Abstract
Hemochorial placentation is characterized by trophoblast-directed uterine spiral artery remodeling. The rat and human both possess hemochorial placentation and exhibit remarkable similarities regarding the depth of trophoblast invasion and the extent of uterine vascular modification. In vitro and in vivo research methodologies have been established using the rat as an animal model to investigate the extravillous/invasive trophoblast lineage. With these research approaches, two signaling pathways controlling the differentiation and invasion of the trophoblast cell lineage have been identified: i) hypoxia/hypoxia inducible factor and ii) phosphatidylinositol 3-kinase/AKT/Fos like antigen I. Dissection of these pathways has facilitated identification of fundamental regulators of the invasive trophoblast cell lineage.
Keywords: Activator protein 1, AKT, FOS like antigen 1, Hypoxia, Hypoxia inducible factor, Phosphatidylinositol 3-kinase, Placentation, Pregnancy, Trophoblast invasion
The maternal-fetal interface is a dynamic site where uterine and placental structures cooperate to promote development of the fetus. The rat, mouse, and human each possess a hemochorial placenta [1–3]. This type of placentation is characterized by erosion of the maternal uterine epithelium and vasculature permitting the direct flow of maternal nutrients to trophoblast cells. In species exhibiting hemochorial placentation, remodeling of the uterine vasculature is critical for the success of pregnancy [4–8]. As gestation progresses, uterine spiral arteries supplying the placenta are modified creatingflaccid, low resistance blood vessels [7–11]. These vessels are the conduit required to meet the nutrient demands of the fetus. Central to uterine spiral artery remodeling is a specialized population of trophoblast cells referred to as invasive trophoblast or alternatively, in humans, as extravillous trophoblast [5, 7, 9, 11]. Defective hemochorial placentation, including impairments in uterine spiral artery remodeling, leads to pregnancy related disorders (preeclampsia, intrauterine growth restriction, preterm birth) that cause significant morbidity and mortality for both mother and fetus [4–7]. In addition, in utero insults have fundamental organizational effects on the developing fetus, which affect postnatal health and susceptibility to adult disease [12, 13].
The barrier for progress in understanding the invasive trophoblast cell lineage is the availability of appropriate experimental models. Scientific approaches using cell culture systems and animal models that permit molecular dissection of mechanisms are required. Our focus is on pathways that control cell differentiation. Therefore a trophoblast stem (TS) cell is the most appropriate cell culture model. TS cells possess the capacity to proliferate and to differentiate into mature trophoblast cell lineages and are ideal models for investigating mechanisms underlying trophoblast cell development [14–16].
Genes implicated in the regulation of placentation exhibit conservation in their expression patterns and actions (17–22]. The mouse has been an exceptional animal model for elucidating the regulation of most aspects of hemochorial placentation [21, 23]. However, the mouse and human exhibit fundamental differences in the extent of intrauterine trophoblast cell invasion and uterine spiral artery remodeling. In the mouse, the depth of trophoblast cell invasion is limited, whereas in the human the process is robust [8, 24, 25]. Organization of rat and human placentation sites show significant conservation, especially regarding trophoblast cell-directed remodeling of the uterine spiral arteries [24, 26–39]. Both species exhibit deep trophoblast invasion. The rat has many of the advantages of the mouse, including the capacity for genetic manipulation [40]. We have established relevant in vitro and in vivo research methods using the rat as an animal model to investigate molecular mechanisms regulating trophoblast cell differentiation and invasion [24, 35–39, 41–48]. Blastocyst-derived rat TS cells and Rcho-1 TS cells have proven to be effective in vitro culture systems for elucidating regulatory pathways controlling trophoblast cell differentiation (41, 44].
Several "candidate" signaling pathways have been implicated in regulating differentiation of the invasive trophoblast cell lineage [21, 49]; however, the significance of most of these pathways during in vivo placentation is unknown. In this short review, we focus on two pathways as probes into the control of the invasive trophoblast cell lineage: i] hypoxia/hypoxia inducible factor (HIF); and ii] phosphatidylinositol3-kinase (PI3K)/AKT/FOS like antigen 1 (FOSLl). We have established the importance of these pathways using both in vitro and in vivo experimentation [38, 43, 50–52].
Hypoxia/HIF Signaling Pathway
Oxygenation is critical to tissue morphogenesis. Oxygen deficits can stimulate vascular development, tissue nutrition and growth, and promote cellular specialization. This is also true for placental morphogenesis. Oxygen tensions tend to be lower during early pregnancy and increase following the establishment of the hemochorial placenta [53–59]. Low oxygen tension (hypoxia) is a physiological regulator of hemochorial placentation, especially for in vivo development of the invasive trophoblast cell lineage. This in vivo response to low oxygen is conserved in rodent and primate placentation [38, 60, 61]. Insights about the role of hypoxia as an intrinsic regulator of placentation have been derived from mutagenesis of genes in the mouse genome controlling cellular responses to O2 deprivation [56]. Central to the cellular response to hypoxia is the HIF transcription factor complex [62–64]. Phenotypes of placentas with null mutations for several genes encoding components of the HIF signaling pathway, including HIFlA, HIF2A, HIFIB (dimerization partner for HIFIA and HIF2A), prolyl hydroxylase domain protein 2, and von Hippel-Lindau genes, are associated with failures in placentation [65–69]. Through in vivo experiments with Holtzman Sprague-Dawley rats and in vitro experiments with rat TS cells [44], our laboratory has demonstrated that development of the invasive trophoblast cell lineage is activated by hypoxia (38, 52; Fig. 1).
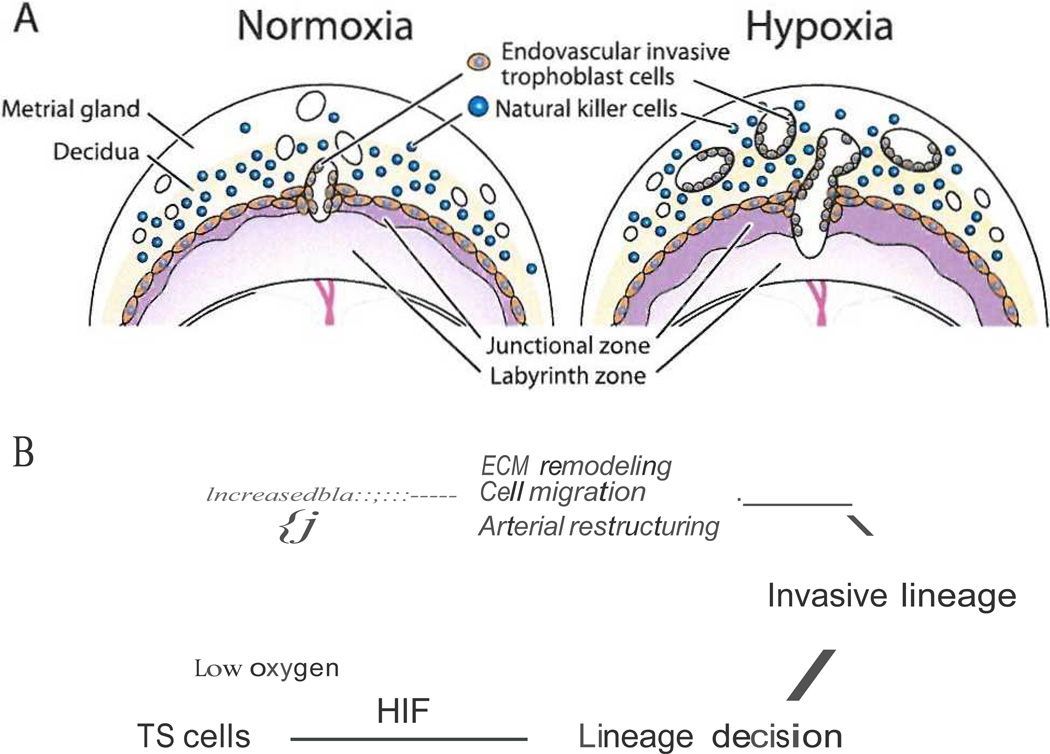
Fig. I.
Hypoxia signaling and activation of the invasive trophoblast cell lineage and trophoblast cell-directed uterine spiral artery remodeling. Panel A} Effects of maternal hypoxia on placentation. Exposure to maternal hypoxia increases uterine vascularity, endovascular invasive trophoblast cell invasion, and cellular allocation to the junctional zone of the chorioallantoic placenta. Panel B) Schematic representation of the effects of oxygen tension on TS cells and HIF-dependent trophoblast cell 1ineage decisions.
In vivo exposure of pregnant rats to hypoxia (− 11% oxygen) from gestation day 6.5 to day 13.5 resulted in an expansion of blood vessel density and volume in the uterine mesometrial compartment, a profound increase in the depth of endovascular trophoblast cell invasion, and a reallocation of trophoblast cells within key compartments of the placenta [38). Rat and mouse chorioallantoic placentas are composed of two compartments Uunctional zone and labyrinth zone) associated with essential functions. The junctional zone is situated at the interface with the uterine decidua, produces hormones that modulate the maternal environment, and is the origin of the invasive trophoblast cell population, whereas the labyrinth zone is located at the fetal interface and contributes to bi-directional nutrient/ waste transport between maternal and fetal vascular systems. Maternal hypoxia leads to a preferential expansion of the junctional zone, which is observed in both the rat and mouse [38, P. Bu and M.J. Soares, unpublished results). Responsiveness to hypoxia is most evident during a critical window of pregnancy (gestation day 8.5 to day 9.5) when essential trophoblast cell lineage decisions are being made [38].
In vitro rat TS cells respond to low oxygen tension (−0.5%) by altering their differentiation state [52). Hypoxia activates an epithelial-mesenchymal-like transformation that is associated with a decrease in the expression of E-cadherin (Cdhl) and increases in the expression of matrix metalloproteinase 9 (Mmp9) and matrix metalloproteinase 12 (Mmpl2) and movement through an extracellular matrix (Matrigel; 53). These responses are dependent on activation of the HIF signaling pathway [52).
In summary, there is a plasticity associated with placentation, which is influenced by hypoxia [38, 52, 70]. TS cells are labile and can differentiate in order to meet challenges within the maternal environment (Fig. 1). Their differentiation is sensitive to oxygen tension and dependent on HIF signaling (38, 52].
PI3K/AKT/FOSL1 Signaling Pathway
A signaling pathway involving PI3K/AKT/FOSL1 controls the differentiation of the invasive trophoblast cell lineage and the trophoblast cell-directed uterine spiral artery remodeling phenotype [43, 50, 51]. This has been determined through in vitro experiments with Rcho-1 TS cells and in vivo experimentation with the Holtzman Sprague-Dawley rat [51].
The PI3K/AKT pathway is a regulator of invasive trophoblast cells [43,51,71–73].Disruption ofPI3K or AKT inhibits the expression of genes associated with the invasive phenotype and trophoblast cell invasion through an extracellular matrix [51]. These actions are mediated, at least in part, through promoting the nuclear accumulation of FOSLl protein [51].FOSLl is a basic region-leucine zipper transcription factor and a contributor to the formation of the activator protein-1(AP-I) transcription factor complex (74). In rat and human placentation sites, FOSLl is expressed in the extravillous/invasive trophoblast cell population [51, 75]. Fosll is also expressed in Rcho-1 TS cells induced to differentiate [43, 51]. FOSLl knockdown in Rcho-1 TS cells inhibits the expression of genes associated with the invasive trophoblast cell phenotype, including carcinoembryonic antigen family 4 (Cgm4), interleukin 17f (l/17j), Mmp9, prolactin family 4-subfamily a-member 1 (Prl4al), and semaphorin 6D (Sema6d) [51; Kubota, Kent and Soares unpublished results). FOSLl occupies regions of the Mmp9 promoter in trophoblast cells critical for the regulation of Mmp9 gene expression [51]. Inhibition of FOSLl expression significantly decreases Rcho-1 trophoblast cell invasion as assessed in vitro through Matrigel-coated filters [51). The in vivo involvement of FOSLl in regulating trophoblast cell invasion was also assessed following trophectoderm-specific lentiviral shRNA delivery [45, 51]. FOSLl knockdown inhibited endovascular trophoblast cell invasion [51]. These FOSLl actions in trophoblast cells are consistent with its prominent role in regulating invasion in other cell types [76–79]. FOSLl is also implicated as a potential regulator of human trophoblast cells. Highly invasive trophoblast cells (HTR-8/ SVneo, Swan71) express higher levels of FOSLl versus trophoblast cells with limited invasiveness (BeWo, JEG3) (Renaud, Kubota, Rumi and Soares, unpublished results). A key role for FOSLl in hemochorial placentation has also been derived from mice possessing a null mutation at the Fosll locus [80].
The actions of FOSLl are dependent upon its interactions with other proteins, especially members of the basic region-leucine zipper transcription factor family (e.g. JUN, JUNB, and JUND; 75). In preliminary experiments, we have observed that FOSLl interacts with JUN and JUNB in rat trophoblast cells (Rumi, Kubota, and Soares, unpublished results). Based on expression and activity in differentiating TS cells, JUNB is a strong candidate for serving as a partner for FOSLl [43, 81]. JUNB is also expressed in human extravillous trophoblast cells [75]. Additionally, Junb null mutations phenocopy FoslJ null mutations, both resulting in defective mouse placental development [80, 82].
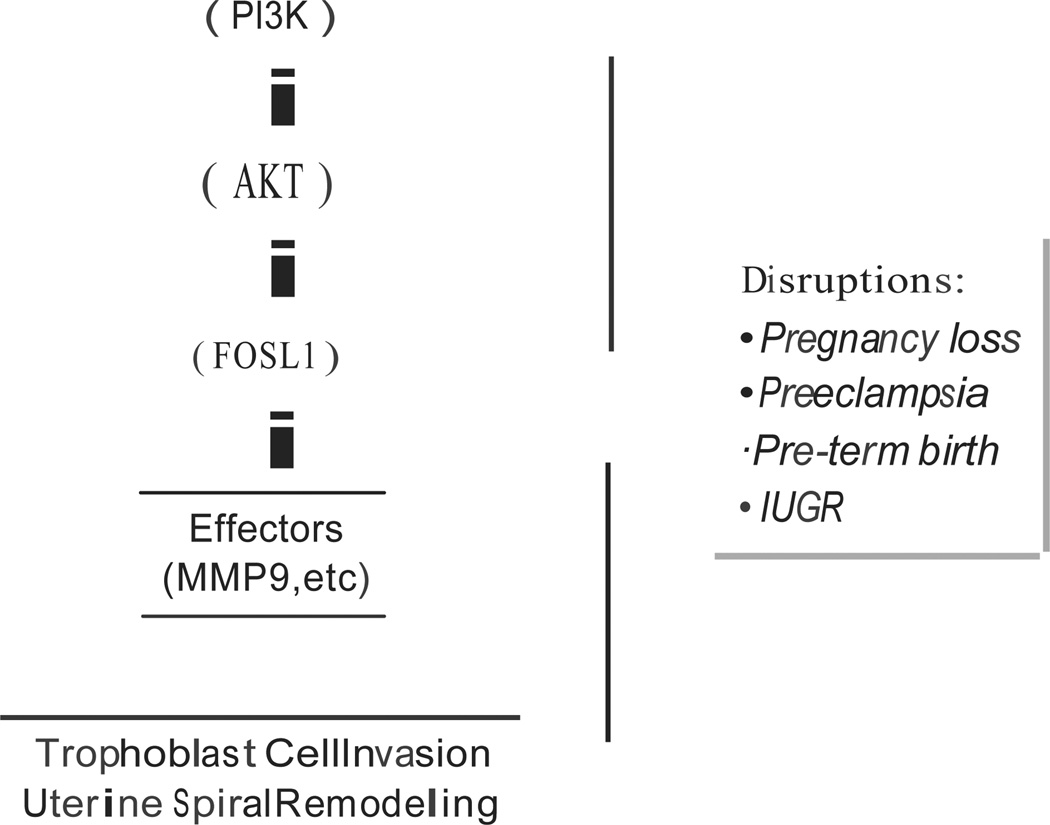
Collectively, the data indicate that FOSLJ is a key downstream effector of the Pl3K/AKT signaling pathway responsible for development of invasive trophoblast cell lineages integral to establishing the maternal-fetal interface (Fig. 2).
Fig. 2.
PBK/AKT/FOSLI pathway regulating the invasive trophoblast cell lineage and trophoblast cell-directed uterine spiral artery remodeling. Disruptions in the PJ3K/AKT/FOSLI pathway leads to pregnancy related disease, including early pregnancy loss, preeclampsia, pre-term birth, and intrauterine growth restriction (IUGR).
Overview
Our understanding of mechanisms regulating the invasive trophoblast cell lineage is minimal. It is now evident that the processes of trophoblast cell invasion and trophoblast cell-directed uterine spiral artery remodeling are conserved in model organisms, especially the rat. Technological advances have created an unprecedented opportunity to make major progress in elucidating regulatory mechanisms controlling development of the invasive trophoblast cell lineage. With these new tools we have determined that hypoxia/HIP and the PI3K/AKT/FOSLJ signaling pathways participate in the control of the invasive trophoblast cell lineage. It is expected that further dissection of these pathways will lead to the identification of fundamental regulators of differentiation and function of the invasive trophoblast cell lineage.
Acknowledgements
This work was supported by the National Institutes of Health (HD20676). DC was supported by a predoctoral fellowship from the American Heart Association. SJR was upported by postdoctoral fellowships from the Lalor Foundation and the Canadian Institute for Health Research. We would like to thank past members of our laboratory for their contributions to the concepts presented in this manuscript.
References
- 1.Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69:542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- 2.Enders AC, Welsh AO. Structural interact ions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool. 1993;266:578–587. doi: 10.1002/jez.1402660608. [DOI] [PubMed] [Google Scholar]
- 3.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placenta. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 4.Kaurmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Bioi Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 5.Red-Horse K, Zhou Y, Genbacev O, Prakobpbol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin lnvest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 9.Whitley GS, Cartwright JE. Cellular and molecular regulation of spital artery remodelling: lessons from the cardiovascular field. Placenta. 2010;31:465–474. doi: 10.1016/j.placenta.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris LK, Aplin JD. Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reprod Sci. 2007;14(Suppl):28–34. doi: 10.1177/1933719107309588. [DOI] [PubMed] [Google Scholar]
- 11.Harris LK. Trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31(Suppl):S93–S98. doi: 10.1016/j.placenta.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 15.Douglas GC, VandeVoort CA, Kumar P, Chang TC, Golos TG. Trophoblast stem cells: models for investigating trophectoderm differentiation and placental development. Endocr Rev. 2009;30:228–240. doi: 10.1210/er.2009-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RM, Fisher SJ. Trophoblast stem cells. Bioi Reprod. 2011;84:412–421. doi: 10.1095/biolreprod.110.088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou z, Romero R, Uddin M, Than NG, Wildman DE. Adaptive history of single copy genes highly expressed in term placenta. Genomics. 2009;93:33–41. doi: 10.1016/j.ygeno.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildman DE. Toward an integrated evolutionary understanding of the mammalian placenta. Placenta. 2011;32(Suppl2):Sl42–Sl45. doi: 10.1016/j.placenta.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox B, Kotlyar M, Evangelou AI, Ignatcheoko V, lgnatchenko A, Whiteley K, Jurisiea I, Adamson SL, Rossant J, Kislinger T. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. 2009;5:279. doi: 10.1038/msb.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemberger M, Udayashankar R, Tesar P, Moore H, Burton GJ. ELFS-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet. 2010;19:2456–2467. doi: 10.1093/hmg/ddq128. [DOI] [PubMed] [Google Scholar]
- 21.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, Fisher SJ. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–2998. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossant J, Cross JC. Extraembryonic lineages. In: Rossant J, Tam PPL, editors. Mouse Development. New York: Academic Press; 2002. pp. 155–180. [Google Scholar]
- 24.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260:176–190. doi: 10.1016/s0012-1606(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 25.Coan PM, Conroy N, Burton GJ, Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 2006;235:3280–3294. doi: 10.1002/dvdy.20981. [DOI] [PubMed] [Google Scholar]
- 26.Pijnenborg R, Robertson WB, Brosens I, Dixon G. Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2:71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- 27.Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta. 2005;26:574–584. doi: 10.1016/j.placenta.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Dtehend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brosen Jfl, Fiebeler A, Schneider W, Caluwaerts S, Vercruysst L, Pijnenborg R, Luft FC, Mueller ON. Agonistic auto antibodies to the ATI receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 29.Vercruysse L, Caluwaerts S, Luyten C, Pijneoborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 2006;27:22–33. doi: 10.1016/j.placenta.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Vtrlohren S, Niehoff M, Hering L, Geusens N, Herse F, Tintu AN, Plagemann A, LeNoble F, Pijnenborh R, Muller DN, Luft FC, Dudenbausen JW, Gollasch M, Dechend R. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension. 2008;51:547–553. doi: 10.1161/HYPERTENSIONAHA.107.103176. [DOI] [PubMed] [Google Scholar]
- 31.Geusens N, Verlobren S, Luyten C, Taube M, Hering L, Vercruysse L, Hanssens M, Dudenhausen JW, Dechend R, Pijnenborg R. Endovascular trophoblast invasion. spiral artery remodelling and uteroplacental haemodynamics in a transgenic rat model of pre-eclampsia. Placenta. 2008;29:614–623. doi: 10.1016/j.placenta.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Hering L, Herse F, Verlohren S, Park JK, Wtllner M, Qadri F, Pijnenborg R, Staff AC, Huppertz B, Muller DN, Luft FC, Dethend R. Trophoblasts reduce the vascular smooth muscle cell proatherogenic response. Hypertension. 2008;51:554–559. doi: 10.1161/HYPERTENSIONAHA.107.102905. [DOI] [PubMed] [Google Scholar]
- 33.Pijnenborg R, Vercruysse L. Shifting concepts of the fetal-maternal interface: a historical perspective. Placenta. 2008;l9(Suppl A):S20–S25. doi: 10.1016/j.placenta.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Geusens N, Hering L, Verlohren S, Luyten C, Drijkoningen K, Taube M, Vercruysse L, Hanssens M, Dechend R, Pijntnborg R. Changes in endovascular trophoblast invasion and spiral artery remodelling at term in a transgenic preeclamptic rat model. Placenta. 2010;31:320–326. doi: 10.1016/j.placenta.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Wiemers DO, Ain R, Ohboshi S, Soares MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J Endocrinol. 2003;179:335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- 36.Arroyo JA, Konno T, Khalili DC, Soares MJ. A simple in vivo approach to investigate invasive trophoblast cells. Int J Dev Biol. 2005;49:977–980. doi: 10.1387/ijdb.051993ja. [DOI] [PubMed] [Google Scholar]
- 37.Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Bioi Reprod. 2001;16:709–718. doi: 10.1095/biolreprod.106.056481. [DOI] [PubMed] [Google Scholar]
- 38.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosario GX, Ain R, Konno T, Soares MJ. Intrauterine fate of invasive trophoblast cells. Placenta. 2009;30:457–463. doi: 10.1016/j.placenta.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob HJ, Lazar J, Dwinell MR, Moreno C, Geurts AM. Gene targeting in the rat: advances and opportunities. Trends Genet. 2010;26:510–518. doi: 10.1016/j.tig.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faria TN, S-oares MJ. Trophoblast cell differentiation: establishment. Characterization and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- 42.Sabgal N, Canham LN, Canham B, Soares MJ. Rcho-1 trophoblast stem cells: a model for studying trophoblast cell differentiation. Methods Mol Med. 2006;121:159–178. [PubMed] [Google Scholar]
- 43.Kent LN, Konno T, Soares MJ. Phosphatidyl inositol 3-kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10:97. doi: 10.1186/1471-213X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asanoma K, Rumi MAK, Kent LN, Chakraborty D, Renaud SJ, Wake N, Lee D-S, Kubota K, Soares MJ. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Bioi. 2011;351:110–119. doi: 10.1016/j.ydbio.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee D-S, Rumi MAK, Konno T, Soares MJ. In vivo genetic manipulation of the rat trophoblast cell lineage using lentiviral vector delivery. Genesis. 2009;47:433–439. doi: 10.1002/dvg.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the placenta in the rat. Methods Mol Med. 2006;121:295–313. doi: 10.1385/1-59259-983-4:293. [DOI] [PubMed] [Google Scholar]
- 47.Konno T, Graham AR, Rempel LA, Ho-Chen JK, Alam SM, Bu P, Rumi MA, Soares MJ. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology. 2010;151:4537–4550. doi: 10.1210/en.2010-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konno T, Rempel LA, Rumi MA, Graham AR, Asanoma K, Renaud SJ, Soares MJ. Chromosome-substituted rat strains provide insights into the genetics of placentation. Physiol Genomics. 2011;43:930–941. doi: 10.1152/physiolgenomics.00069.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.KnOfter M. 2010 Critical growth factors and signaling pathways controlling human trophoblast invasion. Int. J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamei T, Jones SR, Chapman BM, McGonigle KL, Dai G, Soares MJ. The phosphatidylinositol3-kinase/Akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol Endocrinol. 2002;16:1469–1481. doi: 10.1210/mend.16.7.0878. [DOI] [PubMed] [Google Scholar]
- 51.Kent LN, Rumi MAK, Kubota K, Lee DS, Soares MJ. FOSL1 is integral to establishing the maternal-fetal interface. Mol Cell Bioi. 2011;31:4801–4813. doi: 10.1128/MCB.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakraborty D, Rumi MAK, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating HIF dependent trophoblast lineage decisions. Proc Notl Acad Sci USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genbatev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 54.Caniggia I, Mostathfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFp3. J Clin lnvest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burton GJ, Jaunaiux E. Maternal vascularization of the human placenta: does the embryo develop in a hypoxic environment? Gynecol Obstel Fertil. 2001;29:503–508. doi: 10.1016/s1297-9589(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 56.Fryer BH, Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- 57.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anot. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodesch F, Simon P, Donner C, Jauniaur E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 59.Zamudio S. The placenta at high altitude. High Aft Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Y, Chiu K, Brescia RJ, Combs A, Katz MA, Kitzmiller JL, Heilbron DC, Fisher SJ. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. Am J Obstet Gynecol. 1993;169:224–229. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 61.Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- 62.Semenza GL. Hydroxylation of HIF-I: oxygen sensing at the molecular level. Physiology. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 63.Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Semenza GL. Oxygen homeostasis. Wiley lnterdiscip Rev Syst Bioi Med. 2010;l:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 65.Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HJF mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet Pt, Simon MC. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MaUepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ. Hypoxia-inducible factor-dependent histone deacetylase activity deter mines stem cell fate in the placenta. Development. 2005;13l:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- 69.Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Bioi. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho Chen JK, Bustamante JJ, Soares MJ. Prolactin-like protein-F subfamily of placental hormones/cytokines: responsiveness to maternal hypoxia. Endocrinology. 2007;148:559–565. doi: 10.1210/en.2006-1146. [DOI] [PubMed] [Google Scholar]
- 71.Qiu Q, Yang M, Tsang BK, Gruslin A. Both mitrogen-activated protein kinase and phosphatidylinositol kinase signaling are required in epidermal growth factor induced human trophoblast migration. Mol Hum Reprod. 2004;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 72.Qiu Q, Yang BK, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signaling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 73.Sonderegger S, Haslinger' P, Sabri A, Leisser' C, Otten JV, Fiala C, Knofier M. Wingless (Wnt) 3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151:211–220. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eferl R, Wagner EF. AP-I: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 75.Marzioni D, Todros T, Cardaropoli S, Rolfo A, Lorenzi T, Ciarroela R, Romagnoli R, Paulesu L, Castellucci M. Activating protein family of transcription factors in the human placenta complicated by preeclampsia with and without fetal growth restriction. Placenta. 2010;31:919–927. doi: 10.1016/j.placenta.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Kustikova O, Kramerov D, Grigorian M, Berezin V, Bock E, Lukanidin E, Tulcbinsky E. Fral induces morphological transformation and increases in vitro invasiveness and motility of epithelilioid adenocarcinoma cells. Mol Cell Bioi. 1998;18:7095–7105. doi: 10.1128/mcb.18.12.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milde-Langosch K. The Fos family of transcription factors and their role in tumorigenesis. Eur J Cancer. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 78.Verde P, Casalino L, Talotta F, Yaniv M, Weitzman JB. Deciphering API function in tumorigenesis. Fraternizing on target promoters. Cell Cycle. 2007;6:2633–2639. doi: 10.4161/cc.6.21.4850. [DOI] [PubMed] [Google Scholar]
- 79.Young MR, Colburn NH. Fra-1 a target for cancer prevention or intervention. Gene. 2006;379:1–11. doi: 10.1016/j.gene.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularization requires the AP-I component Fral. Development. 2000;127:4937–4948. doi: 10.1242/dev.127.22.4937. [DOI] [PubMed] [Google Scholar]
- 81.Shida MM, Ng YK, Soares MJ, Linzer DI. Trophoblast-specific transcription from the mouse placental lactogenl gene promoter. Mol Endocrinol. 1993;7:181–188. doi: 10.1210/mend.7.2.8469232. [DOI] [PubMed] [Google Scholar]
- 82.Schorpp-Kistner M, Wang Z-Q, Angel P, Wagner EF. JunB is essential formammalian placentation. EMBO J. 1999;18:934–948. doi: 10.1093/emboj/18.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]