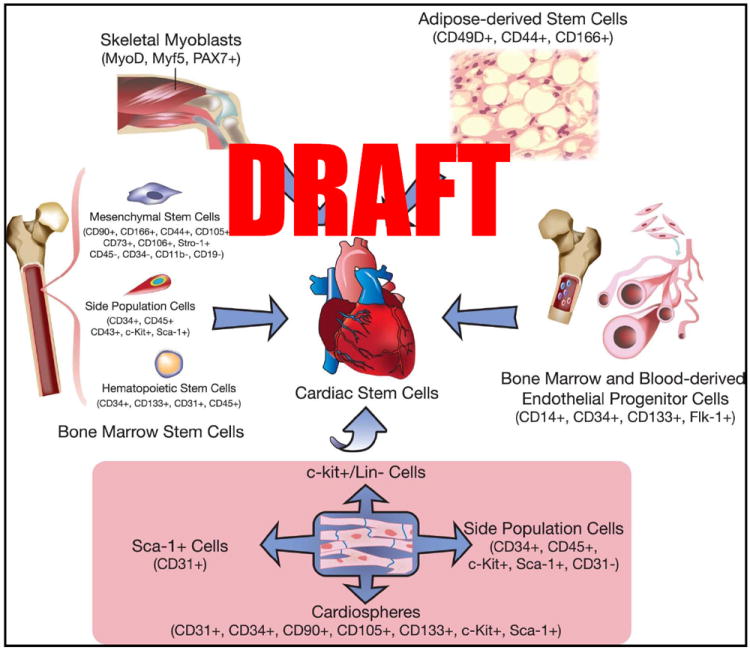
Bone marrow-derived stem cells include a broad range of cells, from mesenchymal stem cells to endothelial progenitor cells, hematopoietic stem cells, and unfractionated mononuclear cells. (Illustration Credit: Ben Smith)
Table 1.
Animal studies of stem cell therapy in heart failure
| Study | Host | Type of heart failure | Time of cell therapy | Dose and route of administration | Follow-up period after cell therapy | Outcomes |
|---|---|---|---|---|---|---|
|
| ||||||
|
SKELETAL MYOBLASTS
| ||||||
| Suzuki et al.46 | Lewis rat | Doxorubicin-induced cardiomyopathy | 4 weeks after last doxorubicin dose | 1×106 cells, intracoronary | 4 weeks | ↓ Mortality |
| Improved hemodynamic parameters | ||||||
|
| ||||||
| Ghostine et al.38 | Sheep | Embolization using absorbable hemostatic gauze | 14 days after MI | 50,000 cells, Intramyocardial | 12 months | ↑ LVEF |
| ↓ LVEDV | ||||||
| Improved global wall motion score | ||||||
|
| ||||||
| Pouly et al.47 | CHF147 Syrian hamster | δ-sarcoglycan deficiency-induced dilated cardiomyopathy | ------ | 5×106 cells, Intramyocardial | 4 weeks | ↑ FAC |
| ↓ Fibrosis | ||||||
|
| ||||||
| Chachques et al.39 | Sheep | Permanent coronary occlusion | 3 weeks after MI | 70×106 cells, intramyocardial | 3 months | ↑ LVEF |
| ↓ LV remodeling | ||||||
|
| ||||||
| He et al.40 | Dog | Coronary microembolization | After hemodynamic confirmation of establishment of heart failure | 270 to 830×106 cells, Intramyocardial | 10 weeks | ↑ LVEF |
| ↓ LV remodeling | ||||||
| Improved hemodynamic parameters | ||||||
|
| ||||||
| Gavira et al.41 | Gottingen mini-pig | Vascular embolization in the intermediate branch of first or second marginal artery | 8 weeks after MI | 407.55 ± 115×106, intramyocardial or intracoronary | 3 months | ↑ LVEF |
| ↓ Fibrosis | ||||||
| ↑ Vasculogenesis | ||||||
|
| ||||||
| Farahmand et al.36 | Lewis rat | Permanent coronary occlusion | either 5 days after MI or 30 days after MI | 5×106 cells, Intramyocardial | 30 days | ↑ LVFS |
| ↓ LV remodeling | ||||||
| Improved hemodynamic parameters | ||||||
| Attenuated matrix remodeling | ||||||
|
| ||||||
| Fukushima et al.37 | Sprague Dawley rat | Permanent coronary occlusion | 3 weeks after MI | 5×106 cells, intramyocardial or intracoronary | 84 days | ↑ LVEF |
| Improved physical activity | ||||||
| ↔ Mortality | ||||||
|
| ||||||
|
BONE MARROW MONONUCLEAR CELLS
| ||||||
| Tomita et al.71 | Sprague Dawley rat | Cryosurgery | 3 weeks after surgery | 1×106 cells, Intramyocardial | 3 weeks | Improved hemodynamic parameters |
| ↓ LV remodeling | ||||||
| ↑ Angiogenesis | ||||||
| Cardiac differentiation + | ||||||
|
| ||||||
| Bel et al.68 | Sheep | Ligation of circumflex artery | 3 weeks after MI | 422×106 cells, Intramyocardial | 2 months | ↔ LVEF |
| ↔ LV remodeling | ||||||
| No differentiation into endothelial cells or cardiomyocytes | ||||||
|
| ||||||
| Waksman et al.69 | Pig | Permanent coronary occlusion | 4 weeks after MI | 24×106 cells, Intramyocardial | 4 weeks | ↔ Global wall motion score |
| ↓ Infarct size | ||||||
| ↑ Angiogenesis | ||||||
|
| ||||||
|
BONE MARROW AND ADIPOSE-DERIVED MESENCHYMAL CELLS
| ||||||
| Nagaya et al. (Bone-marrow MSCs) 98 | Lewis rat | Myosin-induced autoimmune myocarditis | 5 weeks after immunization | 5×106 cells, Intramyocardial | 4 weeks | Improved hemodynamic parameters |
| ↑ Angiogenesis | ||||||
| Cardiomyocyte differentiation + | ||||||
| ↓ Fibrosis | ||||||
|
| ||||||
| Silva et al. (Bone-marrow MSCs) 93 | Dog | Ameroid-induced chronic coronary occlusion | 30 days after MI | 100×106 cells, Intramyocardial | 30 days | ↑ LVEF |
| Neovascularization + | ||||||
|
| ||||||
| Miyahara et al. (Adipose- derived MSCs) 113 | Sprague Dawley rat | Permanent coronary occlusion | 4 weeks after MI | 5-8×105 cells as monolayered grafts into myocardium | 4 weeks | ↓ Mortality |
| Improved hemodynamic parameters | ||||||
| Cardiac regeneration + | ||||||
|
| ||||||
| Liu et al. (Bone-marrow MSCs) 95 | Sprague Dawley rat | Permanent coronary occlusion | 4 weeks after MI | 1×106 cells, Intramyocardial | 4 weeks | ↓ Infarct size |
| ↓ LV remodeling | ||||||
| ↑ LVEF | ||||||
| ↓ Fibrosis | ||||||
| Cardiac differentiation + | ||||||
| ↑ Angiogenesis | ||||||
|
| ||||||
| Mazo et al. (Adipose-derived MSCs) 114 | Sprague Dawley rat | Permanent coronary occlusion | 5 weeks after MI | 1×106 cells, Intramyocardial | 3 months | ↑ LVEF |
| Improved tissue metabolism | ||||||
| ↓ Infarct size | ||||||
| ↓ Fibrosis | ||||||
| Neovascularization + | ||||||
|
| ||||||
| Li et al. (Bone-marrow MSCs) 96 | Wistar rat | Isoproterenol-induced heart failure | 4 weeks after isoproterenol injection | 3×106 cells, Intramyocardial | 4 weeks | ↑ LVEF |
| ↓ Fibrosis | ||||||
|
| ||||||
| Schuleri et al. (Bone-marrow MSCs) 94 | Gottingen pig | Ischemia/reperfusion injury | 12 weeks after MI | 20×106 to 200×106 cells, Intramyocardial | 24 weeks | High dose: |
| ↑ LVEF | ||||||
| ↓ Infarct size | ||||||
| Both high and low dose: | ||||||
| ↑ regional contractility and myocardial blood flow | ||||||
|
| ||||||
| Mazo et al. (Bone-marrow MSCs) 97 | Sprague Dawley rat | Permanent coronary occlusion | 4 weeks after MI | 1×106 cells, Intramyocardial | 4 wk | ↑ LVEF |
| ↓ Fibrosis | ||||||
| ↑ Angiogenesis | ||||||
|
| ||||||
|
CARDIAC STEM CELLS
| ||||||
| Rota et al. (c-kit+ cells) 126 | Fischer 344 rat | Permanent coronary occlusion | 20 days after MI | 40,000 cells, Intramyocardial | 2 weeks | ↑ LVEF |
| Attenuated matrix remodeling | ||||||
| ↓ Fibrosis | ||||||
| Cardiac regeneration + | ||||||
| Neovascularization + | ||||||
| Improved hemodynamic parameters | ||||||
| ↓ LV remodeling | ||||||
|
| ||||||
| Johnston et al. (CDCs) 136 | Mini-pig | Permanent coronary occlusion | 4 weeks after MI | 10×106 cells, intracoronary | 8 weeks | ↓ Infarct size |
| Improved hemodynamic parameters | ||||||
| ↔ LVEDV | ||||||
| ↓ LV remodeling | ||||||
| Cardiac regeneration + | ||||||
|
| ||||||
| Tang et al. (c-kit+ cells) 127 | Fischer 344 rat | Ischemia/reperfusion injury | 30 days after MI | 40,000 cells, intracoronary | 35 days | ↑ LVEF |
| Improved hemodynamic parameters | ||||||
| Attenuated matrix remodeling | ||||||
| ↓ Fibrosis | ||||||
| ↓ LV remodeling | ||||||
| Cardiac regeneration + | ||||||
|
| ||||||
| Lee et al. (Cardiospheres) 138 | Mini-pig | Permanent coronary occlusion | 4 weeks after MI | 1×106 cells, intracoronary | 8 weeks | ↑ LVEF |
| ↓ LV remodeling | ||||||
|
| ||||||
| Bolli et al. (c-kit+ cells) 128 | Pig | Ischemia/reperfusion injury | 90 days after MI | 500,000 cells, intracoronary | 31 days | ↑ LVEF |
| Improved hemodynamic parameters | ||||||
| ↓ Fibrosis | ||||||
| ↓ LV remodeling | ||||||
| Cardiac regeneration + | ||||||
| Angiogenesis + | ||||||
CDC, cardiosphere-derived cell; FAC, fractional area change; LV, left ventricular; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVFS, LV fractional shortening; MI, myocardial infarction; MSC, mesenchymal stem cell. ↑, increased; ↓, decreased; ↔, no change.
Table 2.
Clinical trials of stem cell therapy in heart failure
| Study / Name of the trial | Study design | Number of patients | Delivery method | Cell Dose | End-point evaluation method | Follow-up period | Outcomes | Side effects in cell-treated patients |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
|
SKELETAL MYOBLASTS
| ||||||||
| Menasche et al.48 | Non-randomized, uncontolled study | Cell treatment =10 No controls |
Intramyocardial injection during CABG | 871×106 cells | Echocardiography | 10.9 months | ↑ LVEF | Ventricular arrhythmias in 4/10 patients, 2 deaths |
| ↑ regional wall motion | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Smits et al.51 | Non-randomized, uncontrolled pilot study | Cell treatment =5 No controls |
Intramyocardial (Transendocardial) | 196±105×106 cells | MRI, LV angiography, nuclear radiography, echocardiography | 3 to 6 months | ↑ wall thickening | Ventricular arrhythmias in 1/5 patients |
| ↑ LVEF | ||||||||
| ↑ regional wall motion at 3 mth but not at 6 mth | ||||||||
|
| ||||||||
| Herreros et al.50 | Non-randomized, uncontolled study | Cell treatment =12 No controls |
Intramyocardial injection during CABG | 221×106 | Echocardiography, PET scan | 3 months | ↑ LVEF | No major complications reported |
| ↑ myocardial contractility and tissue viability | ||||||||
| ↑ regional wall motion | ||||||||
|
| ||||||||
| Siminiak et al.52 | Non-randomized, uncontolled study | Cell treatment =10 No controls |
Intramyocardial injection during CABG | 4×105 cells | Echocardiography | 12 months | ↑ contractility | Ventricular arrhythmias in 4/10 patients, 1 death |
| ↑ LVEF | ||||||||
| ↑ regional wall motion | ||||||||
|
| ||||||||
| Ince et al.53 | Non-randomized, case-controlled study | Cell treatment =6 Controls =6 |
Intramyocardial (Transendocardial) | 210±150× 106 cells | Echocardiography | 12 months | ↑ LVEF | 2 patients developed early ventricular arrhythmias, which was not sustained |
| ↑ walking distance | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Siminiak et al. [POZNAN] 55 | Non-randomized, uncontolled study | Cell treatment =10 No controls |
Percutaneous transcoronary-venous | 100×106 cells | Echocardiography | 6 months | ↓ NYHA class | No major complications reported |
| ↑ LVEF | ||||||||
|
| ||||||||
| Dib et al.54 | Non-randomized, uncontolled study | Cell treatment =30 No controls |
Intramyocardial injection during CABG (24 patients) and LVAD (6 patients) | CABG group: 10, 30, 100, 300×106 cells cells; LVAD group: 300×106 cells | Echocardiography, PET scan | 24 months | ↑ LVEF | CABG group: Ventricular arrhythmias in 4/24 patients, 1death and 1 MI; LVAD group: Ventricular arrhythmias in 2/6 patients, 3 deaths |
| ↑ regional wall motion | ||||||||
| ↑ viability | ||||||||
| ↓ LVESV and LVEDV | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Biagini et al.58 | Non-randomized, uncontolled study | Cell treatment =10 No controls |
Intramyocardial (Transendocardial) | 15×106 cells | Echocardiography | 12 months | ↑ LVEF | No major complications reported |
| ↓ LVESV | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Hagege et al.56 | Cohort study | Cell treatment =9 No controls |
Intramyocardial injection during CABG | 62 to 1,100×106 (871×106) cells | Echocardiography | 18-58 (49.4) months | ↑ LVEF | Ventricular arrhythmias in 5/9 patients |
| ↓ NYHA class | ||||||||
|
| ||||||||
| Gavira et al.57 | Non-randomized, contolled study | Cell treatment =12 Controls =14 |
Intramyocardial injection during CABG | 50×106 cells | Echocardiography, PET scan | 12 months | ↑ LVEF | No major complications reported |
| ↑perfusion and viability | ||||||||
| ↑ regional contractility | ||||||||
|
| ||||||||
| Veltman et al.64 | Non-randomized, contolled study | Cell treatment =14 Controls =28 |
Intramyocardial (Transendocardial) | 3 to 50×106 cells | Echocardiography | 4 yrs | ↔ LVEF | Ventricular arrhythmias in 7 cell treated patients, 3 and 11 deaths in cell treated and control groups, respectively. |
| ↔ myocardial performance index | ||||||||
|
| ||||||||
| Menasche et al. [MAGIC] 62 | Randomized, placebo-controlled, double-blind study | Cell treatment =97 (low dose: 33 patients, high dose: 34 patients) Controls =30 |
Intramyocardial injection during CABG |
Low dose: 400×106 High dose: 800×106 cells |
Echocardiography | 6 months | ↔ LVEF | Low dose: 4 patients with ventricular arrhythmias and 5 deaths High dose: 5 patients with ventricular arrhythmias and 4 deaths |
| ↔ regional wall motion | ||||||||
| ↓ LVESV and LVEDV in high dose group | ||||||||
|
| ||||||||
| Dib et al. [CAUSMIC] 59 | Randomized, placebo-controlled, double-blind study | Cell treatment =12 Controls =11 |
Intramyocardial (Transendocardial) | 3 patients/dose group, receiving 30, 100, 300 or 600 ×106 cells | Echocardiography | 12 months | ↓ NYHA class | Ventricular arrhythmias in 6/12 patients |
| ↓ LV dimension | ||||||||
| ↑ LVEF | ||||||||
| ↑ regional wall motion | ||||||||
| ↑ viability | ||||||||
|
| ||||||||
| Duckers et al. [SEISMIC] 65 | Prospective, randomized, open-label study | Cell treatment =26 Controls =14 |
Intramyocardial (Transendocardial) | 150 to 800×106 cells | MUGA scan | 6 months | ↔ LVEF | Ventricular arrhythmias in 12/26 patients, 1death |
| ↑ 6MWD | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Povsic et al.63 | Randomized, double-blind, controlled study | Cell treatment =15 Controls =8 |
Intramyocardial (Transendocardial) |
Low dose: 400×106 High dose: 800×106 cells |
Doubutamine stress echocardiography, MUGA scan | 6 months | ↑ 6MWD | Ventricular arrhythmias in 7/15 cell-treated patients |
|
| ||||||||
|
BONE-MARROW MONONUCLEAR CELLS
| ||||||||
| Perin et al.72 | Prospective, nonrandomized, open-label study | Cell treatment =14 Controls =7 |
Intramyocardial (Transendocardial) | 25.6±6.3× 106 cells | Echocardiography, SPECT | 2 and 4 months | 2 months: ↓ NYHA class, ↓ CCSAS, ↑ LVEF, ↓ LVESV and LVEDV | 1 sudden cardiac death in cell treated group |
| 4 months: ↑ LVEF, ↓ LVESV and LVEDV | ||||||||
|
| ||||||||
| Perin et al.73 | Prospective, nonrandomized, open-label study | Cell treatment =11 Controls =9 |
Intramyocardial (Transendocardial) | 25.6±6.3× 106 cells | Echocardiography, SPECT | 6 and 12 months | ↑ exercise capacity | No major complications reported |
| ↑ perfusion | ||||||||
| ↔ LVEF | ||||||||
|
| ||||||||
| Galinanes et al.75 | Non-randomized, uncontolled study | Cell treatment =14 No Controls |
Intramyocardial injection during CABG | CD34+ (31.5±3.5×106) and CD117+ (0.61±0.1×106) cells | Doubutamine stress echocardiography | 6 weeks and 10 months | ↑ LVEF | No major complications reported |
| Improved wall motion score | ||||||||
|
| ||||||||
| Blatt et al.79 | Non-randomized, uncontolled study | Cell treatment =6 No Controls |
Intracoronary | 16.7×106 cells | Doubutamine stress echocardiography | 4 months | ↑ LVEF | No major complications reported |
| ↓ NYHA class | ||||||||
| Improved wall motion score | ||||||||
|
| ||||||||
| Assmus et al. (TOPCARECHD) 82 | Randomized, controlled study | Cell treatment =52 (28 patients BMCs, 24 patients circulating progenitor cells) Controls =23 |
Intracoronary |
BMCs: 205±110× 106 Circulating progenitor cells: 22±11×106 |
Echocardiography, SPECT, MRI | 3 months | ↑ LVEF (BMCs only) | 1 episode of ventricular arrhythmia and 5 deaths in circulating progenitor cell group |
| ↓ NYHA class (BMCs only) | ||||||||
|
| ||||||||
| Hendrikx et al.77 | Randomized, controlled trial | Cell treatment =10 Controls =10 |
Intramyocardial injection during CABG | 60±31×106 cells | MRI | 4 months | ↔ LVEF | No major complications reported |
| ↑ systolic thickening | ||||||||
| ↓ NYHA class and LVESV | ||||||||
|
| ||||||||
| Gao et al.80 | Non-randomized, contolled study | Cell treatment =14 Controls =14 |
Intracoronary | 28 to 32 ×106 cells | Echocardiography | 3 months | ↑ LVEF | No major complications reported |
| ↓ LVESV | ||||||||
|
| ||||||||
| Seth et al.86 | Pilot study | Cell treatment =24 Controls =120 |
Intracoronary | ~120 ×106 cells | Echocardiography | 3 months | ↑ LVEF | No major complications reported |
| ↓ LVESV | ||||||||
| ↓ NYHA class | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Beeres et al.76 | Non-randomized, uncontolled study | Cell treatment =15 No Controls |
Intramyocardial (Transendocardial) | 94±14×106 cells | SPECT | 3 months | ↑ LVEF | 1 death due to heart failure |
| ↓ NYHA class | ||||||||
| ↑ perfusion | ||||||||
| ↑ regional wall motion | ||||||||
|
| ||||||||
| Yao et al.85 | Randomized, placebo-controlled trial | Cell treatment =24 Controls =23 |
Intracoronary | 12×106 cells | Echocardiography, MRI, SPECT | 6 months | ↔ LVEF | No major complications reported |
| ↔ LVEDV and LVESV | ||||||||
| ↔ perfusion | ||||||||
| ↔ infarct size | ||||||||
|
| ||||||||
| Ang et al.78 | Randomized, controlled, single-blinded trial | Cell treatment =42 (21 intramyocardial, 21 intracoronary) Controls =23 |
Intramyocardial injection during CABG or Intracoronary |
Intramyocardial: 84±56×106 BMCs and 142±166× 103 CD34+/CD 177+ cells Intracoronary: 115±73×106 BMCs and 245±254×103 CD34+/CD 177+ cells |
Echocardiography, MRI | 6 months | ↔ LVEF | No major complications reported |
| ↔ LVEDV and LVESV | ||||||||
| ↔ infarct wall motion | ||||||||
| ↔ infarct size | ||||||||
|
| ||||||||
| Diederichsen et al.81 | Non-randomized, uncontolled study | Cell treatment =32 No Controls |
Repeated intracoronary |
1st infusion: 647±382×106 cells 2nd infusion: 889±361×106 cells |
Echocardiography | 12 months | ↔ LVEF | No major complications reported |
| Improved LV filling | ||||||||
|
| ||||||||
| Perin et al. (FOCUS-HF) 74 | Randomized, double-blinded, controlled study | Cell treatment =20 Controls =10 |
Intramyocardial (Transendocardial) | 30×106 cells | Echocardiography, SPECT | 6 months | ↔ LVEF | No major complications reported |
| ↓ CCSAS | ||||||||
| ↑ perfusion | ||||||||
|
| ||||||||
|
MESENCHYMAL STEM CELLS
| ||||||||
| Hare et al. (POSEIDON) 99 | Randomized pilot Study | Cell treatment =31 No controls |
Intramyocardial (Transendocardial) | Three different doses: 20, 100, 200×106 | Computed tomography | 12 months | ↔ LVEF | 1 patient in each group was hospitalized for HF |
| Improved physical performance | ||||||||
| ↓ LVEDV | ||||||||
|
| ||||||||
|
BONE MARROW PROGENITOR CELLS
| ||||||||
| Patel et al.103 | Randomized, controlled study | Cell treatment =10 Controls =10 |
Intramyocardial injection during CABG | 22×106 cells | Echocardiography, SPECT | 6 months | ↑ LVEF | No major complications reported |
|
| ||||||||
| Manginas et al.106 | Pilot, controlled study | Cell treatment =12 Controls =12 |
Intracoronary |
CD133+: 16.9±4.9× 106 cells CD133-/CD34+: 8±4×106 cells |
Echocardiography | 28±8.7 months | ↑ LVEF | 1 patient developed restenosis at the cell delivery site |
| ↓ LV remodeling | ||||||||
| ↓ LVESV and LVEDV | ||||||||
| ↑ perfusion | ||||||||
|
| ||||||||
| Stamm et al.108 | Nonrandomized, controlled study | Cell treatment =20 Controls =20 |
Intramyocardial injection during CABG | 5.8×106 cells | Echocardiography, SPECT | 6 months | ↑ LVEF | No major complications reported |
| ↑ perfusion | ||||||||
|
| ||||||||
| Fischer-Rasokat et al.87 | Pilot study | Cell treatment =33 No controls |
Intracoronary | 259±135 ×106 cells | MRI, LV angiography | 3 months, 12 months | ↑ LVEF | No major complications reported |
| Improved regional wall motion | ||||||||
|
| ||||||||
| Vrtovec et al.104 | Randomized, controlled study | Cell treatment =28 Controls =27 |
Intracoronary | 123±23×106 cells | Echocardiography | 12 months | ↑ LVEF | 5 patients died of cardiac causes and 5 patients underwent heart transplantation |
| ↑ 6MWD | ||||||||
|
| ||||||||
| Vrtovec et al.105 | Randomized, controlled study | Cell treatment =55 Controls =55 |
Intracoronary | 123±23×106 cells | Echocardiography | 5 years | ↑ LVEF | 27 patients died of cardiac causes and 9 patients underwent heart transplantation |
| ↑ 6MWD | ||||||||
|
| ||||||||
| Perin et al.109 | Randomized, controlled, double-blind study | Cell treatment =10 Controls =10 |
Intramyocardial (Transendocardial) | 2.37±1.31×106 cells | Echocardiography, SPECT | 6 months | ↓ LVEDV | No major complications reported |
| Improved maximal oxygen consumption | ||||||||
|
| ||||||||
|
CARDIAC STEM CELLS
| ||||||||
| Bolli et al. (SCIPIO) 129 | Open label, randomized, controlled study | Cell treatment =16 Controls =7 |
Intracoronary | 1×106 cells | Echocardiography, MRI | 4 and 12 months | ↑ LVEF | No major complications reported |
| ↓ infarct size | ||||||||
| ↓ NYHA class | ||||||||
|
| ||||||||
| Makkar et al. (CADUCEUS) 137 | Randomized, controlled study | Cell treatment =17 Controls =8 |
Intracoronary | 12.5-25×106 cells | MRI | 6 and 12 months | ↔ LVEF | 4 cell-treated patients had serious adverse events |
| ↔ LV volumes | ||||||||
| ↓ scar mass | ||||||||
BMC, bone marrow cell; CABG, coronary artery bypass grafting; CCSAS, Canadian Cardiovascular Society Angina Score; LV, left ventricular; LVAD, LV assist device; LVEDV, LV end-diastolic volume; LVESV, LV end-systolic volume; LVEF, LV ejection fraction; MRI, magnetic resonance imaging; MUGA, Multi gated acquisition scan; MWD, minute walk distance, NYHA, New York Heart Association; PET, positron emission tomography; SPECT, single photon emission computed tomography. ↑, increased; ↓, decreased; ↔, no change.
i) Embryonic stem cells
Embryonic stem cells (ESCs) are pluripotent cells harvested from the inner cell mass of preimplantation-stage blastocysts 7. When cultured as 3-dimensional cystic aggregates (embryoid bodies), both mouse and human (h) ESCs have the capacity to differentiate into cells of all three germ layers, namely, ectoderm, endoderm, and mesoderm (including cardiomyocytes) 8, 9. hESC-derived cardiomyocytes, which can be isolated from embryoid bodies by either mechanical dissection or enzymatic methods 10, exhibit adult cardiomyocyte morphology with properly organized sarcomeric proteins, and express cardiac-specific transcription factors such as Nkx2.5, GATA-4, and MEF2C 11. Also, they display spontaneous beating activity with characteristic atrial, ventricular, and nodal action potentials 12, 13. The strong cardiogenic potential of ESCs and the availability of hESC-derived cardiomyocytes have motivated research into their effects in HF. In the only study of these cells conducted in a large animal model to date, Menard et al. 14 reported that cardiac-committed mouse ESCs, transplanted into infarcted sheep myocardium, differentiated into cardiomyocytes and improved LV function. Similarly, using hESC-derived cardiomyocytes, Caspi et al. 15 and Cai et al. 16 reported formation of stable cardiomyocyte grafts, attenuation of LV remodeling, and improvement in LV systolic function in rat models of old MI (although in the latter study 16 they caused formation of teratomas).
Despite the well-documented capacity of ESCs for cardiac differentiation, both ethical and biological concerns have prevented their use as a treatment modality in patients. Specifically, because of their pluripotency and allogeneic nature, adoptive transfer of ESCs is plagued by teratoma formation 7,17 and graft rejection 17, two formidable problems that essentially preclude the clinical use of these cells. In contemporary clinical research, the margin of tolerance for such catastrophic effects as tumor formation is zero, and no matter how much the probability of tumors is reduced by various ESC manipulations 18-20, it is unlikely that it will be completely eliminated. One teratoma would be sufficient to halt clinical investigation of ESCs for years. On the other hand, the recent emergence of induced pluripotent stem cells (iPSCs), which have pluripotency comparable to ESCs, has provided an alternative that obviates one of the two major problems inherent in ESC-based therapies – graft rejection.
For ESCs, the chasm between promises made and results delivered has been striking. Since the late 1990s 7, these cells have been enthusiastically heralded as a major breakthrough in medicine that will usher in unprecedented opportunities for the treatment of human disease. 21-25 Despite these claims, however, no clinical trial of ESCs in cardiovascular disease has been conducted or even initiated, nor, to the best of our knowledge, is any such trial even being planned. During the same time frame, adult stem cells have been used safely in thousands of patients, with results that were sufficiently encouraging to warrant phase II and phase III trials. Clearly, the expectations raised by the advocates of ECSs have not been met. This sobering realization, coupled with the problems of tumorigenesis and rejection, makes it unlikely that enthusiasm for the therapeutic use of ESCs will continue unabated. The most reasonable interpretation of current knowledge is that ESC-based therapies have no future in terms of clinical application, at least in the next few years, and will probably become obsolete – a thing of the past, which will be remembered as an unfulfilled promise.
ii) Induced pluripotent stem cells
In 2006, Takahashi and Yamanaka 26 produced a population of iPSCs by transducing mouse adult fibroblasts with defined transcription factors (OCT3/4, Sox2, c-Myc, and Klf4) (the “Yamanaka factors”). These iPSCs express ESC surface markers and exhibit morphology and growth properties similar to those of ESCs 26. It was subsequently demonstrated that the cardiogenic potential of iPSCs is very similar to that of ESCs, and that iPSC-derived cardiomyocytes possess functional properties typical of cardiac cells, such as spontaneous beating, contractility, and ion channel expression 27. However, to date, no study has specifically assessed the therapeutic potential of iPSCs in animal models of HF.
Although iPSCs hold great promise for cardiac regeneration, the transcription factors used to generate these cells (c-Myc, Oct4, and Klf4) are known oncogenes that can produce teratomas. Newer methods that involve transient expression of the reprogramming factors may obviate this problem 28, 29, but the pluripotent nature of these cells may still promote tumorigenesis 30. Other problems include the low efficiency of iPSC generation and the variability from one cell line to another 31. Given the rapidly evolving technology in this field, it is possible that these technical hurdles will soon be overcome and iPSC-based approaches will prove to be helpful for the therapy of HF; at present, however, iPSCs are not ready for clinical application.
iii) Skeletal myoblasts
Skeletal myoblasts are derived from satellite cells, a skeletal muscle progenitor cell population present under the basal membrane of myofibers. With muscle injury, these satellite cells undergo proliferation and promote regeneration by differentiating into myotubes and new muscle fibers 32, 33. Because of their ease of procurement from muscle biopsies, rapidity of expansion in vitro, and resistance to hypoxic and ischemic conditions 34, skeletal myoblasts were the first cells to be tested both in preclinical 5 and clinical 6 studies of HF. However, myoblasts transplanted in injured hearts have been found to form skeletal (striated) muscle fibers rather than cardiac muscle 35.
The ability of skeletal myoblasts to promote cardiac repair has been evaluated in small 36, 37 and large 38-42 animals models of HF. Both after intramyocardial and intracoronary administration, these cells have been shown to differentiate into myotubes and form viable skeletal muscle-like grafts in the scarred myocardium, which was associated with attenuation of adverse ventricular remodeling, decreased interstitial fibrosis, and improvement of cardiac performance 36, 43, 44. The reduction in fibrosis has been ascribed to correction of the imbalance between matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) 45. The ability of skeletal myoblasts to improve cardiac function has also been shown in nonischemic cardiomyopathy (induced by doxorubicin and δ-sarcoglycan gene mutation in rats 46 and CHF147 Syrian hamsters, respectively 47); in both studies, intramyocardial injection of myoblasts improved LV function and decreased interstitial fibrosis. In the latter study, the benefits were ascribed to extracellular matrix (ECM) remodeling and activation of cardiac stem cells secondary to the secretion of growth factors 47.
These encouraging results from animal studies were quickly translated into clinical trials in HF. The first human transplantation of myoblasts was performed by Menasche et al. in patients with severe ischemic HF 6, 48 (Fig. 1). In this phase I study, injection of 871 million cells into a scarred LV region at the time of coronary artery bypass grafting (CABG) was associated with a significant improvement in New York Heart Association (NYHA) functional class and LV function. These observations, however, were difficult to interpret because of the confounding effects of concomitant surgical revascularization and lack of a suitable control group. Furthermore, 4 of 10 patients experienced ventricular tachycardia, warranting the use of implantable cardioverter-defibrillators (ICDs). This electrical instability has been ascribed to the lack of electromechanical coupling, due to the failure of differentiated myotubes to express key gap junction proteins such as N-cadherin and connexin-43 49.
After this trial, several small, nonrandomized studies showed augmented LV function 48, 50-59, improved LV remodeling 50, 52, 60, and histological evidence of myoblast survival in the myocardium 61 following intramyocardial injection in patients with ischemic cardiomyopathy. Based on the promising results of these studies, Menasche et al. conducted MAGIC, a phase II randomized, placebo-controlled, double-blind trial that examined the effects of intramyocardial injection of skeletal myoblasts (at two doses: 400 or 800 millions) plus CABG vs. CABG alone (controls) in 97 patients with severe LV dysfunction (LV ejection fraction [EF] between 15-35%). There were no significant differences in cardiac function and occurrence of malignant arrhythmias between patients receiving myoblasts and controls at the end of 6 months; however, in a substudy, it was found that patients treated with 800 million cells had attenuation of LV remodeling and a decrease in LV volumes 62.
Other investigators have used catheter-based intramyocardial injection of skeletal myoblasts in ischemic HF 51, 53, 55, 58, 59. A small (10 patients) phase I study of percutaneous trans-coronary-venous myoblast transplantation (the POZNAN trial) 55 reported an improvement in NYHA class and LVEF at 6 months of follow-up. Other studies in small patient cohorts by Biagini et al. 58 and Dib et al. (CAuSMIC trial) 59 reported improved NYHA functional class and increased LVEF at 1 year after therapy, but in the former study 58, the improvement in LV function was noted only during dobutamine infusion. A double-blind, randomized, placebo-controlled, multicenter study of transcatheter intramyocardial administration of myoblasts in HF (the MARVEL trial), designed to enroll 330 patients, was terminated prematurely because of financial constraints; the preliminary results in 23 patients showed improvement in 6-min walk distance at 3 and 6 months, but also an increase in the occurrence of sustained ventricular tachycardia in 7 of 15 patients 63.
The long-term effects of intramyocardial myoblast injection in patients with ischemic cardiomyopathy have been evaluated in four trials 54, 56, 57, 64 (including a follow-up of the first Menasche study56). Although in three of these trials 54, 56, 57, 64 cardiac function improved, myoblasts were transplanted during surgical revascularization (CABG) or LVAD placement, which, as pointed out above, complicates the interpretation of the outcome. In the fourth study 64, in which myoblasts were delivered percutaneously by transendocardial injection, there was no beneficial effect on global or regional LV function at 4-year follow-up. These findings are consistent with the results of the SEISMIC trial, a recent phase IIa, randomized, open-label trial of percutaneous intramyocardial transplantation of myoblasts in HF patients 65. In this study, myoblast therapy was not associated with any improvement in LVEF at 6-month follow-up, although there was an improvement in 6-min walk distance 65.
In summary, most of the smaller, nonrandomized clinical trials of skeletal myoblasts have yielded encouraging results, but the largest study to date (the MAGIC trial) failed to corroborate these findings. It must also be noted that many of these trials were performed in conjunction with CABG or LVAD procedures, making it difficult to separate the effects of myoblasts from those of revascularization. Because of the negative results of MAGIC, the risk of arrhythmias, and the availability of other cell types, interest in skeletal myoblasts has waned, and it seems unlikely that these cells will play a role in cell therapy of HF.
iv) Bone marrow-derived stem cells
The bone marrow harbors different types of hematopoietic and nonhematopoietic stem cell populations that have the potential to differentiate into diverse phenotypes (Fig. 2). Due to the relatively greater concentration of stem cells in the bone marrow and the ease of procurement of these cells, most of the preclinical and clinical studies in HF have utilized bone marrow-derived stem cells (Fig. 1, Tables 1 and 2).
a) Unfractionated bone marrow mononuclear cells
Bone marrow mononuclear cells (BMMNCs) are a heterogeneous population composed of mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), and more committed cell lineages. As BMMNCs can be easily procured using density gradient centrifugation and as these cells do not require extensive culture techniques, they have been used by many investigators in animal models of acute MI 3, 66, 67. Relatively fewer studies have been performed in the setting of chronic HF, and the results are conflicting. In sheep 68 and pig 69 models of post-infarction HF, BMMNCs (injected directly into the scar tissue) produced no improvement in LV function (although one study reported increased angiogenesis and reduction in infarct size 69). In contrast to these findings, studies in dogs (post-infarction HF) 70 and rats (cryoinjury-induced HF) 71 have reported improvement in myocardial function, reduction in plasma N-terminal pro brain natriuretic peptide (NT-proBNP) levels, and induction of angiogenesis.
Conflicting results have also been obtained in patients with HF. Perin et al. 72, 73 were the first to evaluate the safety and efficacy of autologous BMMNCs, injected transendocardially with a NOGA Myostar catheter, in patients with chronic ischemic HF (Fig. 1). At 2 and 4 months after therapy, there was a significant improvement in LVEF and a reduction in end-systolic volume in cell-treated patients 72. During longer follow-up (6 and 12 months), these patients exhibited not only improved cardiac performance, but also an increase in myocardial perfusion and exercise capacity compared with controls 73, 74. Directionally concordant observations were made by other investigators, who reported that intramyocardial injection of BMMNCs (performed during surgery 75 or percutaneously via a NOGA device 76) was associated with a decrease in HF symptoms and an improvement in LV function in patients with severe ischemic LV dysfunction. In contrast, trials using in-scar injections of BMMNCs in patients with ischemic HF failed to show improved LV function 77, 78. The reasons for these differences are not obvious; one possibility is the site of cell delivery, as in the study by Perin et al. 72, 73 cells were injected into the peri-infarct viable myocardium rather than into the scar itself.
In addition to the intramyocardial route, numerous studies have examined the effect of intracoronary infusion of BMMNCs in patients with HF, again with mixed results. A number of trials have reported an improvement in various parameters of LV function and anatomy 79-81. In the TOPCARE-CHD study, Assmus et al. 82 compared the effects of intracoronary infusion of 22±11×106 circulating EPCs or 205±110×106 BMMNCs on global LV function in 75 patients with chronic ischemic cardiomyopathy. At 3 months after therapy, LVEF improved significantly in patients receiving BMMNCs (+3.7±4.0 absolute EF units) but not in those receiving circulating EPCs (+0.4±3.0 absolute EF units) 82. This difference in response may be due to the functional impairment of circulating EPCs in chronic HF patients 83, which limits their recruitment into the scar tissue, or it may reflect the contribution of cell types other than circulating EPCs. In the TOPCARE–CHD registry, Assmus et al. 84 enrolled 121 patients with ischemic HF and reported a significant reduction of both NT-proBNP and NT-proANP serum levels and a reduction in mortality at 3 months after intracoronary infusion of BMMNCs. However, other trials have failed to confirm the beneficial effects of intracoronary delivery of BMMNCs in HF 78, 85. For example, when BMMNCs were given (intramyocardially or intracoronarily) during CABG surgery 78, there was no improvement in regional or global LV function and no reduction in scar size.
BMMNCs have also been studied in the setting of nonischemic cardiomyopathy 86, 87. In TOPCARE-DCM 87, intracoronary infusion of 259±135×106 BMMNCs in 33 patients with DCM was associated with an improvement in regional contractile and microvascular function and a decrease in NT-proBNP serum levels, suggesting a beneficial effect on LV remodeling. Interestingly, the increase of regional contractile function was directly proportional to the functionality of the infused cells as measured by their colony-forming capacity 87.
In summary, studies of BMMNC administration in patients with chronic ischemic HF have yielded inconsistent results; all of these trials, however, have been small. Larger, phase II trials are needed to achieve definitive conclusions.
b) Mesenchymal stem cells
MSCs, also known as bone marrow stromal cells, are a subset of nonhematopoietic cells that are multipotent and plastic-adherent under culture conditions. MSCs can differentiate into chondrocytes, adipocytes, osteoblasts, and skeletal muscle cells, and have also been reported to differentiate into cardiomyocytes 88, 89 and endothelial cells 90, although this cardiogenic potential remains controversial 91. MSCs typically express CD105, CD73, CD90, and STRO-1 but lack hematopoietic markers (CD45, CD34 and CD14/CD11b) 92.
The results of MSC administration in animal models of chronic HF have been encouraging. Direct epicardial injection of allogeneic MSCs in a dog model of ischemic HF induced by ameroid constriction resulted in differentiation of MSCs into smooth muscle cells and endothelial cells, increased vascularity, and improved myocardial function 93. Similarly, autologous MSCs, injected directly into a myocardial infarct scar, have been reported to attenuate LV remodeling and reduce infarct size in a swine model of ischemic cardiomyopathy 94. These data provided the groundwork for an ongoing randomized, double-blind, placebo-controlled study of autologous MSCs in patients with chronic ischemic LV dysfunction undergoing CABG (PROMETHEUS; NCT00587990) (Table 3). In rat models of both ischemic 95-97 and nonischemic 98 cardiomyopathy, intramyocardial injection of MSCs has been shown to improve cardiac function 95-98, increase angiogenesis 95, 98, and reduce myocardial fibrosis 96, 98. To date, the only clinical study that has examined the effects of MSCs in patients with HF is the POSEIDON trial by Hare et al. 99, which compared three doses of autologous or allogeneic MSCs (20, 100 and 200 × 106 cells) in patients with ischemic cardiomyopathy and demonstrated that all doses favorably affected patient functional capacity, quality of life, and ventricular remodeling (Table 2).
Table 3.
Ongoing clinical trials of stem cell therapy in heart failure registered at clinicaltrials.gov (April 2013)
| Trial Design Phase and title | Cell type | Status | Design | Estimated patient enrollment | Delivery Method | Reference |
|---|---|---|---|---|---|---|
|
Phase I/II; Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) |
Autologous MSCs | Active, not recruiting | Randomized, double-blinded, placebo-controlled | 45 | Intramyocardial | NCT 00587990 |
|
Phase I/II; The Transendocardial Autologous Cells (Hmsc or Hbmc) in Ischemic Heart Failure Trial (TAC-HFT) |
Autologous hMSC or hBMC | Recruiting | Randomized, double-blinded, placebo-controlled | 67 | Intramyocardial (Transendocardial) | NCT 00768066 |
|
Phase I/II; The Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (POSEIDON-DCM) |
Autologous MSCs Allogenic MSCs | Recruiting | Randomized, open-label, pilot study | 36 | Intramyocardial (Transendocardial) | NCT 01392625 |
|
Phase I/II; Autologous Mesenchymal Stromal Cell Therapy in Heart Failure |
Mesenchymal stromal cells | Recruiting | Randomized controlled | 60 | Intramyocardial | NCT 00644410 |
|
Phase II; A Phase II Dose-escalation Study to Assess the Feasibility and Safety of Transendocardial Delivery of Three Different Doses of Allogeneic Mesenchymal Precursor Cells (MPCs)in Subjects With Heart Failure (REVASCOR) |
Mesenchymal Precursor Cells | Active, not recruiting | Dose-escalation study | 60 | Intramyocardial (Transendocardial) | NCT 00721045 |
|
Phase II; Safety and Efficacy Study of Intramyocardial Stem Cell Therapy in Patients With Dilated Cardiomyopathy (NOGA-DCM) |
Autologous BM-HSCs (CD34+ cells) | Recruiting | Randomized, double-blinded, placebo-controlled | 60 | Intramyocardial | NCT 01350310 |
|
Phase I; Cardiac Stem cell Infusion in Patients with Ischemic Cardiomyopathy (SCIPIO) |
c-kit+ Cardiac Progenitor Cells | Active, not recruiting | Randomized, open-label | 33 | Intracoronary | NCT 00474461 |
|
Phase I/II; Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration (ALLSTAR) |
Cardiosphere-Derived Cells (CDCs) | Recruiting | Randomized, double-blind, placebo-controlled | 274 | Intracoronary | NCT 01458405 |
|
Phase III; Safety and Efficacy of Autologous Cardiopoietic Cells for Treatment of Ischemic Heart Failure (CHART-1) |
Bone Marrow-derived Mesenchymal Cardiopoietic Cells (C3BS-CQR-1) | Recruiting | Randomized, double-blind, placebo-controlled | 240 | Intramyocardial | NCT 01768702 |
|
Phase II; An Efficacy, Safety and Tolerability Study of Ixmyelocel-T Administered Via Transendocardial Catheter-based Injections to Subjects With Heart Failure Due to Ischemic Dilated Cardiomyopathy (ixCELL DCM) |
Bone marrow-derived cells, including primarily CD90+ MSCs, CD14+ monocytes and alternatively activated macrophages | Recruiting | Randomized, double-blind, placebo-controlled | 108 | Intramyocardial (Transendocardial) | NCT 01670981 |
BMC, bone marrow cell; BMMNCC, bone marrow mononuclear cell; CSC, cardiac stem cell; MSC, mesenchymal stem cell.
c) Hematopoietic stem cells and endothelial progenitor cells
HSCs reside in the bone marrow and differentiate into cells of both myeloid and lymphoid lineages. EPCs, on the other hand, are mobilized into peripheral blood in response to ischemic injury and promote neovascularization by differentiating into endothelial cells (reendothelialization) 100, 101. CD34 is a typical surface marker of both HSCs and EPCs 102. Thus, CD34+ cells are found in the bone marrow and in the peripheral blood and have the potential to give rise to all blood cell types as well as endothelial cells (<1% of nucleated cells in the blood are CD34+).
Autologous CD34+ cell transplantation has been performed in patients with both ischemic 103 and nonischemic 104, 105 cardiomyopathy (Fig. 1). In the former setting, injection of CD34+ cells into the peri-infarct, viable LV regions during off-pump CABG surgery produced a greater improvement in contractile function than did CABG alone 103. Also, a small pilot study evaluating the safety and feasibility of intracoronary CD133+ or CD133-, CD34+ cell therapy in patients with old anterior MI reported a sustained improvement in regional perfusion and LV remodeling with both cell types 106. In the setting of nonischemic cardiomyopathy, a study by Vrtovec et al. concluded that intracoronary infusion of CD34+ cells led to an increase in LVEF and 6-min walk distance and a decrease in NT-proBNP levels 104. Importantly, these beneficial effects were sustained during long-term follow-up 105. Another surface marker of HSCs and EPCs is CD133 (AC133) 107. Stamm et al. 108 examined the effects of CD133+ cells, given by intramyocardial injection during CABG, in patients with ischemic HF. At 6 months after treatment, LVEF and perfusion of the infarcted myocardium increased to a greater extent in patients who received CABG and CD133+ therapy than in those who received CABG alone.
Recently, Perin et al. 109 investigated a novel population of hematopoietic cells, referred to as aldehyde dehydrogenase-bright (ALDHbr) cells, in 20 patients with ischemic HF (10 control and 10 treated). ALDHbr cells, which have been isolated from human bone marrow and peripheral blood, express CD34, CD117, CD105, CD133, and CD166 and include primitive CD34+/CD38- cells 110. Transendocardial delivery of ALDHbr cells produced a significant decrease in LV end-systolic volume at 6 months and a trend toward improved maximal oxygen consumption 109.
In summary, the initial experience with CD34+ and CD133+ cells in HF (both of ischemic and nonischemic origin) is encouraging but limited by the small size of the trials. As is the case for other cells, larger studies will be necessary to evaluate the role of these cell types in the treatment of HF.
v) Adipose-derived MSCs
Adipose tissue contains a pool of multipotent stem cells, designated as adipose-derived MSCs that are able to replicate as undifferentiated cells, to develop as mature adipocytes, and to differentiate into other cell types along the mesenchymal lineage. Reports that adipose-derived MSCs can differentiate into cardiomyocytes 111 and endothelial cells 112 have motivated studies in animal models of HF. Using a cell sheet technology, Miyahara et al. 113 reported that transplantation of monolayered MSCs into scarred myocardium reversed wall thinning in the scar area and improved cardiac function. In another study 114, the effects of transplanting undifferentiated or cardiac pre-differentiated adipose-derived MSCs were compared with those of BMMNCs in a rat model of chronic MI. One month after transplantation, adipose-derived MSCs induced an improvement in LVEF, an increase in angiogenesis, and a decrease in fibrosis that were significantly greater than those effected by adipose-derived cardiomyogenic cells or BMMNCs 114. Additionally, intramyocardial injection of adipose stem cells at 1 week after coronary occlusion has been reported to mitigate the deterioration in cardiac contractile function and enhance angiogenesis in infarcted rat hearts 115.
In the clinical arena, no full report of adipose-derived MSCs in HF is available yet. The preliminary results of the PRECISE trial by Perin et al. 116 in 27 patients indicate that administration of adipose-derived cells resulted in stabilization of infarct size and improvement in maximal oxygen consumption.
vi) Cardiac stem cells
One of the most dramatic developments in the history of cardiac biology has been the recent recognition that the adult heart undergoes a continuous turnover of its cellular components (including myocytes) 117. This process is thought to be underlain by a population of resident stem cells that possess the capacity to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells 117 (Fig. 2). The discovery that the heart is a self-renewing organ has not only refuted the long-held doctrine that the myocardium is a postmitotic tissue (composed of cells that have withdrawn from the cell cycle and are terminally differentiated), but has also opened exciting therapeutic avenues.
a) c-kit+ Cardiac stem cells
In 2003, Beltrami et al. described a population of cells isolated from the adult rat heart that expressed the tyrosine kinase receptor c-kit (a marker of stemness) but lacked any markers of hematopoietic lineage 118. These c-kit+ cardiac stem cells (CSCs) were shown to be self-renewing, clonogenic, and multipotent, exhibiting the ability to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells both in vitro and in vivo 118-120. Four years later, a similar population of c-kit+ CSCs was identified in the adult human heart 120. Injection of human CSCs into infarcted rodent myocardium resulted in improvement of LV function and structure and formation of a chimeric heart that contained human myocardium composed of myocytes and coronary vessels 120.
In the past decade, the ability of human and rodent CSCs to alleviate LV dysfunction and remodeling and promote regeneration has been repeatedly demonstrated by several laboratories in various preclinical animal models of acute MI 119, 121-124. Evidence that ischemic cardiomyopathy is associated with loss of functionally competent CSCs 125 has ignited interest in investigating the effects of CSCs in the setting of chronic HF as well. Intramyocardial injection of c-kit+ CSCs at the borders of an infarct 20 days after a permanent coronary occlusion in rats was reported to result in replacement of ~42% of the scar with new myocardium, attenuation of LV dilation, and preservation of LV function 126. However, in contemporary medicine, most infarcts are reperfused. Furthermore, from a practical standpoint, the technique most conducive to widespread use of CSCs in patients with HF would be intracoronary delivery. To address these issues, Tang et al. 127 investigated whether administration of CSCs is effective in regenerating cardiac tissue and alleviating postinfarction LV remodeling and dysfunction when these cells are infused intracoronarily in the setting of an old MI produced by a temporary coronary occlusion followed by reperfusion. One month after coronary occlusion/reperfusion, rats received an intracoronary infusion of vehicle or EGFP-labeled CSCs. Thirty-five days later, CSC-treated rats exhibited more viable myocardium in the risk region, less fibrosis in the noninfarcted region, and improved LV function 127. However, the number of EGFP+ cells expressing markers of cardiogenic commitment was too small to account for the augmentation of LV function (EGFP+ cells accounted for only 2.6±1.1% of the region at risk and 1.1±0.4% in the noninfarcted region). These observations suggest that an important mechanism whereby CSCs produced their salutary effects was the secretion of cytokines/growth factors that exerted paracrine actions on endogenous cells, particularly endogenous CSCs, which in turn proliferated and differentiated into adult cardiac cells. In support of this hypothesis was the finding that the pool of endogenous CSCs expanded to a greater degree in CSC-treated than in control rats 127.
The efficacy of CSCs in chronic ischemic cardiomyopathy 126, 127 was surprising, as a scar would seem to be a very hostile environment to the homing and survival of transplanted cells, and the signals (adhesion molecules and growth factors) that attract and activate CSCs soon after ischemia-reperfusion would be expected to have largely abated once the healing process is complete. To verify these rat findings 126, 127 in a large, clinically-relevant species, a similar study was performed in pigs that underwent a 90-min coronary occlusion followed by reperfusion 128. At the time of occlusion, the right atrial appendage was harvested for isolation and expansion of c-kit+ CSCs; 3 months after MI, 1 million autologous CSCs were infused into the infarct-related artery using a balloon catheter. Similar to the results obtained in rats, one month later the pigs treated with CSCs exhibited an increase in LVEF and systolic thickening fraction in the infarcted LV wall, as well as a decrease in LV end-diastolic pressure (LVEDP) and an increase in LV dP/dtmax 128. The encouraging results of these studies of intracoronary CSC infusion in the setting of an old MI 127, 128 laid the groundwork for SCIPIO, the first clinical trial of CSCs (Fig. 1).
SCIPIO was a phase I, randomized, open-label trial of autologous CSCs for the treatment of ischemic HF. The target population consisted of patients with LVEF ≤40% who underwent CABG. Approximately 4 months after CABG, 1 million autologous CSCs (isolated and expanded from myocardial tissue harvested during surgery) were administered by intracoronary infusion; controls were not given any treatment. Although the two-year follow-up has not been completed, the interim results are very encouraging 129, 130. In 20 CSC-treated patients, LVEF (measured by 3-D echo) increased from 29.0 ± 1.7% before CSC infusion to 36.0 ± 2.5% at 4 months after infusion. By contrast, in 13 control subjects, LVEF did not change. The salubrious effects of CSCs persisted and, if anything, became even more pronounced at 1 year (LVEF: +8.1% vs. baseline, n=17) and 2 years (LVEF: +12.9%, n=8) 131. In nine CSC-treated patients in which MRI could be performed, there was a profound reduction in infarct size at 4 months (from 34.9±2.3 to 21.6±2.7 g [-38.1%]) and even more at 1 year (from 33.9±3.0 to 18.7±3.6 g [-44.8%]) 129. These salubrious effects were associated with a significant improvement in the NYHA functional class and in the quality of life (measured by the Minnesota Living with Heart Failure Questionnaire).
Aside from the setting of ischemic cardiomyopathy, CSCs have also been found to exert salutary effects in a rat model of anthracycline-induced cardiomyopathy 132.
In summary, several studies have documented the ability of CSCs to promote regeneration and alleviate LV dysfunction and remodeling in various preclinical models of post-MI cardiomyopathy. The results of the first clinical trial (SCIPIO) are consistent with this preclinical work and suggest that intracoronary infusion of autologous CSCs results in a substantial and sustained improvement in LV systolic function, in a reduction in infarct size, and in clinical improvement in patients with ischemic HF. These promising observations warrant larger, phase II studies. It is important to note that although in SCIPIO CSCs were isolated from the right atrial appendage, it is now possible to isolate and expand these cells from endomyocardial biopsy specimens 133, which makes the use of autologous CSCs potentially applicable to most patients with HF.
b) Cardiospheres and cardiosphere-derived cells
Cardiospheres were first described by Messina et al. 134 in 2004. Using subcultures of atrial or ventricular human biopsy samples and murine hearts, these authors isolated a population of cells that grew as self-adherent clusters and could differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells. Messina et al. termed these clusters “cardiospheres” 134. Three years later, Smith et al. 135 presented a method in which cardiospheres obtained from percutaneous endomyocardial biopsy specimens were plated to yield cardiosphere-derived cells (CDCs). These CDCs were reported to differentiate into electrically stable cardiomyocytes in vitro and, when injected into a murine infarct model, to promote cardiac regeneration and improved cardiac function 135. In 2009, Johnston et al. reported that intracoronary delivery of human CDCs in pigs with old MI resulted in cardiac regeneration, reduction in “relative” infarct size, attenuation of adverse LV remodeling, and improvement in cardiac function 136.
Phenotypically, cardiospheres and CDCs are a heterogeneous mixture of many different cell types, including cells that express endothelial (KDR [human]/ flk-1 [mouse], CD31), stem cell (CD34, c-kit, Sca-1), and mesenchymal (CD105, CD90) antigenic markers 134 (Fig. 2). Which of these cells type(s) is responsible for the observed effects on cardiac function and remodeling is unknown. In CADUCEUS, 98% of CDCs infused were positive for CD105, suggesting a mesenchymal nature 137. In a recent study by the same group 138, the safety and efficacy of direct intramyocardial injection of CDCs and cardiospheres were compared in a porcine model of post-MI HF; although CDCs and cardiospheres had equivalent effects on LVEF, the latter were superior in improving hemodynamics and regional function and in mitigating ventricular remodeling. The enhanced potency of cardiospheres for myocardial repair has been attributed to enhanced “stemness” and cell-matrix interactions 139.
This preclinical work was translated by Makkar et al. 137 into a phase I, randomized trial (CADUCEUS) in patients with a recent MI and LVEF≤45% but ≥25%. At 1.5-3 months after MI, 17 patients received an intracoronary infusion of escalating doses of autologous CDCs (12.5, 17.3, or 25 million cells), which were produced from an endomyocardial biopsy. (However, the amount of tissue used to produce CDCs was reported to be 276 mg [SD 177, range 93–891 mg] 137, which is all but impossible to obtain with endomyocardial biopsies.) Eight control patients received standard care. In two patients, CDCs were found to be aneuploid (trisomy 8) and had to be discarded. At 12 months of follow-up, CDC-treated patients exhibited a 42% reduction in scar size (from 24% to 12% of the left ventricle), concomitant with an increase in viable tissue and regional systolic wall thickening in the infarcted region. However, CDC therapy failed to increase LVEF, reduce LV volumes, and improve NYHA functional class or quality of life as assessed with the MLHFQ 137. Although the increase in non-gadolinium enhanced tissue in CDC-treated patients was claimed to be proof of cardiac regeneration 137, it could also be accounted for by other changes unrelated to regeneration, such as hypertrophy, decreased interstitial space, reduced vascular permeability, and/or improved perfusion 140-144.
In summary, CDCs are a mixture of different cell types (predominantly expressing mesenchymal markers) that have been reported to promote regeneration and alleviate post-MI dysfunction and remodeling in various preclinical models 135, 136, 138, 145, 146. The clinical effects of CDCs are unclear. The MRI data reported in CADUCEUS are consistent with regeneration (but they do not prove it); however, evidence that CDCs have beneficial effects on global LV function and clinical status is still lacking. Given the heterogeneous nature of this cell preparation, it will be difficult to identify which component(s) accounts for the salubrious effects. As is the case of c-kit+ CSCs, larger Phase II studies are needed to evaluate the therapeutic potential of CDCs.
c) Other cardiac progenitor cells
Sca-1+ cardiac stem cells
The existence of Sca-1+ progenitors in the adult mouse heart was reported by Oh et al. 147. These cells expressed CD31 and cardiogenic transcription factors (GATA-4, MEF2C, and MEF-1) but lacked blood lineage markers, c-kit, Flt-1, Flk-1, vascular endothelial cadherin, von Willebrand factor, and hematopoietic stem cell markers (CD45 and CD34) 147. In vitro, Sca-1+ cells have the ability to express cardiac structural genes and differentiate into beating cardiomyocytes upon treatment with 5-azacytidine 147 and oxytocin 148 Transplantation of Sca-1+ cells into the peri-infarct and infarct zones in a murine model of MI resulted in endothelial and cardiomyogenic differentiation of these cells with attenuation of LV remodeling 149. However, the effects of these cells in the setting of chronic HF remain to be determined; further, the lack of a human homologue of Sca-1 makes translation difficult.
Side population cells
The so-called side population (SP) cells are characterized by their ability to exclude the Hoechst 33342 dye via the ATP binding transporters Bcrp1/Abcg2 and MDR1 150. First identified in murine bone marrow as HSCs 151, SP cells were subsequently isolated by Martin et al. 152 from adult as well as embryonic mouse hearts and characterized as CD31-, Sca-1high, c-kitlow, CD34low, and CD45low. Although cardiac SP cells have been reported to differentiate into mature cardiomyocytes, endothelial cells, and smooth muscle cells and to regenerate cryoinjured myocardium 153, their ability to induce cardiac repair has not been tested.
Islet-1+ cells
During cardiogenesis, Isl-1+ cells give rise to cardiac muscle, the conduction system, and endothelial and smooth muscle cells in the heart compartments 154. Laugwitz et al. 155 proposed that Isl-1+ cells represent endogenous cardiac progenitors that display conversion to a mature cardiac phenotype, with intact calcium dynamics and action potentials 155; however, the ability of these cells to repair injured myocardium in vivo has never been demonstrated. Importantly, these cells do not exist in the postnatal ventricular myocardium, either under normal conditions or after MI, making it unlikely that they serve as cardiac progenitors or will have any clinical application 156.
Potential mechanisms of actions of stem cells in HF
Taken together, the studies reviewed above (Tables 1 and 2) suggest that at least some types of cell therapy are likely to improve cardiac function in chronic HF. What remains largely unknown, however, is the mechanism(s) responsible for these beneficial effects. Below we discuss briefly the various hypotheses that have been proposed (Fig. 3).
Figure 3. Potential mechanisms of action of stem cells.
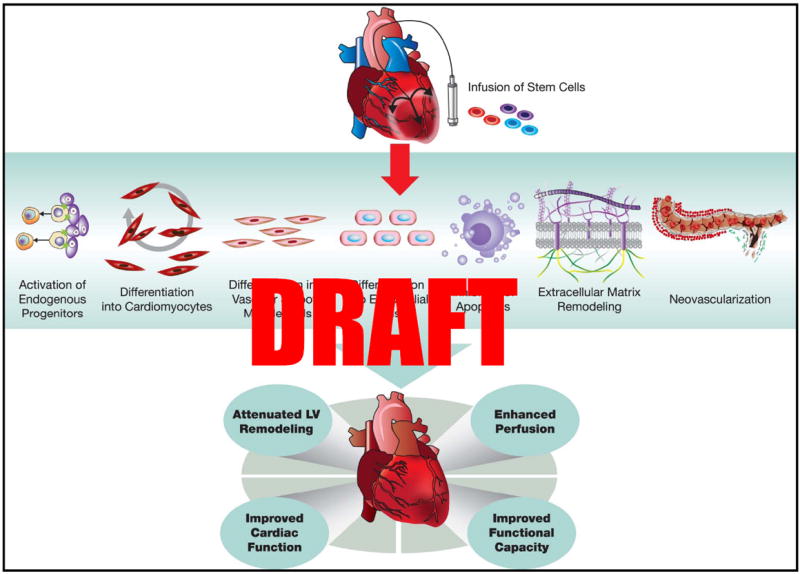
Implantation of stem cells in the injured heart initiates myocardial repair via several direct and indirect mechanisms: activation of endogenous precursors, differentiation into cardiac and vascular cells, promotion of neovascularization, favorable modulation of the extracellular matrix, and inhibition of apoptosis. Together these events reduce adverse cardiac remodeling and hypertrophy, increase perfusion, and improve cardiac function, leading to improvement in clinical status. (Illustration Credit: Ben Smith)
(Trans)differentiation of transplanted cells into cardiac cells
Although this may seem the most obvious explanation for the salubrious effects of stem cells, the evidence obtained thus far does not support (trans)differentiation of transplanted cells as the only, or even the major, mechanism of action. As mentioned above, Reinecke et al. 157 found that transplanted skeletal myoblasts differentiate into skeletal muscle fibers and do not express cardiac-specific genes. Transdifferentiation of bone marrow cells into cardiac myocytes remains highly controversial, with studies both supporting 3, 71, 158 and refuting 66, 67 this concept. Others have suggested fusion of bone marrow cells with resident cardiomyocytes as the responsible mechanism 159, 160, but this has also been refuted 161, 162. Similarly, transdifferentiation of human peripheral blood CD34+ cells into cardiomyocytes and vascular smooth muscle cells remains controversial 163, 164. Although the therapeutic benefits of MSCs have been ascribed to differentiation towards cardiac and vascular lineages 88, 89, 98, 165, most studies have not supported this concept, suggesting instead that the major actions of MSCs are paracrine 166-168.
A similar uncertainty applies to cardiac-derived cells. As discussed above, CSCs are multipotent, being able to differentiate into myocytes, endothelial cells, and vascular smooth muscle cells in vitro 118. When transplanted in injured hearts, CSCs give rise to vascular cells and to cells that express myocyte-specific proteins (although these cells are usually small and do not resemble adult myocytes) 121, 124, 126-128. In some studies, particularly in models of acute MI, the magnitude of this regenerative process has been found to be substantial 118, 119, 169, 170. However, in a rat 127 and pig 128 model of chronic post-MI HF, differentiation of transplanted CSCs into myocytes or myocyte-like cells was quantitatively insufficient to account for the improvement in LV function. In the case of CDCs, differentiation into cardiac cells has been reported to be either a minor mechanism of action 171 or non-existent 172, 173.
In summary, differentiation of transplanted cells along the cardiac lineage may occur. However, the key issue is the magnitude of this phenomenon vis-à-vis the improvement in function. In most of the studies reported to date, the functional benefits appear to be disproportionate to the relatively small number of new cardiac cells formed by differentiation of transplanted cells; consequently, the former cannot be accounted for solely by the latter. Other mechanisms must be at work.
Formation of new blood vessels from transplanted cells
Differentiation of transplanted cells into new blood vessels has been reported with various cells (e.g., MSCs 93, adipose-derived cells 174, 175, CD34+ cells 176, 177 and CSCs 118, 178). Experimentally, this phenomenon may be important in models of chronic coronary occlusion, which can be associated with the presence of ischemic but viable myocardium 118, 119, 169, 170, but not in models in which the artery that supplies the infarcted/scarred myocardium is patent 127, 128. Clinically, formation of new vessels may contribute to improved cardiac performance in some patients with ischemic heart disease, but it is difficult to envision how it could do so in the setting of nonischemic cardiomyopathy or in patients with ischemic heart disease who do not have flow-limiting coronary lesions (e.g., revascularized patients).
Paracrine mechanisms
The inability to explain the salutary effects of transplanted stem cells on the basis of their differentiation has led to the “paracrine hypothesis” 167, that is, the concept that transplanted cells induce myocardial repair by releasing signals (cytokines, chemokines, growth factors, possibly exosomes or microparticles) into the surrounding tissue, which in turn promote a number of restorative processes including activation of endogenous CSCs, neovascularization, inhibition of apoptosis, inhibition of hypertrophy, and favorable alterations of the ECM. Collectively, these actions result in enhanced LV function, improved perfusion, and myocardial repair 167.
Activation of endogenous CSCs. In the aforementioned study by Tang et al. 127 in a rat model of chronic HF, infusion of exogenous CSCs was found to promote proliferation of endogenous CSCs in both the infarcted and noninfarcted regions, suggesting that activation of the endogenous pool of CSCs via paracrine mechanisms was a major mechanism of benefit. It is known that CSCs secrete growth factors (such as hepatocyte growth factor [HGF] and insulin growth factor-1 [IGF-1]) that stimulate other CSCs to migrate through the myocardial interstitium, proliferate, and differentiate into myocytes and vascular structures. 126, 168. Activation of endogenous CSCs has also been suggested to be an important mechanism underlying the beneficial effects of other cell types, including MSCs 168.
Induction of neovascularization. Many stem cells can induce neovascularization by secreting chemokines (stromal cell-derived factor-1 [SDF-1]) 70, 179, 180 and proangiogenic factors (vascular endothelial growth factor [VEGF]), basic fibroblast growth factor [FGF], HGF, IGF-1, tissue growth factor-β [TGF-β], and angiopoietin-1) 45, 98, 181, 182. EPCs recruited to the ischemic area can also secrete the endothelial and inducible isoforms of nitric oxide synthase (eNOS and iNOS) and promote proliferation of endothelial cells 183. The resulting neovascularization may improve blood supply to the viable cells that remain in the infarcted region and thus improve cardiac function in settings of chronic coronary occlusion; as mentioned above, however, this mechanism would not account for improved function in experimental models of reperfused infarction, where no residual ischemia is present, or in patients without persistent ischemia.
Inhibition of apoptosis. A number of studies suggest that paracrine factors (such as IGF-1) released by stem cells following transplantation inhibit cardiomyocyte death by apoptosis (e.g., 98). In vitro and in vivo data in models of acute MI suggest that Akt overexpressing MSCs decrease cardiomyocyte apoptosis 167, 182. Combined transplantation of skeletal myoblasts and AC133+ cells was also reported to improve cardiac function by reducing myocardial apoptosis 44.
Inhibition of hypertrophy. Administration of stem cells in models of HF is associated with a reduction in the hypertrophic response of surviving myocytes 36, 71, 95, 126, 127. It remains uncertain, however, whether this is a primary action of transplanted cells or it is secondary to improved cardiac performance.
Remodeling of the extracellular matrix. Stem cells can modulate various constituents of the ECM, thereby limiting infarct expansion, LV remodeling, and myocardial fibrosis. Skeletal myoblasts have been reported to preserve matrix collagen architecture 36, to reduce fibrosis in the peri-infarct and infarct-remote regions, 37 and to modulate MMP-2 and TIMP-4 levels 45, suggesting a favorable effect on the ECM metabolism. The importance of ECM alterations in CSC-dependent repair is underscored by the findings of Rota et al. 126, who reported that CSCs increased MMP-2, MMP-9, and MMP-14 levels and decreased TIMP-4 levels in a rat model of post-MI HF.
Cell fusion
In 2004, spontaneous cell fusion was proposed as an alternative mechanism by which transplanted bone marrow cells produce apparent regeneration of various adult tissues 66, 67, 160. This concept was based on work by Alvarez-Dolado et al., who used a method based on Cre-Lox recombination for detecting cell fusion events of bone marrow cells with cardiomyocytes 159. Subsequent studies 161, 162, however, concluded that c-kit+ bone marrow cells differentiated into myocytes and coronary vessels independent of cell fusion. The use of Cre-Lox recombination as an appropriate model to study cell fusion has been challenged, as the unmodified Cre-recombinase in the progenitor cells can cross the membrane of the recipient cell 184, thus mimicking cell fusion. The notion that cells fusion is an important mechanism underlying the salubrious effects of stem cells has lost support in recent years.
Current challenges, unresolved issues, and future directions
Taken together, the preclinical and clinical work performed to date suggests that administration of stem cells has considerable potential to improve cardiac function and regenerate viable myocardium in HF. Despite these encouraging results, however, no cell type has been conclusively demonstrated to be effective in alleviating HF in patients. It is clear that in order to unleash the full potential of cell-based therapies and proceed toward clinical translation, a number of major unresolved issues will have to be resolved; for example, what are the optimal cell type(s), the optimal cell dose, the optimal route of cell administration, and the optimal frequency of treatment? These questions can be answered only by conducting careful preclinical and clinical studies.
Unfortunately, the current environment does not support studies that compare cells, doses, routes of administration, and frequency of treatment. At the preclinical level, this type of work is likely to receive low priority scores by peer review groups because it is, by definition, descriptive and lacks mechanistic insights and conceptual novelty. In the clinical arena, comparisons of different cell types or doses are expensive and time-consuming. It is hoped that sponsors and funding agencies will recognize that this type of research is indispensable to translate cell base-therapies to humans and will identify it as a priority for funding.
i) Cell type
It is unknown which, among the many different types of stem/progenitor cells that have been studied to date (Tables 1 and 2), is most effective in a given pathophysiological setting. Despite the obvious importance of this question, very few studies have directly compared different cell types with respect to the outcomes of therapy 45, 70, 97, 185. Such studies are difficult because they require that the dose-response relationships for each cell type be defined and compared (as simply comparing one dose of cells would be inadequate). This has not been done heretofore. For example, the claim that CDCs are “superior” to CSCs is untenable because it is predicated upon the use of one dose of cells 185. Similarly, the few studies that have compared different cell types 45, 70, 97 have not evaluated the dose-response relationships for each cell type.
A related and unresolved issue is whether combinations of different cell types may be more efficacious than a single cell type. Theoretical considerations, as well as preclinical studies of BMMNCs, skeletal myoblasts 44, 186, 187, MSCs, and CSCs 188, suggest that the former approach may offer advantages, as the actions of different cells may be complementary or even synergistic 188.
ii) Cell dose
It is evident from Tables 1 and 2 that the doses of cells used to treat chronic HF have varied enormously. Although it seems obvious that the effects of cell-based therapies will depend on the number of cells administered, the nature of this relationship is still unknown for most cell types. In the clinical realm, only two studies have addressed the dose-dependency of the effects of stem cells in HF. In the MAGIC trial 62, a higher dose (8 × 106) of skeletal myoblasts was more effective in decreasing LV volumes and reversing LV remodeling than a low dose (4 × 106), although neither dose improved LV function. In the POSEIDON trial, Hare et al. 99 compared three doses of autologous or allogeneic MSCs (20, 100, and 200 × 106 cells) in patients with ischemic cardiomyopathy and demonstrated that all doses favorably affected patient functional capacity, quality of life, and ventricular remodeling, although 200 × 106 was (unexpectedly) less effective than 20 × 106. These results differ from those obtained by these investigators in a swine model of ischemic cardiomyopathy, in which both a high dose (200 × 106 cells) and a low dose (20 × 106 cells) of MSCs increased regional function, but only the high dose effected reverse remodeling 94. To address this important issue, an ongoing phase II dose-escalation study (REVASCOR) is assessing the feasibility and safety of transendocardial delivery of three doses of allogeneic mesenchymal precursor cells (25, 75, 150 × 106 cells) in patients with HF (NCT00721045) (Table 3). Similar studies of the dose-response relationship are needed for other cell types.
iii) Route of administration
As is the case for the optimal cell type and dose, the most effective technique to deliver cells to the heart is still unknown. The major routes used to date are direct injection into the LV wall (transendocardially or transepicardially) and intracoronary infusion. Transepicardial injection is performed during cardiac surgery 54, 62; this method offers direct visualization of the scarred regions but is limited by the requirement for surgery. With transendocardial injection, cells can be delivered directly into the LV wall by using an injection catheter advanced across the aortic valve and positioned against the endocardial surface. The advantages of this technique over intracoronary infusion are that: i) electromechanical mapping of the endocardial surface with a NOGA system can be used to trace viable, ischemic, and scarred myocardium, thereby enabling targeted injection of cells into the scar or into the border zone, and ii) cells can be delivered to a scarred region even if the coronary artery supplying it is totally occluded. Because of these advantages, transendocardial injection has been used extensively in the clinical arena 51, 53, 58, 59, 63-65, 72-74, 76. However, intramyocardial injections may disrupt tissue architecture and create cell clumps that lack adequate blood supply, resulting in cell death. Further, the distribution of cells within the infarcted region is usually inhomogeneous 124, 189.
Intracoronary delivery involves the infusion of cells into a coronary artery, usually during a brief coronary occlusion produced by inflating a balloon at the tip of the catheter. The rationale for stopping flow is to prevent the rapid “washout” of the cells and to facilitate their extravasation into the interstitium. Compared with transendocardial injection, intracoronary delivery offers several advantages: i) it results in a much more uniform distribution of cells within the infarcted region 124, ii) it does not require specialized training or the purchase of specialized equipment, and iii) it is technically easier, and therefore more practical for widespread utilization in clinical practice. The widespread distribution of cells within the infused vascular bed has also the theoretical advantage of enabling them to “decide where to go” in response to local cues. However, intracoronary delivery has also certain disadvantages vs. transendocardial injection: i) the immediate retention of cells is lower 190, 191 (e.g., 2.6±0.3% after intracoronary infusion compared with 11±3% after intramyocardial injection 192), presumably because of rapid wash out of cells, ii) microvascular occlusion can occur when large cells, such as MSCs (10-20 μm) 193, 194, skeletal myoblasts (~20 μm) 195, and CDCs (~21 μm) 134, 136, 139 are infused (this problem is not encountered when smaller cells, such as CSCs and BMMNCs, are used), and iii) delivery of cells to a myocardial region supplied by an occluded artery is not possible.
To date, relatively few studies have compared different routes of cell delivery 37, 41, 78, 124, 191, 194, 196-198, with discrepant results. None of them has used a range of doses, which, as discussed above, is necessary to achieve valid conclusions. Comparisons of the intracoronary and transendocardial delivery routes in large animal models using a range of doses of cells are needed to resolve this issue.
iv) Frequency of administration
There is no a priori reason to posit that the effects of a single cell administration cannot be improved by a repeated administration. Most stem cells can be frozen, stored, and re-used at a later time. Consequently, it seems rather curious that almost every study performed heretofore has used a single injection of cells to determine whether this therapy is efficacious in HF. This would be tantamount to determining the effect of an antibiotic on an infectious disease by giving only one dose. The lack of studies evaluating repeated cell injections is all the more perplexing when one considers that there is evidence suggesting a dose-dependent response relationship between number of cells injected and functional benefit 62, 94, as discussed above. The effects of stem cells in HF patients should not be labeled as “negative”, “modest”, or “small” on the basis of the results obtained with a single treatment; in our opinion, the effects of repeated administrations of stem cells need to be compared with those of a single administration, lest a cell therapy may be inappropriately dismissed as ineffective.
The few available data do support the concept that repeated injections of cells are more efficacious than a single injection. In animal models of old MI, repeated injections of skeletal myoblasts were more effective than single injections in increasing LVEF 42, 199 and vasculogenesis and in decreasing fibrosis 42. Clearly, further studies are necessary to determine the relationship between the number/frequency of cells administered and their effects on cardiac function.
While it is appreciated that the issues discussed above (items i-iv) are not conceptually challenging, it is our opinion that they have enormous practical importance and need to be addressed. It is unlikely that optimal clinical application of cell therapy will be achieved until we have an answer to these questions.
v) Cell retention, survival, long-term engraftment, and lineage commitment
Stem cell studies have consistently shown very low rates of long-term cell engraftment: regardless of cell type, dose, and mode of delivery, more than 90% of injected cells disappear in the first few days and <2% can still be found 4 weeks after transplantation 200, 201. This massive cell loss is the result of two sequentially distinct events. During or immediately after delivery, there is significant loss due to failure of cells to extravasate (intracoronary infusion) or leakage through transepicardial/transendocardial puncture holes coupled with removal through the venous system (intramyocardial injection). For example, in the acute phase of MI, only ~10% of CSCs 201 and <10% of MSCs 202 were found in the myocardium 24 hours after intramyocardial injection in mice and only 2–5% of BMMNCs a few hours after intracoronary infusion in humans 203. In a porcine model of cardiopulmonary bypass, only 10% of epicardially injected microspheres approximating the size of MSCs were retained within the sites of injection after 30 min 204. Then, during the first weeks after transplantation, most of the cells that were initially retained die because of ischemia caused by poor vascularization of the injected region, inflammation with attendant oxidative stress and release of cytotoxic cytokines, immune destruction of allogeneic cells, and apoptosis following disengagement of anchorage-dependent cells from their extracellular matrix (anoikis).
Clearly, the massive loss of transplanted cells is a major unresolved problem that limits the efficacy of any type of cell therapy. Improving cell homing, survival, and engraftment in the hostile ischemic environment is therefore important for optimizing therapeutic benefits. Several strategies are currently under investigation, including pretreatment of the target tissue, ex vivo pretreatment of cells (genetic modifications205; physical or pharmacologic preconditioning), and implantation of cells included in scaffolds made of biocompatible matrix. Pretreatment of the host tissue has been accomplished with ultrasound-mediated destruction of microbubbles in the coronary circulation (which improves recruitment of BMMNCs and MSCs, probably by creating capillary pores 206, 207) and extracorporeal shock wave treatment (which has shown benefit in patients with ischemic heart failure receiving intracoronary BMMNCs in the CELLWAVE trial 208). With regard to ex vivo pretreatment of stem cells, many promising strategies have emerged. One is the overexpression of antiapoptotic genes, such as heme oxygenase 1, Bcl-2, Akt, or Pim-1, which has been shown to increase the survival and function of MSCs 202, 209, 210 and CSCs 122 including their capacity to secrete paracrine mediators 209. Augmenting either the expression of SDF-1 in the myocardium or that of its receptor, CXCR4, on stem cells increases cell recruitment 205, 211, 212. Preconditioning EPCs with antibodies, HMGB-1, or small molecules increases their neovascularization capacity by activating β2 integrins 213, 214. Similarly, preconditioning human EPCs and BMMNCs with the endothelial nitric oxide synthase transcription enhancer AVE9488 improves their migratory and neovascularization potential 215. Many studies have found that preconditioning MSCs and EPCs with simulated ischemia upregulates prosurvival, angiogenic, and migratory proteins, such as HIF-1α, Akt-1, Bcl-2, Ang-1, VEGF, as well as the receptors CXCR4 and c-Met, and imparts beneficial effects 212, 216, 217. Preconditioning human CSCs with the HO-1 inducer CoPP significantly enhances their resistance to apoptosis 218.
The importance of promoting the lineage commitment of transplanted cells is illustrated by the recently reported C-CURE (Cardiopoietic stem Cell therapy in heart failure) trial, in which lineage specification of MSCs was achieved by exposing them to a cardiogenic cocktail regimen that triggered expression and nuclear translocation of cardiac transcription factors; in this study, administration of autologous bone marrow-derived mesenchymal cardiopoietic cells was found to effect favorable LV remodeling and improve cardiac function in patients with ischemic HF 219.
Embedding cells in natural (e.g. matrigel, collagen, fibrin, alginate) or synthetic (e.g. peptide nanofibers) biomaterials is another means of enhancing stem cell function. Biomaterials promote cell engraftment, retention, and differentiation because of their low viscosity and their similarity to myocardial extracellular matrix, which preserves cell-to-matrix signals 220. The two main approaches in cardiac tissue engineering are in vitro engineering, which consists of seeding cells on pre-formed porous scaffolds that are cultivated in vitro and then applied on the epicardial surface, and in vivo engineering, in which a mixture of biomaterials and cells is injected and the formation of a biocomplex occurs in situ 221, 222. Conceptually, biomaterials could be designed to release growth factors in a controlled manner that promotes survival and engraftment of cells, and also guides cell phenotype decisions 221, 222.
In summary, improving cell survival and engraftment is crucial to the progress of cell therapy and thus should be a high priority area for research. The strategies summarized above (pretreatment of target tissue, pretreatment of cells, embedding cells in a matrix) are not mutually exclusive and may have additive or even synergistic effects.
Ongoing clinical trials
At the time of this writing, ClinicalTrials.gov lists ten clinical trials that are testing the safety and efficacy of stem cells in HF patients (Table 3).
To evaluate the effects of intramyocardial injection of BMMNCs and MSCs in patients with ischemic cardiomyopathy, three phase I/II randomized, double-blind, placebo-controlled trials are being conducted at the University of Miami. The primary end-point of Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) is to test the safety of intramyocardial injection of autologous human MSCs in patients with chronic MI undergoing CABG. The Transendocardial Autologous Cells (hMSCs or hBMCs) in Ischemic Heart Failure Trial (TAC-HFT) is directly comparing hMSCs and hBMMNCs in a prospective manner. The recently published preliminary data from the phase I pilot study of TAC-HFT suggest that transendocardial injection of autologous bone marrow progenitor cells (hMSCs or hBMMNCs) improves regional contractility in a myocardial scar and reverse LV remodeling 223, 224. Owing to the absence of major histocompatibility complex class II, MSCs are immunoprivileged and suppress T-cell proliferation. These cells are being evaluated in the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (POSEIDON-DCM), which is comparing allogeneic MSCs with autologous MSCs in patients with nonischemic dilated cardiomyopathy. In the early-stage study of patients with ischemic cardiomyopathy, POSEIDON demonstrated that transendocardial injection of allogeneic and autologous MSCs favorably affected patient functional capacity, quality of life, and ventricular remodeling 99.
Cardio3 BioSciences is currently recruiting patients in its phase III trial (CHART-1) to examine autologous bone marrow-derived mesenchymal cardiopoietic cells (C3BS-CQR-1) in patients with chronic HF. In this study, the investigators are using a unique ‘cardiopoietic cocktail’ of growth factors (transforming growth factor-β1, bone morphogenetic protein-4, activin A, retinoic acid, insulin-like growth factor-1, fibroblast growth factor-2, alpha-thrombin, and interleukin-6), which has been reported to engage MSCs to differentiate into cardiac stem cells 225. Using a patient-specific multicellular therapy expanded from a small sample of a patient’s own bone marrow, Aastrom Biosciences is using Ixmyelocel-T (primarily CD90+ MSCs, CD14+ monocytes and alternatively activated macrophages) to evaluate the efficacy, safety and tolerability of transendocardial injection in subjects with HF due to ischemic dilated cardiomyopathy. The NOGA-DCM (Safety and Efficacy Study of Intramyocardial Stem Cell Therapy in Patients with Dilated Cardiomyopathy) study is using CD34+ cells in HF patients. This study is being conducted by Dr. Vrtovec’s group, who has recently demonstrated that intracoronary stem cell transplantation is associated with improved ventricular function, exercise tolerance, and long-term survival (up to 5-years) in patients with dilated cardiomyopathy 105. NOGA-DCM is designed to directly compare the effects of intracoronary and intramyocardial stem cell delivery in non-ischemic dilated cardiomyopathy at 1-year follow-up. Aside from these studies using bone marrow-derived cells, ALLSTAR (Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration), sponsored by Capricor Inc., is a phase I/II study that tests the safety and efficacy of intracoronary delivery of allogeneic CDCs in patients with an anterior MI and HF.
Conclusions
When considering the current status of cell-based therapies for HF, it is important to keep a historical perspective. We are still at the dawn of the era of regenerative medicine. Only 15 years ago, suggesting that it was possible to regenerate dead myocardium would have been considered science fiction. Notwithstanding the many mechanistic, pathophysiological, and practical issues that remain unresolved, it is important to remember that tremendous progress has been made in a relatively short time. Many promising candidates for cell therapy have been identified, both in experimental animals and in humans, and several studies are ongoing in patients with chronic HF (Fig. 1, Tables 1-3). Never has an idea been translated from preclinical models to humans so quickly. Importantly, cell therapy appears to be safe – to date, no adverse effect of stem/progenitor cells has been reported.
It is true that the precise mechanism of action of stem cells remains unclear, and their efficacy in HF has not been proven. But wouldn’t it be surprising if a conclusive answer to these complex questions had been achieved in just a decade? How long did it take for reperfusion therapy to become a routine part of the management of acute MI? And do we understand the mechanism of action of all therapies that we use daily? We must not succumb to irrational impatience or premature nihilism. When a novel therapy comes along, the clinical trials conducted in the first few years are generally small and inconclusive. This has indeed been the case for stem cells in HF; nevertheless, the results are encouraging, and the therapy appears safe. What is important now is: i) to resolve issues concerning optimal cell type, dosage, and route and timing of administration, and ii) to proceed with rigorous, large-scale, rationally-designed, randomized clinical trials. With this approach, we believe that cell-based therapies are likely to become a clinical reality that may revolutionize the management of HF.
Acknowledgments
We gratefully acknowledge Ms. Heather L. Jones for expert assistance with graphics design.
Sources of Funding
Work discussed in this paper was supported in part by NIH grants R01-HL-68088, HL-70897, HL-76794, HL-78825, HL-55757, HL-74351, and HL-91202.
Nonstandard Abbreviations and Acronyms
- ALDHbr
aldehyde dehydrogenase-bright
- BMMNCs
Bone marrow mononuclear cells
- CABG
coronary artery bypass grafting
- CDCs
cardiosphere-derived cells
- CSCs
cardiac stem cells
- ECM
extracellular matrix
- EPCs
endothelial progenitor cells
- ESCs
embryonic stem cells
- FGF
fibroblast growth factor
- HF
Heart failure
- HGF
hepatocyte growth factor
- HSCs
hematopoietic stem cells
- ICD
implantable cardioverter-defibrillators
- IGF-1
insulin growth factor-1
- IPSCs
induced pluripotent stem cells
- LV
left ventricular
- LVEDP
LV end-diastolic pressure
- MMPs
matrix metalloproteinases
- MSCs
mesenchymal stem cells
- NYHA
New York Heart Association
- NT-proBNP
N-terminal pro brain natriuretic peptide
- SDF-1
stromal cell-derived factor-1
- TIMPs
tissue inhibitors of matrix metalloproteinases
- TGF-β
tissue growth factor-β
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None.
References
- 1.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 6.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 7.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 9.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 12.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 13.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: A molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 14.Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, Bel A, Messas E, Bissery A, Bruneval P, Desnos M, Puceat M, Menasche P. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 15.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 16.Cai J, Yi FF, Yang XC, Lin GS, Jiang H, Wang T, Xia Z. Transplantation of embryonic stem cell-derived cardiomyocytes improves cardiac function in infarcted rat hearts. Cytotherapy. 2007;9:283–291. doi: 10.1080/14653240701247838. [DOI] [PubMed] [Google Scholar]
- 17.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 18.Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells. 2003;21:257–265. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 19.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M, Sasai Y, Hashimoto N. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- 21.Gearhart J. New human embryonic stem-cell lines--more is better. N Engl J Med. 2004;350:1275–1276. doi: 10.1056/NEJMp048045. [DOI] [PubMed] [Google Scholar]
- 22.Daley GQ. Missed opportunities in embryonic stem-cell research. N Engl J Med. 2004;351:627–628. doi: 10.1056/NEJMp048200. [DOI] [PubMed] [Google Scholar]
- 23.Okie S. Stem-cell politics. N Engl J Med. 2006;355:1633–1637. doi: 10.1056/NEJMp068206. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz RS. The politics and promise of stem-cell research. N Engl J Med. 2006;355:1189–1191. doi: 10.1056/NEJMp068180. [DOI] [PubMed] [Google Scholar]
- 25.Cohen IG, Adashi EY. Human embryonic stem-cell research under siege--battle won but not the war. N Engl J Med. 2011;364:e48. doi: 10.1056/NEJMp1105088. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Pfannkuche K, Liang H, Hannes T, Xi J, Fatima A, Nguemo F, Matzkies M, Wernig M, Jaenisch R, Pillekamp F, Halbach M, Schunkert H, Saric T, Hescheler J, Reppel M. Cardiac myocytes derived from murine reprogrammed fibroblasts: Intact hormonal regulation, cardiac ion channel expression and development of contractility. Cell Physiol Biochem. 2009;24:73–86. doi: 10.1159/000227815. [DOI] [PubMed] [Google Scholar]
- 28.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riggs JW, Barrilleaux BL, Varlakhanova N, Bush KM, Chan V, Knoepfler PS. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev. 2013;22:37–50. doi: 10.1089/scd.2012.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma T, Xie M, Laurent T, Ding S. Progress in the reprogramming of somatic cells. Circ Res. 2013;112:562–574. doi: 10.1161/CIRCRESAHA.111.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckingham M, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev. 2008;18:330–336. doi: 10.1016/j.gde.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Chachques JC, Acar C, Herreros J, Trainini JC, Prosper F, D’Attellis N, Fabiani JN, Carpentier AF. Cellular cardiomyoplasty: Clinical application. Ann Thorac Surg. 2004;77:1121–1130. doi: 10.1016/j.athoracsur.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 35.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farahmand P, Lai TY, Weisel RD, Fazel S, Yau T, Menasche P, Li RK. Skeletal myoblasts preserve remote matrix architecture and global function when implanted early or late after coronary ligation into infarcted or remote myocardium. Circulation. 2008;118:S130–137. doi: 10.1161/CIRCULATIONAHA.107.757617. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima S, Coppen SR, Lee J, Yamahara K, Felkin LE, Terracciano CM, Barton PJ, Yacoub MH, Suzuki K. Choice of cell-delivery route for skeletal myoblast transplantation for treating post-infarction chronic heart failure in rat. PLoS One. 2008;3:e3071. doi: 10.1371/journal.pone.0003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghostine S, Carrion C, Souza LC, Richard P, Bruneval P, Vilquin JT, Pouzet B, Schwartz K, Menasche P, Hagege AA. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation. 2002;106:I131–136. [PubMed] [Google Scholar]
- 39.Chachques JC, Duarte F, Cattadori B, Shafy A, Lila N, Chatellier G, Fabiani JN, Carpentier AF. Angiogenic growth factors and/or cellular therapy for myocardial regeneration: A comparative study. J Thorac Cardiovasc Surg. 2004;128:245–253. doi: 10.1016/j.jtcvs.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 40.He KL, Yi GH, Sherman W, Zhou H, Zhang GP, Gu A, Kao R, Haimes HB, Harvey J, Roos E, White D, Taylor DA, Wang J, Burkhoff D. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J Heart Lung Transplant. 2005;24:1940–1949. doi: 10.1016/j.healun.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Gavira JJ, Perez-Ilzarbe M, Abizanda G, Garcia-Rodriguez A, Orbe J, Paramo JA, Belzunce M, Rabago G, Barba J, Herreros J, Panizo A, de Jalon JA, Martinez-Caro D, Prosper F. A comparison between percutaneous and surgical transplantation of autologous skeletal myoblasts in a swine model of chronic myocardial infarction. Cardiovasc Res. 2006;71:744–753. doi: 10.1016/j.cardiores.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Gavira JJ, Nasarre E, Abizanda G, Perez-Ilzarbe M, de Martino-Rodriguez A, Garcia de Jalon JA, Mazo M, Macias A, Garcia-Bolao I, Pelacho B, Martinez-Caro D, Prosper F. Repeated implantation of skeletal myoblast in a swine model of chronic myocardial infarction. Eur Heart J. 2010;31:1013–1021. doi: 10.1093/eurheartj/ehp342. [DOI] [PubMed] [Google Scholar]
- 43.Atkins BZ, Lewis CW, Kraus WE, Hutcheson KA, Glower DD, Taylor DA. Intracardiac transplantation of skeletal myoblasts yields two populations of striated cells in situ. Ann Thorac Surg. 1999;67:124–129. doi: 10.1016/s0003-4975(98)01197-7. [DOI] [PubMed] [Google Scholar]
- 44.Bonaros N, Rauf R, Wolf D, Margreiter E, Tzankov A, Schlechta B, Kocher A, Ott H, Schachner T, Hering S, Bonatti J, Laufer G. Combined transplantation of skeletal myoblasts and angiopoietic progenitor cells reduces infarct size and apoptosis and improves cardiac function in chronic ischemic heart failure. J Thorac Cardiovasc Surg. 2006;132:1321–1328. doi: 10.1016/j.jtcvs.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 45.Shintani Y, Fukushima S, Varela-Carver A, Lee J, Coppen SR, Takahashi K, Brouilette SW, Yashiro K, Terracciano CM, Yacoub MH, Suzuki K. Donor cell-type specific paracrine effects of cell transplantation for post-infarction heart failure. J Mol Cell Cardiol. 2009;47:288–295. doi: 10.1016/j.yjmcc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki K, Murtuza B, Suzuki N, Smolenski RT, Yacoub MH. Intracoronary infusion of skeletal myoblasts improves cardiac function in doxorubicin-induced heart failure. Circulation. 2001;104:I213–217. doi: 10.1161/hc37t1.094929. [DOI] [PubMed] [Google Scholar]
- 47.Pouly J, Hagege AA, Vilquin JT, Bissery A, Rouche A, Bruneval P, Duboc D, Desnos M, Fiszman M, Fromes Y, Menasche P. Does the functional efficacy of skeletal myoblast transplantation extend to nonischemic cardiomyopathy? Circulation. 2004;110:1626–1631. doi: 10.1161/01.CIR.0000142861.55862.15. [DOI] [PubMed] [Google Scholar]
- 48.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 49.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol. 2000;149:731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herreros J, Prosper F, Perez A, Gavira JJ, Garcia-Velloso MJ, Barba J, Sanchez PL, Canizo C, Rabago G, Marti-Climent JM, Hernandez M, Lopez-Holgado N, Gonzalez-Santos JM, Martin-Luengo C, Alegria E. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J. 2003;24:2012–2020. doi: 10.1016/j.ehj.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzezniczak J, Rozwadowska N, Kurpisz M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase i clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 53.Ince H, Petzsch M, Rehders TC, Chatterjee T, Nienaber CA. Transcatheter transplantation of autologous skeletal myoblasts in postinfarction patients with severe left ventricular dysfunction. J Endovasc Ther. 2004;11:695–704. doi: 10.1583/04-1386R.1. [DOI] [PubMed] [Google Scholar]
- 54.Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, Binkley P, Buchele D, Anand I, Swingen C, Di Carli MF, Thomas JD, Jaber WA, Opie SR, Campbell A, McCarthy P, Yeager M, Dilsizian V, Griffith BP, Korn R, Kreuger SK, Ghazoul M, MacLellan WR, Fonarow G, Eisen HJ, Dinsmore J, Diethrich E. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: Four-year follow-up. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 55.Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kalmucki P, Kurpisz M. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: The poznan trial. Eur Heart J. 2005;26:1188–1195. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- 56.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P. Skeletal myoblast transplantation in ischemic heart failure: Long-term follow-up of the first phase i cohort of patients. Circulation. 2006;114:I108–113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 57.Gavira JJ, Herreros J, Perez A, Garcia-Velloso MJ, Barba J, Martin-Herrero F, Canizo C, Martin-Arnau A, Marti-Climent JM, Hernandez M, Lopez-Holgado N, Gonzalez-Santos JM, Martin-Luengo C, Alegria E, Prosper F. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J Thorac Cardiovasc Surg. 2006;131:799–804. doi: 10.1016/j.jtcvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 58.Biagini E, Valgimigli M, Smits PC, Poldermans D, Schinkel AF, Rizzello V, Onderwater EE, Bountioukos M, Serruys PW. Stress and tissue doppler echocardiographic evidence of effectiveness of myoblast transplantation in patients with ischaemic heart failure. Eur J Heart Fail. 2006;8:641–648. doi: 10.1016/j.ejheart.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Dib N, Dinsmore J, Lababidi Z, White B, Moravec S, Campbell A, Rosenbaum A, Seyedmadani K, Jaber WA, Rizenhour CS, Diethrich E. One-year follow-up of feasibility and safety of the first u.S., randomized, controlled study using 3-dimensional guided catheter-based delivery of autologous skeletal myoblasts for ischemic cardiomyopathy (causmic study) JACC Cardiovasc Interv. 2009;2:9–16. doi: 10.1016/j.jcin.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Zhang F, Chen Y, Yang Z, Gao X, Ma W, Li C, Kao RL. Cellular cardiomyoplasty for a patient with heart failure. Cardiovasc Radiat Med. 2003;4:43–46. doi: 10.1016/s1522-1865(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 61.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 62.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The myoblast autologous grafting in ischemic cardiomyopathy (magic) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 63.Povsic TJ, O’Connor CM, Henry T, Taussig A, Kereiakes DJ, Fortuin FD, Niederman A, Schatz R, Spencer Rt, Owens D, Banks M, Joseph D, Roberts R, Alexander JH, Sherman W. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J. 2011;162:654–662 e651. doi: 10.1016/j.ahj.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 64.Veltman CE, Soliman OI, Geleijnse ML, Vletter WB, Smits PC, ten Cate FJ, Jordaens LJ, Balk AH, Serruys PW, Boersma E, van Domburg RT, van der Giessen WJ. Four-year follow-up of treatment with intramyocardial skeletal myoblasts injection in patients with ischaemic cardiomyopathy. Eur Heart J. 2008;29:1386–1396. doi: 10.1093/eurheartj/ehn171. [DOI] [PubMed] [Google Scholar]
- 65.Duckers HJ, Houtgraaf J, Hehrlein C, Schofer J, Waltenberger J, Gershlick A, Bartunek J, Nienaber C, Macaya C, Peters N, Smits P, Siminiak T, van Mieghem W, Legrand V, Serruys PW. Final results of a phase iia, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: The seismic trial. EuroIntervention. 2011;6:805–812. doi: 10.4244/EIJV6I7A139. [DOI] [PubMed] [Google Scholar]
- 66.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 67.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 68.Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, Hagege AA, Menasche P. Transplantation of autologous fresh bone marrow into infarcted myocardium: A word of caution. Circulation. 2003;108(Suppl 1):II247–252. doi: 10.1161/01.cir.0000089040.11131.d4. [DOI] [PubMed] [Google Scholar]
- 69.Waksman R, Fournadjiev J, Baffour R, Pakala R, Hellinga D, Leborgne L, Yazdi H, Cheneau E, Wolfram R, Seabron R, Horton K, Kolodgie F, Virmani R, Rivera E. Transepicardial autologous bone marrow-derived mononuclear cell therapy in a porcine model of chronically infarcted myocardium. Cardiovasc Radiat Med. 2004;5:125–131. doi: 10.1016/j.carrad.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, Metens T, da Costa AM, Mahmoudabady M, Egrise D, Blocklet D, Mazouz N, Naeije R, Heyndrickx G, McEntee K. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg. 2009;138:646–653. doi: 10.1016/j.jtcvs.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 71.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 72.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 73.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FO, Assad JA, Carvalho AC, Branco RV, Rossi MI, Dohmann HJ, Willerson JT. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:II213–218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 74.Perin EC, Silva GV, Henry TD, Cabreira-Hansen MG, Moore WH, Coulter SA, Herlihy JP, Fernandes MR, Cheong BY, Flamm SD, Traverse JH, Zheng Y, Smith D, Shaw S, Westbrook L, Olson R, Patel D, Gahremanpour A, Canales J, Vaughn WK, Willerson JT. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (focus-hf) Am Heart J. 2011;161:1078–1087 e1073. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 75.Galinanes M, Loubani M, Davies J, Chin D, Pasi J, Bell PR. Autotransplantation of unmanipulated bone marrow into scarred myocardium is safe and enhances cardiac function in humans. Cell Transplant. 2004;13:7–13. doi: 10.3727/000000004772664842. [DOI] [PubMed] [Google Scholar]
- 76.Beeres SL, Bax JJ, Dibbets-Schneider P, Stokkel MP, Fibbe WE, van der Wall EE, Schalij MJ, Atsma DE. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. Am J Cardiol. 2007;100:1094–1098. doi: 10.1016/j.amjcard.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 77.Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation. 2006;114:I101–107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 78.Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT, Galinanes M. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during cabg versus cabg alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 79.Blatt A, Cotter G, Leitman M, Krakover R, Kaluski E, Milo-Cotter O, Resnick IB, Samuel S, Gozal D, Vered Z, Slavin S, Shapira MY. Intracoronary administration of autologous bone marrow mononuclear cells after induction of short ischemia is safe and may improve hibernation and ischemia in patients with ischemic cardiomyopathy. Am Heart J. 2005;150:986. doi: 10.1016/j.ahj.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 80.Gao LR, Wang ZG, Zhu ZM, Fei YX, He S, Tian HT, Zhang NK, Chen Y, Xu HT, Yang Y. Effect of intracoronary transplantation of autologous bone marrow-derived mononuclear cells on outcomes of patients with refractory chronic heart failure secondary to ischemic cardiomyopathy. Am J Cardiol. 2006;98:597–602. doi: 10.1016/j.amjcard.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 81.Diederichsen AC, Moller JE, Thayssen P, Videbaek L, Saekmose SG, Barington T, Kassem M. Changes in left ventricular filling patterns after repeated injection of autologous bone marrow cells in heart failure patients. Scand Cardiovasc J. 2010;44:139–145. doi: 10.3109/14017430903556294. [DOI] [PubMed] [Google Scholar]
- 82.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 83.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. Cd34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 84.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schachinger V, Dimmeler S, Zeiher AM. Transcoronary transplantation of functionally competent bmcs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: Results of the topcare-chd registry. Circ Res. 2007;100:1234–1241. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 85.Yao K, Huang R, Qian J, Cui J, Ge L, Li Y, Zhang F, Shi H, Huang D, Zhang S, Sun A, Zou Y, Ge J. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–1153. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]
- 86.Seth S, Narang R, Bhargava B, Ray R, Mohanty S, Gulati G, Kumar L, Reddy KS, Venugopal P. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: Clinical and histopathological results: The first-in-man abcd (autologous bone marrow cells in dilated cardiomyopathy) trial. J Am Coll Cardiol. 2006;48:2350–2351. doi: 10.1016/j.jacc.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 87.Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, Schachinger V, Tonn T, Martin H, Dimmeler S, Zeiher AM. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: Final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail. 2009;2:417–423. doi: 10.1161/CIRCHEARTFAILURE.109.855023. [DOI] [PubMed] [Google Scholar]
- 88.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 89.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 90.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. The international journal of biochemistry & cell biology. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 94.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu JF, Wang BW, Hung HF, Chang H, Shyu KG. Human mesenchymal stem cells improve myocardial performance in a splenectomized rat model of chronic myocardial infarction. J Formos Med Assoc. 2008;107:165–174. doi: 10.1016/S0929-6646(08)60130-8. [DOI] [PubMed] [Google Scholar]
- 96.Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep. 2009;36:725–731. doi: 10.1007/s11033-008-9235-2. [DOI] [PubMed] [Google Scholar]
- 97.Mazo M, Gavira JJ, Abizanda G, Moreno C, Ecay M, Soriano M, Aranda P, Collantes M, Alegria E, Merino J, Penuelas I, Garcia Verdugo JM, Pelacho B, Prosper F. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19:313–328. doi: 10.3727/096368909X480323. [DOI] [PubMed] [Google Scholar]
- 98.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 99.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 102.Krause DS, Fackler MJ, Civin CI, May WS. Cd34: Structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 103.Patel AN, Geffner L, Vina RF, Saslavsky J, Urschel HC, Jr, Kormos R, Benetti F. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: A prospective randomized study. J Thorac Cardiovasc Surg. 2005;130:1631–1638. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 104.Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, Haddad F, Torre-Amione G. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail. 2011;17:272–281. doi: 10.1016/j.cardfail.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary cd34+ stem cell transplantation in non-ischemic dilated cardiomyopathy patients: 5-year follow up. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 106.Manginas A, Goussetis E, Koutelou M, Karatasakis G, Peristeri I, Theodorakos A, Leontiadis E, Plessas N, Theodosaki M, Graphakos S, Cokkinos DV. Pilot study to evaluate the safety and feasibility of intracoronary cd133(+) and cd133(-) cd34(+) cell therapy in patients with nonviable anterior myocardial infarction. Catheter Cardiovasc Interv. 2007;69:773–781. doi: 10.1002/ccd.21023. [DOI] [PubMed] [Google Scholar]
- 107.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. Ac133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 108.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of cd133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 109.Perin EC, Silva GV, Zheng Y, Gahremanpour A, Canales J, Patel D, Fernandes MR, Keller LH, Quan X, Coulter SA, Moore WH, Herlihy JP, Willerson JT. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase-bright stem cells in patients with ischemic heart failure. Am Heart J. 2012;163:415–421. 421 e411. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 110.Gentry T, Deibert E, Foster SJ, Haley R, Kurtzberg J, Balber AE. Isolation of early hematopoietic cells, including megakaryocyte progenitors, in the aldh-bright cell population of cryopreserved, banked uc blood. Cytotherapy. 2007;9:569–576. doi: 10.1080/14653240701466347. [DOI] [PubMed] [Google Scholar]
- 111.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 112.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 113.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 114.Mazo M, Planat-Benard V, Abizanda G, Pelacho B, Leobon B, Gavira JJ, Penuelas I, Cemborain A, Penicaud L, Laharrague P, Joffre C, Boisson M, Ecay M, Collantes M, Barba J, Casteilla L, Prosper F. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 115.Wang L, Deng J, Tian W, Xiang B, Yang T, Li G, Wang J, Gruwel M, Kashour T, Rendell J, Glogowski M, Tomanek B, Freed D, Deslauriers R, Arora RC, Tian G. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: An mr imaging study of rat hearts. Am J Physiol Heart Circ Physiol. 2009;297:H1020–1031. doi: 10.1152/ajpheart.01082.2008. [DOI] [PubMed] [Google Scholar]
- 116.Perin ECS PL, Ruiz RS, Perez-Cano R, Lasso J, Alonso-Farto JC, Fernandez-Pina L, Serruys PW, Duckers HJ, Kastrup J, Chameleau S, Zheng Y, Silva GV, Milstein AM, Martin MT, Willerson JT, Aviles FF. First in man transendocardial injection of autologous adipose-derived stem cells in patients with non revascularizable ischemic myocardium (precise) Circulation. 2010;122:A17966. [Google Scholar]
- 117.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: A paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 119.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res. 2011;108:1226–1237. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O’Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–864. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Late-breaking clinical trial abstracts. Circulation. 2012;126:2776–2799. doi: 10.1161/CIR.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 132.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.D’Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, Welt FG, Givertz MM, Mitchell RN, Leri A, Kajstura J, Pfeffer MA, Anversa P. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 135.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 136.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 1077 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R, Marban E. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 139.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, Marban E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced mri. Potential mechanisms. Circulation. 1995;92:1117–1125. doi: 10.1161/01.cir.92.5.1117. [DOI] [PubMed] [Google Scholar]
- 141.Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006–1014. doi: 10.1161/01.cir.98.10.1006. [DOI] [PubMed] [Google Scholar]
- 142.Saeed M, Lund G, Wendland MF, Bremerich J, Weinmann H, Higgins CB. Magnetic resonance characterization of the peri-infarction zone of reperfused myocardial infarction with necrosis-specific and extracellular nonspecific contrast media. Circulation. 2001;103:871–876. doi: 10.1161/01.cir.103.6.871. [DOI] [PubMed] [Google Scholar]
- 143.Debl K, Djavidani B, Buchner S, Lipke C, Nitz W, Feuerbach S, Riegger G, Luchner A. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: Visualisation of focal fibrosis. Heart. 2006;92:1447–1451. doi: 10.1136/hrt.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Breuckmann F, Mohlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jockel KH, Heusch G, Erbel R, Barkhausen J. Myocardial late gadolinium enhancement: Prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–57. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 145.Carr CA, Stuckey DJ, Tan JJ, Tan SC, Gomes RS, Camelliti P, Messina E, Giacomello A, Ellison GM, Clarke K. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks--an mri study. PLoS One. 2011;6:e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye J, Boyle A, Shih H, Sievers RE, Zhang Y, Prasad M, Su H, Zhou Y, Grossman W, Bernstein HS, Yeghiazarians Y. Sca-1+ cardiosphere-derived cells are enriched for isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One. 2012;7:e30329. doi: 10.1371/journal.pone.0030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 149.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/cd31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 150.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The abc transporter bcrp1/abcg2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 151.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the atp-binding cassette transporter, abcg2, identifies cardiac sp cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 153.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: A single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- 155.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weinberger F, Mehrkens D, Friedrich FW, Stubbendorff M, Hua X, Muller JC, Schrepfer S, Evans SM, Carrier L, Eschenhagen T. Localization of islet-1-positive cells in the healthy and infarcted adult murine heart. Circ Res. 2012;110:1303–1310. doi: 10.1161/CIRCRESAHA.111.259630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 158.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 160.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 161.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 162.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood cd34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 164.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood cd34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 165.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 166.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: Potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S21–26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 167.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 170.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 171.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO molecular medicine. 2012 doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 175.Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. Ifats collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Botta R, Gao E, Stassi G, Bonci D, Pelosi E, Zwas D, Patti M, Colonna L, Baiocchi M, Coppola S, Ma X, Condorelli G, Peschle C. Heart infarct in nod-scid mice: Therapeutic vasculogenesis by transplantation of human cd34+ cells and low dose cd34+kdr+ cells. FASEB J. 2004;18:1392–1394. doi: 10.1096/fj.03-0879fje. [DOI] [PubMed] [Google Scholar]
- 177.Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, Bankson JA, Shpall E, Willerson JT, Gelovani JG, Yeh ET. Human cd34+ cells in experimental myocardial infarction: Long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–1911. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 180.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 181.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 182.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 183.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Will E, Klump H, Heffner N, Schwieger M, Schiedlmeier B, Ostertag W, Baum C, Stocking C. Unmodified cre recombinase crosses the membrane. Nucleic Acids Res. 2002;30:e59. doi: 10.1093/nar/gnf059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ott HC, Bonaros N, Marksteiner R, Wolf D, Margreiter E, Schachner T, Laufer G, Hering S. Combined transplantation of skeletal myoblasts and bone marrow stem cells for myocardial repair in rats. Eur J Cardiothorac Surg. 2004;25:627–634. doi: 10.1016/j.ejcts.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 187.Memon IA, Sawa Y, Miyagawa S, Taketani S, Matsuda H. Combined autologous cellular cardiomyoplasty with skeletal myoblasts and bone marrow cells in canine hearts for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:646–653. doi: 10.1016/j.jtcvs.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 188.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: Promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mitchell AJ, Sabondjian E, Sykes J, Deans L, Zhu W, Lu X, Feng Q, Prato FS, Wisenberg G. Comparison of initial cell retention and clearance kinetics after subendocardial or subepicardial injections of endothelial progenitor cells in a canine myocardial infarction model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:413–417. doi: 10.2967/jnumed.109.069732. [DOI] [PubMed] [Google Scholar]
- 191.George JC, Goldberg J, Joseph M, Abdulhameed N, Crist J, Das H, Pompili VJ. Transvenous intramyocardial cellular delivery increases retention in comparison to intracoronary delivery in a porcine model of acute myocardial infarction. Journal of interventional cardiology. 2008;21:424–431. doi: 10.1111/j.1540-8183.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 2005;112:I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 193.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 194.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 195.Suzuki K, Brand NJ, Smolenski RT, Jayakumar J, Murtuza B, Yacoub MH. Development of a novel method for cell transplantation through the coronary artery. Circulation. 2000;102:III359–364. doi: 10.1161/01.cir.102.suppl_3.iii-359. [DOI] [PubMed] [Google Scholar]
- 196.Vela DC, Silva GV, Assad JA, Sousa AL, Coulter S, Fernandes MR, Perin EC, Willerson JT, Buja LM. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009;57:167–176. doi: 10.1369/jhc.2008.952507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van der Spoel TI, Vrijsen KR, Koudstaal S, Sluijter JP, Nijsen JF, de Jong HW, Hoefer IE, Cramer MJ, Doevendans PA, van Belle E, Chamuleau SA. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: A study on delivery efficiency. J Cell Mol Med. 2012;16:2768–2776. doi: 10.1111/j.1582-4934.2012.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hong SJ, Hou D, Brinton TJ, Johnstone B, Feng D, Rogers P, Fearon WF, Yock P, March KL. Intracoronary and retrograde coronary venous myocardial delivery of adipose-derived stem cells in swine infarction lead to transient myocardial trapping with predominant pulmonary redistribution. Catheter Cardiovasc Interv. 2012 doi: 10.1002/ccd.24659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Premaratne GU, Tambara K, Fujita M, Lin X, Kanemitsu N, Tomita S, Sakaguchi G, Nakajima H, Ikeda T, Komeda M. Repeated implantation is a more effective cell delivery method in skeletal myoblast transplantation for rat myocardial infarction. Circ J. 2006;70:1184–1189. doi: 10.1253/circj.70.1184. [DOI] [PubMed] [Google Scholar]
- 200.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 201.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 203.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 204.Hudson W, Collins MC, deFreitas D, Sun YS, Muller-Borer B, Kypson AP. Beating and arrested intramyocardial injections are associated with significant mechanical loss: Implications for cardiac cell transplantation. The Journal of surgical research. 2007;142:263–267. doi: 10.1016/j.jss.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 205.Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102:1471–1482. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zen K, Okigaki M, Hosokawa Y, Adachi Y, Nozawa Y, Takamiya M, Tatsumi T, Urao N, Tateishi K, Takahashi T, Matsubara H. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 207.Ghanem A, Steingen C, Brenig F, Funcke F, Bai ZY, Hall C, Chin CT, Nickenig G, Bloch W, Tiemann K. Focused ultrasound-induced stimulation of microbubbles augments site-targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J Mol Cell Cardiol. 2009;47:411–418. doi: 10.1016/j.yjmcc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 208.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave-facilitated intracoronary cell therapy on lvef in patients with chronic heart failure: The cellwave randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;309:1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 209.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 210.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered mscs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 211.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 212.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing cxcr4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 214.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111:2640–2646. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 215.Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, Rossig L, Koehl U, Koyanagi M, Mohamed A, Brandes RP, Martin H, Zeiher AM, Dimmeler S. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial no synthase enhancer ave9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci U S A. 2006;103:14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Akita T, Murohara T, Ikeda H, Sasaki K, Shimada T, Egami K, Imaizumi T. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Laboratory investigation; a journal of technical methods and pathology. 2003;83:65–73. doi: 10.1097/01.lab.0000050761.67879.e4. [DOI] [PubMed] [Google Scholar]
- 217.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, Tang XL, Rokosh G, Bhatnagar A, Bolli R. The heme oxygenase 1 inducer (copp) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (erk)/nrf2 signaling pathway and cytokine release. J Biol Chem. 2012;287:33720–33732. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, Nakadi BE, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure the c-cure multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 220.Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 221.Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (igf-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (tac-hft) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161:487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 224.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]


