Abstract
Objective
LRP1 is a large endocytic and signaling receptor that is abundant in vascular smooth muscle cells (SMC). Mice in which the lrp1 gene is deleted in SMC (smLRP1-/-) on an LDLr-deficient background display excessive PDGF signaling, SMC proliferation, aneurysm formation, and increased susceptibility to atherosclerosis. The objectives of the current studies were to examine the potential of LRP1 to modulate vascular physiology under non-atherogenic conditions.
Approach and Results
We found smLRP1-/- mice to have extensive in vivo aortic dilatation accompanied by disorganized and degraded elastic lamina along with medial thickening of the arterial vessels resulting from excess matrix deposition. Surprisingly, this was not due to excessive PDGF signaling. Rather, quantitative differential proteomic analysis revealed that smLRP1-/- vessels contain a 4-fold increase in protein levels of high-temperature requirement factor A1 (HtrA1) which is a secreted serine protease that is known to degrade matrix components and to impair elastogenesis resulting in fragmentation of elastic fibers. Importantly, our studies discovered that HtrA1 is a novel LRP1 ligand. Proteomics analysis also identified excessive accumulation of connective tissue growth factor (CTGF), an LRP1 ligand and a key mediator of fibrosis.
Conclusions
Our findings suggest a critical role for LRP1 in maintaining the integrity of vessels by regulating protease activity as well as matrix deposition by modulating HtrA1 and CTGF protein levels. These studies highlight two new molecules, CTGF and HtrA1, which contribute to detrimental changes in the vasculature and therefore represent new target molecules for potential therapeutic intervention to maintain vessel wall homeostasis.
Keywords: LRP1, CTGF, HtrA1, elastic lamina, collagen
Introduction
The LDL receptor related protein 1 (LRP1) is a large endocytic and signaling receptor that mediates the endocytosis of several ligands including proteases and protease-inhibitor complexes1. LRP1 regulates a number of physiological processes including tPA-induced disruption of the blood brain barrier2 and macrophage migration3,4. Genome wide association studies reveal that the LRP1 gene represents a susceptibility locus for elevated plasma lipids5, for abdominal aortic aneurysms6 and for coronary heart disease7. Mechanisms by which LRP1 modulates the development and progression of cardiovascular disease are currently unknown, but studies in vascular smooth muscle cells reveal that LRP1 can mediate the uptake of aggregated LDL8-10 which contributes to lipid loading in these cells. Genetic studies in mice demonstrate that the lrp1 gene expressed in vascular smooth muscle cells11-13 and macrophages14-16 protects the vessels from the development of atherosclerosis and restenosis, revealing that LRP1 is a key receptor that regulates the integrity of the vasculature during vessel wall remodeling. Mice deficient in SMC LRP1 when on an LDL receptor-deficient background display excessive SMC proliferation resulting in enlarged aortas, aneurysms formation and increased susceptibility to cholesterol-induced atherosclerosis. This results from excess PDGF-signaling in smLRP1-/- mice, and total and phospho-PDGFR-β levels are significantly increased in the vessels of these mice11,12. Mice deficient in SMC LRP1 not on an LDL receptor-deficient background have also been generated, and SMC isolated from these mice display accelerated growth rates and are depleted of calponin, a marker for contractile SMC phenotype13. Interestingly, these mice display increased injury-induced neointimal hyperplasia.
Integral to the normal physiologic function of the large elastic arteries is the extracellular matrix, especially collagen and elastin which are the major matrix components of these vessels17. Collagen provides strength and prevents failure of the vessels at high pressure while elastin provides reversible extensibility during cyclic loading of the cardiac cycle17. Collagens turnover continually throughout life18, and as we age, collagen deposition increases, leading to stiffening of the vessels and contributing to the development of cardiovascular disease, including hypertension19. In contrast, elastin does not turnover in normal, healthy arteries20; thus damage or degradation of elastin results in severe consequences, including aneurysms, and aortic dissections.
Mechanisms associated with fragmentation and degradation of the elastic fibers in the vasculature are not fully understood, but it is clear that a major contributor to this process results from the accumulation of excess protease activity. MMP2 in concert with MMP921 and MT1-MMP22 have been identified to contribute to the development of aneurysms in mouse models. Interestingly, the levels of MMP2 and MMP9 are both regulated by LRP123,24. In the current investigation, we initiated studies to define mechanisms by which SMC LRP1 maintains the integrity of the vasculature. Our findings reveal that LRP1 protects the vasculature by regulating matrix deposition and protease activity in the vessel wall.
Material and Methods
Materials and Methods are available in the online-only Supplement
Results
Effective deletion of LRP1 from SMC
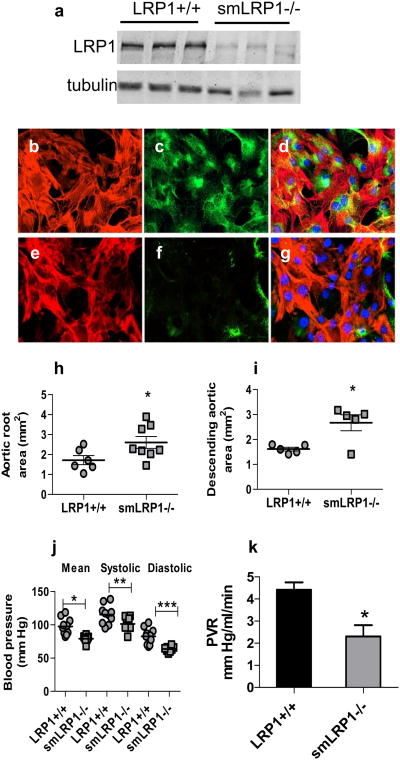
To investigate the role of LRP1 expressed in vascular smooth muscle cells on the integrity of the vasculature, we generated smooth muscle lrp1 knockout mice by crossing sm22 promoter-driven cre transgenic mice with lrp1 flox/flox mice. Immunoblotting of extracts from the aorta revealed an effective deletion of the lrp1 gene (Fig 1a) while immunofluorescence studies on aortic SMC isolated from WT (Fig 1b-d) and smLRP1-/- (Fig 1e-g) mice confirmed an effective deletion of the lrp1 gene in SMC isolated from smLRP1-/- mice.
Figure 1. SMC LRP1 deficiency induces aortic dilatation.
a) Immunoblot analysis of aortas (adventitia removed) from LRP1+/+ and smLRP1-/- mice. a/b tubulin was used as a loading control. b–g) Immunofluorescent analysis of SMC isolated from aortas of LRP1+/+ (b, c, d) and smLRP1-/- (e, f, g) mice. Cells were stained for αSMA (red, b, e) and LRP1 (green, c, f). Merged images are shown in d and g. h) Aortic root area (*p=0.04) and i) thoracic aortic area (*p=0.01) as measured by echocardiography in 20 weeks old mice; j) blood pressure measurements were performed by cannulating the right carotid artery and recording the blood pressure continuously (*p=0.001, **p=0.03, ***p=0.0006); k) peripheral vascular resistance for LRP1+/+ and smLRP1-/- mice. *p=0.014, n=4)
Aortic dilatation and reduced basal blood pressure in smLRP1-/- mice
We initially measured several physiological parameters to determine if genetic deletion of lrp1 has an impact on aortic integrity. Echocardiographic measurements revealed substantial increases in the area of the aortic root (Fig 1h) as well as in the area of the ascending aorta (Fig 1i) in smLRP1-/- mice (51% and 55% increase, respectively). Our studies also revealed a significant decrease in the mean blood pressure in smLRP1-/- mice, resulting from a decrease in both the systolic and diastolic blood pressure (Fig 1j).
Since the sm22 (taln) gene is transiently expressed in the heart during development25 and since LRP1 is expressed in the heart with unknown function, we studied left ventricular geometry and function using biomicroscopy (high frequency ultrasound). We did not detect any difference in the dimensions or the wall thickness of the left ventricle (LV) either in systole or diastole between smLRP1-/- and WT mice. This indicates normal LV geometry in smLRP1-/- mice, and thus these mice have no geometric deficiencies such as myocardial hypertrophy or ventricular remodeling (Supplemental Table I). Moreover, there is no significant change in contractile function of the LV in smLRP1-/- mice when compared to WT littermates, as indicated by LV fractional shortenings and LV ejection fraction (Supplemental Table I). The maximal mitral E wave velocity and the mitral E/A wave ratio were significantly greater in smLRP1-/-, with no change in the mitral A wave velocity (Supplemental Table I). Additional work is required to determine why the mitral E wave velocity is increased in smLRP1-/- mice. We conclude from all of the measurements that there is no defect in cardiac function as a consequence of transient deletion of the lrp1 gene in cardiac myocytes during development. Therefore, the main cause of hypotension observed in smLRP1-/- mice is a result of decreased peripheral vascular resistance (Fig 1k), most likely due to impaired vessel contraction that were previously noted in studies employing aortic rings from smLRP1-/- mice13.
Deregulation of connective tissue growth factor and increased matrix deposition in the aortas of smLRP1-/- mice
To identify the potential mechanisms by which SMC LRP1 regulates the integrity of the vessel wall, we employed quantitative proteomics to assist in the identification of key molecules that might contribute to the phenotype observed in smLRP1-/- mice. In these experiments, we subjected 5 mm sections of the ascending aortas pooled from WT and smLRP1-/- mice to the differential extraction protocol of Didangelos et al.26 after which the final extract, containing extracellular matrix proteins, was subjected to proteomics analysis. The results identified a number of proteins (Supplemental Table III), some of which were altered in smLRP1-/- mice. Strikingly, the levels of several matrix proteins are altered in smLRP1-/- aortas (Table 1). These include various collagens, extracellular matrix proteoglycans, cell adhesion proteins, proteins involved in elastogenesis as well as a matrix-associated protease. The proteomics findings were confirmed by staining sections of aorta from both WT and smLRP1-/- mice with Masson's Trichrome staining (Supplemental Fig I), which revealed increased collagen staining in both the media and adventitia of vessels from smLRP1-/- mice. These results are in agreement with previous work27.
Table 1. Changes in matrix and cell adhesion proteins found in smLRP1-/- mice.
| Uniprot gene ID | Name | Unique peptides found | aprotein ratio | bFDR adjusted p | Gene Symbol |
|---|---|---|---|---|---|
| Extracellular Matrix | |||||
| ECM Proteoglycans | |||||
| ASPN_MOUSE | Asporin | 9/6 | 3.37 ± 1.12 | 0.022 | Aspn |
| PGCA_MOUSE | Aggrecan core protein | 36 | 4.01 ± 0.65 | 0.003 | Acan |
| PGS2_MOUSE | Decorin | 17 | 0.43 ± 0.12 | 0.003 | Dcn |
| ECM Collagens, fibrillogenesis and collagen remodeling | |||||
| COIA1_MOUSE | Isoform 2 of Collagen alpha-1(XVIII) chain | 35 | 3.15 ± 0.34 | 0.001 | Col4a6 |
| CO5A2_MOUSE | Collagen alpha-2(V) chain | 5/5 | 1.74 ± 0.30 | 0.016 | Col5a2 |
| CO6A1_MOUSE | Collagen alpha-1(VI) chain | 29 | 1.42 ± 0.03 | 0.0004 | Col6a1 |
| DERM_MOUSE | Dermatopontin | 10 | 1.34 ± 0.20 | 0.047 | Dpt |
| LUM_MOUSE | Lumican | 9 | 0.66 ± 0.07 | 0.002 | Lum |
| MIME_MOUSE | Osteoglycin | 11 | 0.65 ± 0.19 | 0.027 | Ogn |
| POSTN_MOUSE | Periostin, osteoblast specific factor | 21 | 0.66 ± 0.01 | 0.0004 | Postn |
| CTGF_MOUSE | Connective tissue growth factor | 15 | 8.58 ± 3.56 | 0.021 | Ctgf |
| ECM Matrix assembly and Other functions | |||||
| LAMA4_MOUSE | Laminin subunit alpha-4 | 20 | 1.59 ± 0.11 | 0.002 | Lama4 |
| LAMA5_MOUSE | Laminin subunit alpha-5 | 16 | 1.88 ± 0.40 | 0.021 | Lama5 |
| LAMB2_MOUSE | Laminin subunit beta-2 | 32 | 2.03 ± 0.22 | 0.003 | Lamb2 |
| NID1_MOUSE | Nidogen 1 | 35 | 3.02 ± 0.48 | 0.004 | Nid1 |
| NID2_MOUSE | Nidogen 2 | 15 | 3.40 ± 0.73 | 0.007 | Nid2 |
| THSD4_MOUSE | Thrombinspondin type-1 domain containing protein4 | 24 | 2.12 ± 0.14 | 0.0008 | Thsd4 |
| TENA_MOUSE | Tenascin C | 58 | 8.69± 3.42 | 0.019 | Tnc |
| Elastogenesis | |||||
| A2AQ53_MOUSE | Fibrillin 1 | 4 | 2.11± 0.15 | 0.002 | Fbn1 |
| EMIL1_MOUSE | Elastin microfibril interface 1 | 16 | 1.53 ± 0.24 | 0.022 | Emilin1 |
| HTRA1_MOUSE | Serine protease HTRA1 | 15 | 4.23± 0.68 | 0.003 | Htra1 |
| Cell Adhesion | |||||
| WISP2_MOUSE | WNT1-inducible-signaling pathway protein 2 | 10 | 3.02 ± 0.28 | 0.001 | Wisp2 |
| ITA8_MOUSE | Integrin alpha 8 | 11 | 0.70 ± 0.18 | 0.032 | Itga8 |
| ITAV_MOUSE | Integrin alpha V | 5 | 0.59 ± 0.14 | 0.014 | Itgav |
| ITB1_MOUSE | Integrin beta 1 | 9 | 1.35 ± 0.05 | 0.003 | Itgb1 |
| IBP7_MOUSE | Insulin-like growth factor binding protein 7 | 7 | 1.52 ± 0.13 | 0.004 | Igfbp7 |
| MFGM_MOUSE | Milk fat globule-EGF factor 8 protein | 21 | 2.06 ± 0.37 | 0.011 | Mfge8 |
| VINC_MOUSSE | Vinculin | 48 | 0.58 ± 0.10 | 0.003 | Vcl |
| VTNC_MOUSE | Vitronectin | 7 | 1.46 ± 0.03 | 0.0004 | Vtn |
| FBLI1_MOUSE | Filamin binding LIM protein 1 | 22 | 1.76 ± 0.38 | 0.027 | Fblim1 |
Gene ontology analysis was performed using the DAVID bioinformatics resource (http://david.abcc.ncifcrf.gov/).
Protein ratio: smLRP1-/-/LRP1+/+
p values were calculated by Students t-test (n=3) and values adjusted for false discovery rate calculated by the approach of Benjamini and Hochberg's FDR controlling methods (Benjamini & Hochberg (1995) Journal of the Royal Statistical Soc. Series B (Methodological) 57, 289-300; Benjamini & Hochberg (2000) J. Education and Behavorial Statistics, 25, 60-83)
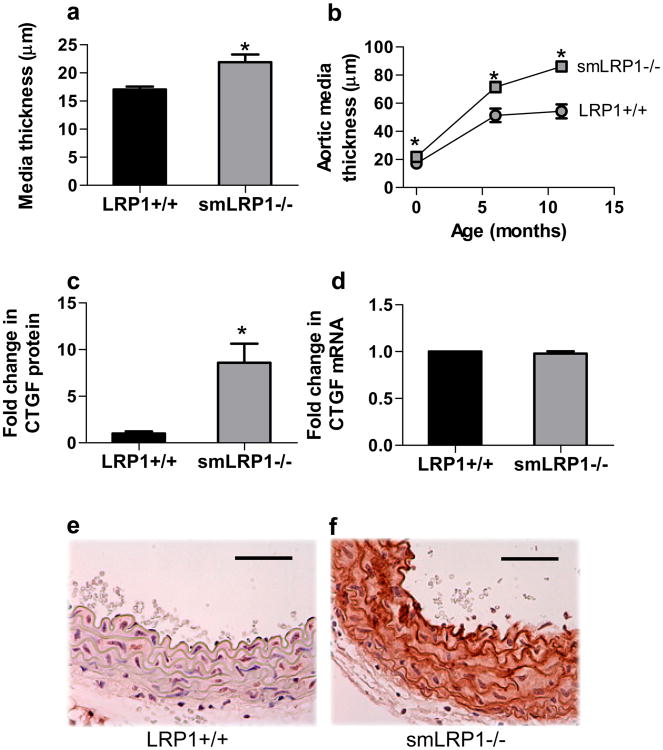
Consistent with increased matrix deposition in vessels of smLRP1-/- mice, we also noted increased thickening of the media of the aorta in these mice. This was even apparent in newborn mice (Fig 2a), and increased as the mice aged (Fig 2b). Examination of the proteomics data revealed a potential mechanism by which deletion of LRP1 in SMC regulates matrix deposition, as the levels of connective tissue growth factor (CTGF) were found to be significantly elevated in smLRP1-/- mice (Fig 2c Table 1). CTGF is a key mediator of fibrosis28 and is known to induce collagen synthesis in SMC and fibroblasts. In addition, CTGF is an LRP1 ligand29. We also measured levels of CTGF mRNA in the aorta, which revealed no differences between WT and smLRP1-/- mice (Fig 2d). As expected for normal vessels, immunohistochemical analysis of aortic tissue revealed very little expression CTGF in WT mice (Fig 2e), but interestingly, abundant expression of CTGF was detected in the media of smLRP1-/- mice (Fig 2f). Together, these results reveal that genetic deletion of LRP1 in SMC leads to excessive accumulation of CTGF, a known mediator of matrix production. Given the known role of CTGF in promoting matrix deposition, we hypothesize that the collagen deposition seen in smLRP1-/- mice as well as age-dependent medial thickening results from excess accumulation of CTGF in the vessel wall.
Figure 2. LRP1 modulates matrix deposition by regulating CTGF levels in the vessel wall.
a) Media thickness of the descending aorta from newborn mice was quantified using NIH image-J software (p=0.005, n=6) b) age-dependent increase in aortic medial thickening in smLRP1-/- mice (p<0.002, n=6); c) fold-increase in CTGF protein expression in smLRP1-/- mice relative to WT littermate controls was quantified by mass-spectrometry (p=0.021, n=3); d) qRT-PCR analysis of CTGF expression (n=3); e,f) immunohistochemistry analysis of CTGF expression in aortic vessel wall of WT (e) or smLRP1-/- (f) mice. Magnification, 40×, scale bars: 50 μm.
Deregulated protease levels in aortas of smLRP1-/- mice
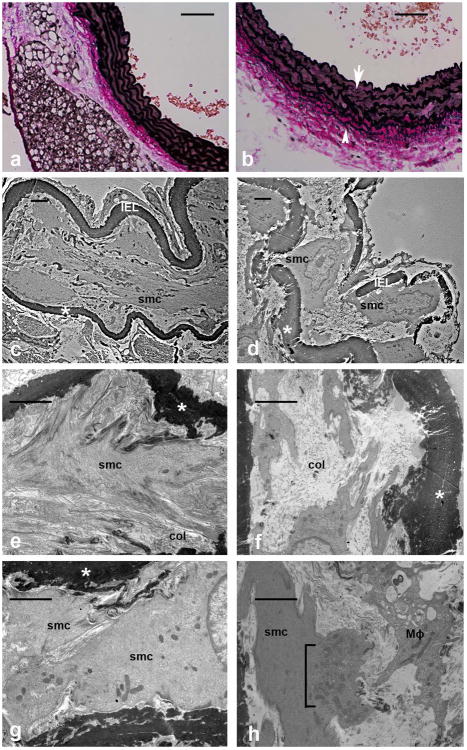
Immunohistochemical analysis of aortas from smLRP1-/- mice also revealed severe disorganization and degradation of the elastic lamina (Fig 3b, arrow) consistent with reports from earlier studies11. With elastic Van Gieson staining, elastin fibers in smLRP1-/- mice were characterized by random breaks and unusually thinner elastic fiber deposition both in medial and adventitial layers of aortic vessel wall (Fig 3b, arrowhead). We also detected an unusual architecture of elastin fibers in smLRP1-/- in comparison to wild type suggesting a change in the functional properties of these fibers. To gain insight into structural defects, we performed ultrastructural analysis of the vessels. As expected, vessels from WT mice displayed an aortic elastic laminae (*) structure that is highly organized (Fig 3c) and in close contact with a well-organized medial extracellular matrix (ECM) which includes fibrillin and collagen fibers (col) (Fig 3e). In addition, the elastic laminae is also in close contact with contractile SMC (smc) (Fig 3e), which establish cell-cell contact in the aortic wall of WT mice (Fig 3g). In contrast, the elastic laminae in smLRP1-/- mice is disrupted and degraded (Fig 3d) and in loose contact with disarrayed and unorganized ECM molecules (Fig 3f). Further, SMC extend cytoplasmic processes through the disrupted elastic laminae (Fig 3d). Disruptions of the elastic laminae as well as the rest of medial ECM is associated with infiltration of macrophage(s) (Mϕ) which are involved in clearing degradation products of ECM by large phagocytic vesicles (Fig 3h). Vascular SMC in smLRP1-/- mice have prominent synthetic organelles including rough endoplasmic reticulum, ribosomes and synthetic vesicles (Fig 3h, bracket), which correlates with the significant thickening of aortic media and the increase in matrix molecules in these mice (Table I).
Figure 3. Genetic deletion of lrp1 from SMC results in extensive disruptions in the vessel wall.
a,b) Elastic Van Gieson staining of sections from thoracic aorta showing wild type (a) and smLRP1-/- (b) aortic wall. Note the disrupted lamella (arrow) and additional elastin deposition (arrowhead) in the adventitia. Magnification, 40×, scale bars: 50 μm. c-h) Electron microscopy images (bar = 2 μm for all images) support the intact (c) vs. disrupted (d) lamella (*) including the internal elastic lamina (IEL). Compare higher magnification (e) of vascular smooth muscle cells (smc) in contact with organized EMC with collagen fiber (col) in WT aorta with SMC loosely in contact with unorganized collagen (f) in smLRP1-/- aorta. Medial SMC in WT aorta are contractile and in contact with each other (g) and with the vascular ECM (c,e). LRP1 deficient SMCs have prominent synthetic organelles (h, bracket). Proteolytic products of ECM in smLRP1-/- mice are cleared by phagocytosis by a macrophage (Mϕ) (h)
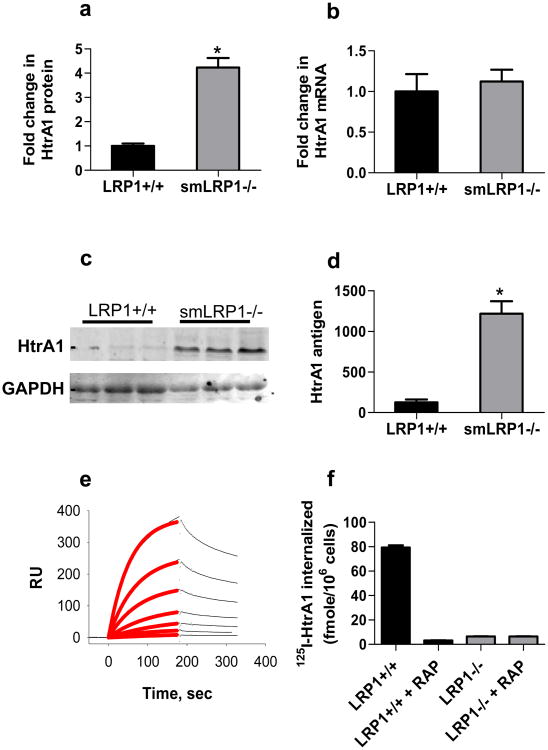
Our quantitative proteomics data identified a key protease that is de-regulated in the vessels of smLRP1-/- mice (Fig 4a). This enzyme, high-temperature requirement factor A1 (HtrA1) is a secreted serine protease that degrades several matrix components including decorin, fibronectin, aggrecan, type II collagen30,31 and impairs elastogenesis by cleaving fibulin 530. Interestingly, while HtrA1 protein levels were increased in smLRP1-/- aortic extracts, mRNA levels for htra1 were not altered in these mice (Fig 4b). To confirm the proteomics results, we performed immunoblot analysis on extracts from the descending aorta of WT and smLRP1-/- mice. The results (Fig 4c,d) confirm increased expression of HtrA1 in aortic extracts from smLRP1-/- mice.
Figure 4. De-regulation of HtrA1 in vessels from smLRP1-/- mice.
a) Fold-change in HtrA1 expression in smLRP1-/- mice relative to WT sibling controls was quantified by mass-spectrometry (p=0.001, n=3); b) qRT-PCR analysis of htra1 expression (n=3); (c) Immunoblot analysis using anti-HtrA1 of aortic extracts from smLRP1-/- and WT sibling controls; d) immunoblots were analyzed by densitometry using NIH Image software (p<0.05); e) Binding of different concentrations of HtrA1 (6, 12, 15, 50, 100 and 200 nM) to LRP1 immobilized on surface of Biacore chip. The red line shows the best fit to a pseudo-first order process. f) Uptake of 125I-labeled HtrA1 (25 nM) by LRP1-expressing and LRP1-deficient cells in the absence or presence of 2 μM RAP.
The fact that HtrA1 protein levels are significantly elevated in aortas from smLRP1-/- mice while mRNA levels of htra1 are unchanged raise questions about how the protein levels of HtrA1 might be regulated. One mechanism we considered is the possibility that HtrA1 might be an LRP1 ligand, and if so, removal of the protease by LRP1-mediated endocytosis might represent a viable mechanism by which LRP1 regulates HtrA1 levels. To test this proposal, we employed surface plasmon resonance experiments to determine if HtrA1 binds to LRP1. The results revealed a concentration-dependent binding of HtrA1 to the LRP1-coated sensor chip (Fig 4e). To determine the affinity of the interaction, the association data were fit to a pseudo-first order process to obtain values of Req for each concentration of HtrA1. These Req values were then plotted as function of total concentration of HtrA1 from which a KD value of 70 ± 6 nM was obtained by non-linear regression analysis. These data reveal that HtrA1 is capable of binding to LRP1 with high affinity, and raise the possibility that LRP1 regulates HtrA1 levels in the vasculature by binding this protease and mediating its endocytosis and subsequent degradation. To determine this, we used LRP1-expressing and LRP1-deficient cells to measure the internalization of 125I-labeled HtrA1. The results (Fig 4f) reveal specific internalization of HtrA1 by LRP1-expressing cells, but not by cells genetically deficient in LRP1. Importantly, the internalization was blocked by RAP, an LRP1 antagonist. Together, these results confirm that HtrA1 is a novel LRP1 ligand.
Increased inflammation in the vessel wall of smLRP1-/- mice
Proteolytic products of elastin32, fibrillin-133, and fibronectin34 have all been reported to recruit macrophages into the vessel wall. Since we detected macrophages in the vessel wall of smLRP1-/- mice in ultrastructural studies, we stained sections of the descending aorta to determine the magnitude of macrophage infiltration using anti-Mac2 IgG. The results revealed substantial recruitment of macrophages in the media of aortas from smLRP1-/- mice (Fig 5b). As expected, we were unable to detect any Mac2-positive staining in sections from WT mice.
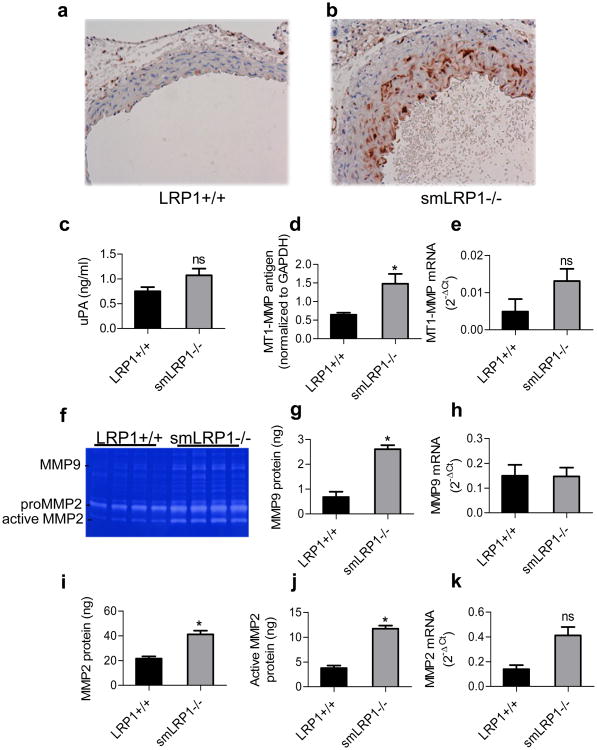
Figure 5. Increased macrophage infiltration and proteinases expression in aortas from smLRP1-/- mice.
Abundant Mac-2 expression was detected in smLRP1-/- aortic vessel wall (b) while none was detected in WT (a) aortas. c) Expressions of uPA determined by ELISA; d) MT1-MMP levels were quantified by immunoblot analysis of aortic extracts; e) MT1-MMP mRNA levels determined by qRT-PCR analysis; f) Gelatin zymography analysis of aortic extracts reveal increased levels of MMP9, pro-MMP2, and active MMP2; g,i,j) levels of MMP9, MMP2 and active MMP2 were quantified by gelatin zymography using purified proteins as standards. (g, *p=0.0004, n=4; i, *p=0.001, n=4; j, *p<0.0001, n=4); h) mmp9 mRNA levels determined by qRT-PCR analysis; (*p=0.002); k) mmp2 mRNA levels determined by qRT-PCR analysis (FDR adjusted p=0.08).
Since macrophages are a source of protease activity, we quantified the levels of several proteases known to be expressed by macrophages in the aortas of WT and smLRP1-/- mice. By employing an ELISA specific for murine uPA, we found a trend towards increased expression of uPA in smLRP1-/- mice (Fig 5c), but the data did not quite reach significance (p=0.07, n=5). MT1-MMP (MMP14) levels were quantified by immunoblot analysis, and the results reveal a significant increase in MT1-MMP antigen levels in the aorta of smLRP1-/- mice (Fig 5d). To measure gene expression levels of these and other proteases in the vessel wall, we employed quantitative RT-PCR arrays to identify changes in mRNA levels of proteases and other matrix genes (see Supplemental Table II). These results revealed that while there appeared to be an increase in the mRNA levels of MT1-MMP (Fig 5e), the results did not reach significance (p=0.305, n=3).
MMP2 and MMP9 are the gelatinases produced by macrophages, and thus we measured the levels of these MMPs in the aortas of WT and smLRP1-/- mice by employing gelatin zymography. These studies demonstrated increased activities of MMP9, MMP2 and active forms of MMP2 (Fig 5f). By employing purified MMP2 and MMP9 as standards in this assay, we quantified the levels of these metalloproteinases, which indicated increased levels of MMP9 (Fig 5g), pro-MMP2 (Fig 5i) and active MMP2 (Fig 5j) in the aortas of smLRP1-/- mice when compared to WT mice. Interestingly, mRNA levels of mmp9 in the aorta were not increased in smLRP1-/- mice (Fig 5h). In contrast, mRNA levels of mmp2 in the vessel wall of smLRP1-/- mice appear elevated with a trend toward significance (p 0.08) (Fig 5k). In summary, deletion of the lrp1 gene in SMC is associated with increased inflammation in the vessel wall, which we hypothesize results in increased protease activity and levels leading to increased elastic lamina degradation.
Increased ERK activation in aortas from smLRP1-/- mice
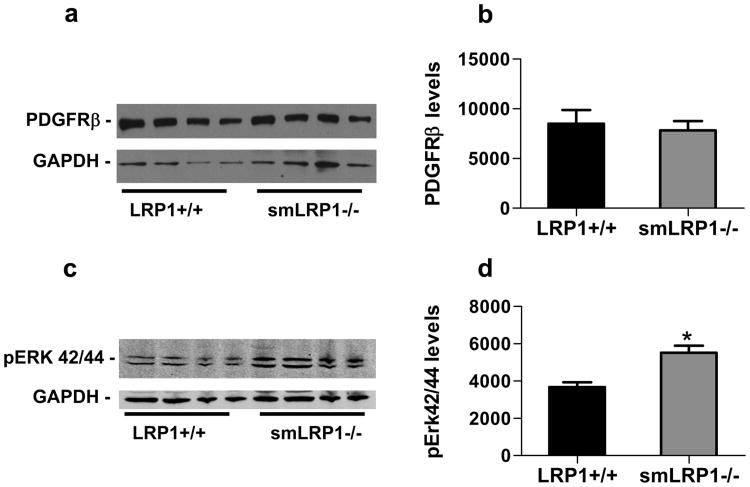
Prior studies examining smLRP1-/- mice in an atherosclerotic model (LDLr-/-, Western diet) observed overexpression of the PDGFRβ11 and abnormal activation of the PDGF11 and TGFβ12 signaling pathways. Activation of the PDGFRβ was also reported in smLRP1-/- mice not on an LDLr-/- background, although the magnitude of the effect was minimal13. We examined smLRP1-/- mice for evidence that the PDGF and TGFβ signaling pathways were abnormally activated. The results of these studies are shown in Fig 6, and demonstrate that total PDGFRβ levels in smLRP1-/- mice are identical with those in WT mice (Fig 6a,b). In contrast, we noted slightly elevated levels of phospho-ERK42/44 (Fig 6c,d), suggesting activation of the PDGF pathway. However, we were unable to confirm that phosphorylated forms of PDGFRβ were elevated in smLRP1-/- mice, as immunoblot analysis revealed very low levels of phospho-PDGFRβ with no significant difference between WT and smLRP1-/- mice. Finally, levels of phospho-SMAD2/3, which were also low, appeared similar between WT and smLRP1-/- mice (data not shown).
Figure 6. Increased Erk activation in aortic extracts from smLRP1-/- mice.
a) Immunoblot analysis of PDGFRβ levels in WT and smLRP1-/- mice. GAPDH levels are also shown as a loading control. b) Analysis of the immunoblot results in (a) using NIH ImageJ software. c) Immunoblot analysis of pERK42/44 levels in aortic extracts from WT and smLRP1-/- mice using GAPDH as a loading control. d) Analysis of the immunoblot results in (c) using NIH ImageJ software (*p=0.0063, n=4).
Discussion
Prior studies11,12 revealed that deletion of the lrp1 gene in SMC significantly enhances the development of atherosclerosis in mice when on an LDL receptor-deficient background and fed a “Western” diet. This was attributed to deregulation of the PDGF signaling pathway, and was confirmed by demonstrating that the total PDGFR-β and phospho-PDGFR-β levels were significantly increased in the vessel wall of these mice. In addition to the rapid development of atherosclerosis, smLRP1-/- and LDLR-deficient mice also demonstrated elastic lamina degradation and aneurysm formation. SMC LRP1-deficient mice also display a degraded elastic lamina, even when not on an LDL receptor-deficient background13,27. However, it is not clear that deregulation of the PDGF signaling pathway contributes to the phenotype observed in these mice as the changes in PDGFR and phospho-PDGFR levels in the aorta are rather modest13,27. Further, our studies employing smLRP1-/- mice failed to detect any changes in PDGFR-β or phosho-PDGFR-β levels in the aorta, indicating that the aortic dilatation, elastic lamina fragmentation, and matrix deposition that we report in the current study results from mechanisms distinct from excess PDGF-mediated signaling. The objectives of the current studies were to define mechanisms by which LRP1 modulates these processes.
We noted significant aortic dilatation in smLRP1-/- mice, which we attribute to degradation and disorganization of the elastic laminae. Degradation of the vessel wall extracellular matrix likely results from deregulation of protease activity in smLRP1-/- mice, and proteomics analysis discovered excessive levels of HtrA1 in smLRP1-/- mice that was validated by immunoblot analysis of aortic extracts. HtrA1 is a secreted serine protease that is ubiquitously expressed and is especially abundant in vascular smooth muscle cells35. In vitro studies reveal that HtrA1 can degrade several matrix proteins, including fibronectin, type II collagen, decorin, aggrecan, and fibulin 530,31. A SNP in the HtrA1 promoter region resulting in increased HtrA1 transcript levels has been associated with the progression of age-related macular degeneration36,37, suggesting a role for HtrA1 in matrix degradation. A direct in vivo role for HtrA1 in fragmentation of the elastic layer is revealed from studies in which HtrA1 was overexpressed in the pigment epithelial layer of the retina in transgenic mice. HtrA1 overexpression resulted in a fragmented and less continuous elastic layer of Bruch's membrane30, much like the results obtained in the present study. Thus the studies of Vierkotten et al30 confirm a role for HtrA1 in fragmentation and disorganization of the elastic layer.
In addition to its ability to degrade matrix, HtrA1 also regulates the TGFβ signaling pathway. This was discovered when mutations in the HTRA1 gene were associated with familial ischemic cerebral small-vessel disease (cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy – CARASIL)38 that result from deregulation of TGFβ signaling. The mechanism by which HtrA1 regulates TGFβ signaling has been identified in studies by Shiga et al.39, who demonstrated that HtrA1 mediates the proteolysis of the pro-domain of proTGF-β1 within the endoplasmic reticulum. Cleaved proTGF-β1 is then degraded by ER-associated degradation39. Consequently, the impact of HtrA1 expression is to reduce the amount of mature TGF-β1 secreted by cells.
Our findings that LRP1 regulates levels of HtrA1 in the vessel wall identify a new function for LRP1 in maintaining vessel wall integrity. Interestingly, while protein levels of HtrA1 were increased in the vessel wall of smLRP1-/- mice, mRNA levels were unaltered, suggesting that the regulation of HtrA1 by LRP1 does not occur at the transcription level. Indeed, we found that purified LRP1 binds HtrA1with high affinity and that LRP1-expressing cells internalize HtrA1 in a RAP-sensitive manner. These studies identify HtrA1 as a novel LRP1 ligand and further reveal that LRP1 can regulate HtrA1 levels by binding and mediating the rapid endocytosis of this protease. In addition to HtrA1, we noted excess MMP9, MMP2 and MT1-MMP in the vessel wall of smLRP1-/- mice, all of which contribute to degradation of elastic fibers and the development of aneurysms in mouse models21,22. Both MMP923 and MMP224 levels are regulated by LRP1; MMP9 directly binds to LRP1, whereas MMP2 binds to thrombospondin-2, which in turn binds LRP1.
Proteomic analysis also revealed excess matrix deposition in the vessel wall of smLRP1-/- mice. Consistent with this, we noted medial thickening in these mice, which became more severe as the mice age. Increased collagen deposition causes stiffening of the vessels, which normally results in hypertension. However, smLRP1-/- mice are hypotensive, which likely results from impaired vasoconstriction in vessels of smLRP1-/- mice as Basford et al.13 found impaired contractility in aortic rings from smLRP1-/- mice. We attribute excess matrix deposition to our discovery of significant elevation of CTGF in vessels of smLRP1-/- mice. CTGF is a member of the CCN family of secreted matricellular proteins40 that is a key mediator of fibrosis28 and plays an important role in vascular development. CTGF is expressed in endothelial cells as well as SMC in the developing vasculature, and CTGF-deficient mice exhibit vascular defects beginning at day E14.5. In the larger vessels, minor enlargement of the vessels and disorganization of the tunica media in CTGF-deficient mice was noted. In the aortic media of CTGF null mice, SMC exhibited a different morphology from WT mice and were not organized into distinct layers41 suggesting that CTGF modulates SMC physiology.
The molecular mechanisms by which CTGF elicits cellular responses are not clear, but CTGF may carry out some of these functions by modulating the TGFβ signaling pathway since CTGF synergizes with TGFβ to induce matrix deposition. Abreu et al.42 demonstrated that CTGF directly binds BMP4 and TGFβ1 and modulates their signaling properties by inhibiting BMP4-mediated signaling and enhancing TGFβ1-mediated signaling. CTGF is a ligand for LRP129 which mediates the rapid endocytosis of this molecule. The present data reveal a critical role for LRP1 in regulating levels of CTGF in the vasculature, and in the absence of SMC LRP1, CTGF levels accumulate. Since mRNA levels of ctgf, like htra1, were not changed in smLRP1-/- mice, the data are consistent with an important role for LRP1 in the clearance of CTGF.
The relationship between disorganized elastic lamina, collagen accumulation in the media of the vessel wall, and hypotension is interesting and not predicted, as excess collagen deposition is normally associated with hypertension. We hypothesize that genetic deletion of LRP1 from SMC alters their normal physiology in the vessel wall, which in turn impacts their contractile properties as well as their regulation of extracellular matrix deposition. This is supported by ultrastructural studies revealing that SMC in the vessel wall of smLRP1-/- mice have prominent synthetic organelles revealing a synthetic state. SMC are highly specialized cells that express a unique assortment of contractile proteins43 and function to regulate vascular tone and diameter. During development, SMC are responsible for the deposition of matrix proteins and for the correct assembly of elastic fibers, the largest and most complex structures of the extracellular matrix44. Ultrastructural studies in smLRP1-/- mice reveal a disorganized matrix. We further propose that accumulation of excess HtrA1 in smLRP1-/- vessels results in a disruption of normal elastic fiber and extracellular matrix formation that occurs during vessel development as well as during postnatal and adult life. The accumulation of extracellular matrix degradation products throughout adult life has the potential to recruit macrophages32-34, which are detected in the vessel wall of smLRP1-/- mice. These inflammatory cells are a source of additional proteases capable of degrading the elastic fibers.
In summary, we have discovered pathways in the vessel wall in which LRP1 regulates fibrosis by controlling CTGF levels and vascular structural integrity by modulating HtrA1 levels. Our findings suggest a critical role for LRP1 in maintaining an intact elastic lamina and appropriate vessel function by reducing protease activity and collagen deposition. Understanding mechanisms by which LRP1 protects the vasculature and regulates SMC physiology will give important insight into mechanisms regulating vessel wall homeostasis and pathology. Further, our studies highlight two new molecules (CTGF and HtrA1) that appear to play critical roles in changes in the vasculature and therefore represent new target molecules for potential therapeutic intervention.
Supplementary Material
Significance.
We discovered that deletion of the LRP1 in SMC leads to extensive aortic dilatation resulting from a disorganized and degraded elastic lamina in our KO mice. This is a serious problem, as it leads to aneurysms. In addition, we noted medial thickening of the arterial vessels resulting from excess matrix deposition. Quantitative differential proteomic analysis of vessels from WT and KO mice revealed accumulation HtrA1 in KO mice. HtrA1 is a secreted protease that is known to degrade matrix components and to impair elastogenesis. Proteomics analysis also identified accumulation of connective tissue growth factor (CTGF) in KO mice. CTGF is a known LRP1 ligand and a key mediator of fibrosis. Our studies highlight two new molecules (CTGF and HtrA1) that contribute to detrimental changes in the vasculature and therefore represent new target molecules for potential therapeutic intervention.
Acknowledgments
We thank Elizabeth Smith and Leisha Coats for excellent technical assistance. The authors would also like to thank Dr. Hegang Chen (University of Maryland School of Medicine) for assistance in statistical analysis.
Sources of Funding: This work was supported by grants P01 HL054710 (DKS) and HL050784 (DKS). SCM and SB were supported by T32 HL007698.
Footnotes
Disclosures: All authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL Receptor-Related Protein 1: Unique Tissue-Specific Functions Revealed by Selective Gene Knockout Studies. Physiol Rev. 2008 Jul;88(3):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yepes M, Sandkvist M, Moore E, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Inves. 2003;112(10):1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranganathan S, Cao C, Catania J, Migliorini M, Zhang L, Strickland DK. Molecular basis for the interaction of the LDL receptor-related protein 1 (LRP1) with the integrin alpambeta2: identification of binding sites within alphambeta2 for LRP1. J Biol Chem. 2011 Jun 15; doi: 10.1074/jbc.M111.265413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006 May 3;25(9):1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010 Aug 5;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bown MJ, Jones GT, Harrison SC, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011 Nov 11;89(5):619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy JJ, Parker A, Salem R, Moliterno DJ, Wang Q, Plow EF, Rao S, Shen G, Rogers WJ, Newby LK, Cannata R, Glatt K, Topol EJ. Large scale association analysis for identification of genes underlying premature coronary heart disease: cumulative perspective from analysis of 111 candidate genes. J Med Genet. 2004 May;41(5):334–341. doi: 10.1136/jmg.2003.016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llorente-Cortes V, Martinez-Gonzalez J, Badimon L. LDL receptor-related protein mediates uptake of aggregated LDL in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1572–1579. doi: 10.1161/01.atv.20.6.1572. [DOI] [PubMed] [Google Scholar]
- 9.Llorente-Cortes V, Otero-Vinas M, Camino-Lopez S, Costales P, Badimon L. Cholesteryl esters of aggregated LDL are internalized by selective uptake in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006 Jan;26(1):117–123. doi: 10.1161/01.ATV.0000193618.32611.8b. [DOI] [PubMed] [Google Scholar]
- 10.Llorente-Cortes V, Otero-Vinas M, Badimon L. Differential role of heparan sulfate proteoglycans on aggregated LDL uptake in human vascular smooth muscle cells and mouse embryonic fibroblasts. Arterioscler Thromb Vasc Biol. 2002 Nov 1;22(11):1905–1911. doi: 10.1161/01.atv.0000035391.46201.9a. [DOI] [PubMed] [Google Scholar]
- 11.Boucher P, Gotthardt M, Li WP, Anderson RGW, Herz J. LRP: Role in Vascular Wall Integrity and Protection from Atherosclerosis. Science. 2003 Apr 11;300(5617):329. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 12.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, Anderson RG, Herz J. LRP1 Functions as an Atheroprotective Integrator of TGFbeta and PDFG Signals in the Vascular Wall: Implications for Marfan Syndrome. PLoS ONE. 2007;2:e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basford JE, Moore ZW, Zhou L, Herz J, Hui DY. Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2009 Nov;29(11):1772–1778. doi: 10.1161/ATVBAHA.109.194357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007 Mar 16;100(5):670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 15.Hu L, Boesten LS, May P, Herz J, Bovenschen N, Huisman MV, Berbee JF, Havekes LM, van Vlijmen BJ, Tamsma JT. Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler Thromb Vasc Biol. 2006 Dec;26(12):2710–2715. doi: 10.1161/01.ATV.0000249641.96896.e6. [DOI] [PubMed] [Google Scholar]
- 16.Muratoglu SC, Belgrave S, Lillis AP, Migliorini M, Robinson S, Smith E, Zhang L, Strickland DK. Macrophage LRP1 suppresses neo-intima formation during vascular remodeling by modulating the TGF-β signaling pathway. PloS One. 2011;6(12):e28846. doi: 10.1371/journal.pone.0028846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mecham RP. Elastin synthesis and fiber assembly. Ann N Y Acad Sci. 1991;624:137–146. doi: 10.1111/j.1749-6632.1991.tb17013.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Feo JA, Sluijter JP, de Kleijn DP, Pasterkamp G. Modulation of collagen turnover in cardiovascular disease. Curr Pharm Des. 2005;11(19):2501–2514. doi: 10.2174/1381612054367544. [DOI] [PubMed] [Google Scholar]
- 19.Faury G, Chabaud A, Ristori MT, Robert L, Verdetti J. Effect of age on the vasodilatory action of elastin peptides. Mech Ageing Dev. 1997 Apr;95(1-2):31–42. doi: 10.1016/s0047-6374(96)01842-8. [DOI] [PubMed] [Google Scholar]
- 20.Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 21.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002 Sep;110(5):625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W, Knispel R, MacTaggart J, Greiner TC, Weiss SJ, Baxter BT. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem. 2009 Jan 16;284(3):1765–1771. doi: 10.1074/jbc.M806239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The Low Density Lipoprotein Receptor-related Protein Modulates Levels of Matrix Metalloproteinase 9 (MMP-9) by Mediating Its Cellular Catabolism. J Biol Chem. 2001 May 4;276(18):15498–15503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Strickland DK, Bornstein P. Extracellular MMP2 levels are regulated by the LRP scavenger receptor and thrombospondin 2. J Biol Chem. 2000;276(11):8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996 Feb;78(2):188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 26.Didangelos A, Yin X, Mandal K, Saje A, Smith A, Xu Q, Jahangiri M, Mayr M. Extracellular matrix composition and remodeling in human abdominal aortic aneurysms: a proteomics approach. Mol Cell Proteomics. 2011 Aug;10(8):M111. doi: 10.1074/mcp.M111.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. PloS One. 2009;4(9):e6922. doi: 10.1371/journal.pone.0006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)--a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol. 2010;114(3):e83–e92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 29.Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR, III, Carmichael DF. The Low Density Lipoprotein Receptor-related Protein/alpha 2- Macroglobulin Receptor Is a Receptor for Connective Tissue Growth Factor. J Biol Chem. 2001 Nov 2;276(44):40659–40667. doi: 10.1074/jbc.M105180200. [DOI] [PubMed] [Google Scholar]
- 30.Vierkotten S, Muether PS, Fauser S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch's membrane via cleavage of extracellular matrix components. PloS One. 2011;6(8):e22959. doi: 10.1371/journal.pone.0022959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadfield KD, Rock CF, Inkson CA, Dallas SL, Sudre L, Wallis GA, Boot-Handford RP, Canfield AE. HtrA1 inhibits mineral deposition by osteoblasts: requirement for the protease and PDZ domains. J Biol Chem. 2008 Feb 29;283(9):5928–5938. doi: 10.1074/jbc.M709299200. [DOI] [PubMed] [Google Scholar]
- 32.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006 Mar;116(3):753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, Hecht J, Mundlos S, Robinson PN. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006 Oct 24;114(17):1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- 34.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40(6-7):1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De LA, De FM, Severino A, Campioni M, Santini D, Baldi F, Paggi MG, Baldi A. Distribution of the serine protease HtrA1 in normal human tissues. J Histochem Cytochem. 2003 Oct;51(10):1279–1284. doi: 10.1177/002215540305101004. [DOI] [PubMed] [Google Scholar]
- 36.Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006 Nov 10;314(5801):989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006 Nov 10;314(5801):992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 38.Hara K, Shiga A, Fukutake T, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009 Apr 23;360(17):1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- 39.Shiga A, Nozaki H, Yokoseki A, et al. Cerebral small-vessel disease protein HTRA1 controls the amount of TGF-beta1 via cleavage of proTGF-beta1. Hum Mol Genet. 2011 May 1;20(9):1800–1810. doi: 10.1093/hmg/ddr063. [DOI] [PubMed] [Google Scholar]
- 40.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008 Oct;33(10):461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall-Glenn F, De Young RA, Huang BL, van HB, Hofmann JJ, Chen TT, Choi A, Ong JR, Benya PD, Mikkola H, Iruela-Arispe ML, Lyons KM. CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS ONE. 2012;7(2):e30562. doi: 10.1371/journal.pone.0030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002 Aug;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012 Jul 15;95(2):156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietz HC, Mecham RP. Mouse models of genetic diseases resulting from mutations in elastic fiber proteins. Matrix Biol. 2000 Nov;19(6):481–488. doi: 10.1016/s0945-053x(00)00101-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.