Abstract
Repetitive transcranial magnetic stimulation (rTMS) is emerging as a potentially valuable intervention to augment the effects of behavioral therapy for stroke. When used in conjunction with other therapies, rTMS embraces the concept of metaplasticity. Due to homeostatic mechanisms inherent to metaplasticity, interventions known to be in isolation to enhance excitability can interact when applied successively under certain timing conditions and produce enhanced or opposite effects. Similar to “muscular wisdom,” with its self-protective mechanisms, there also appears to be “synaptic wisdom” in neural networks with homeostatic processes that prevent over- and under-excitability. These processes have implications for both enhancing and suppressing the excitability effects from behavioral therapy. The purpose of this article is to relate the concept of metaplasticity, as derived from studies in humans who are healthy, to stroke rehabilitation and consider how it can be leveraged to maximize stroke outcomes.
Rehabilitation following stroke can follow the strategy of substitution or restitution. For the upper limb, substitution may involve activation of the contralesional hemisphere to direct movements with the nonparetic limb or use of adaptive maneuvers or devices to substitute for the impairments in the paretic limb. Restitution involves effort to reinstate function in the ipsilesional hemisphere to maximize recovery of the paretic limb. Depending on stroke size and location, a substitution strategy may be the only option. However, given that greater improvement in functional recovery is correlated with elevated excitability in the ipsilesional primary motor area (M1),1 whereas elevated excitability of contralesional M1 is associated with poorer prognosis for recovery of the affected hand,2 a restitution strategy is generally preferred. Repetitive transcranial magnetic stimulation (rTMS) is an emerging intervention in stroke rehabilitation used to condition the brain to make subsequent behavioral therapy more effective. As will be described, however, conditioning of neural networks is complex. Changes in brain plasticity resulting from the conditioning of neural networks may change the brain in unexpected ways—some more favorable and some less favorable for promoting recovery. Such changeable plasticity (ie, the plasticity of synaptic plasticity) is known as metaplasticity.3 The purpose of this article is to elucidate some of the principles of metaplasticity and how these principles can be used to synergize rTMS with behavioral therapy to optimize a return of ipsilesional excitability in M1 and promote recovery from stroke.
The Plastic Brain in Stroke
To understand the application of rTMS in stroke neurorehabilitation, it is first helpful to understand the adaptive and maladaptive factors that influence synaptic plasticity and recovery following stroke. In addition to neuronal death, stroke alters neurotransmitter activity, particularly gamma-aminobutyric acid-A (GABAA). GABAA is an inhibitory neurotransmitter known to decrease neuronal firing probability.4 During the acute phase of stroke recovery, the peri-infarct region demonstrates diminished excitability attributed to increased GABAA activity.5 This initial GABAA upregulation early in stroke may serve as a neuroprotective mechanism to minimize excitotoxicity and neuronal death. Yet, in the subacute stage, downregulation of GABAA signaling occurs.6 Related to stroke recovery, downregulation of GABAA is integral to both structural7 and electrophysiological8 reorganization in animals and reduced intracortical inhibition in humans.9,10
Interestingly, even with small strokes induced in animals, reduced GABAA inhibition is widespread to include not only the peri-infarct zone but also the contralesional hemisphere.6 Reduced intracortical inhibition in the contralesional hemisphere enhanced the use of and reliance on compensatory motor learning strategies in rodents.11 Indeed, studies in rats have shown that induced lesions to one sensorimotor cortex resulted in increased synaptogenesis and dendritic arborization in the contralesional sensorimotor cortex12,13 and were related to improved motor skill in the nonparetic forelimb.12,13 Hsu and Jones14 and Allred et al15 demonstrated similar results in rodents and cautioned that such early synaptic changes in the contralesional hemisphere, combined with motor training of the nonparetic limb, could diminish the recovery of the paretic limb. The corollary in humans is that such reorganization may facilitate substitutive motor relearning strategies involving contralesional hemispheric control of both nonparetic and paretic limbs. A reorganization scheme involving ipsilateral control of the paretic limb is detrimental to a restitution strategy because of an exaggeration of a phenomenon known as interhemispheric inhibition (IHI).
Interhemispheric Inhibition
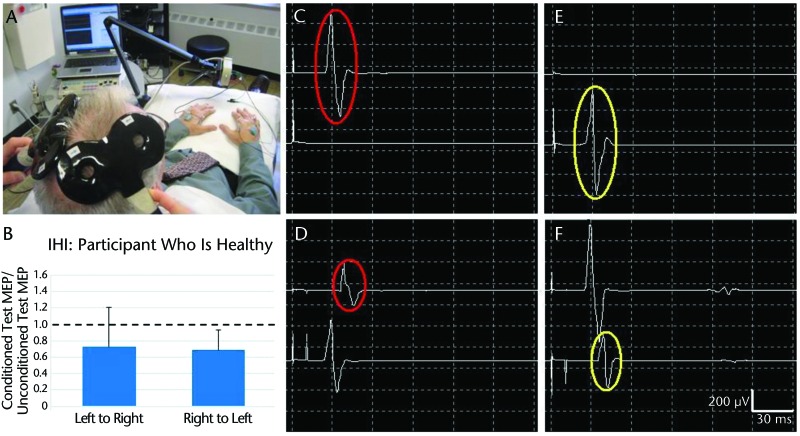
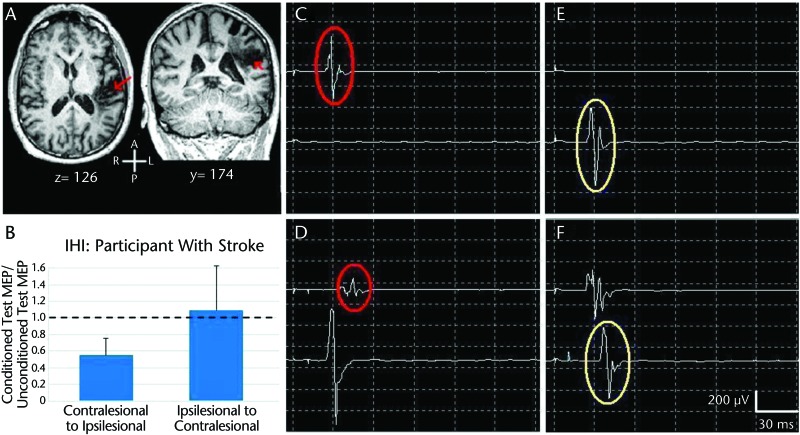
Ferbert et al16 demonstrated IHI by applying a conditioning pulse of transcranial magnetic stimulation (TMS) to M1 of one hemisphere and a second TMS pulse, referred to as a test pulse, to contralateral M1 a short time later. Compared with unconditioned test pulse responses, Ferbert et al16 found reduced corticospinal excitability in response to the test pulse when a conditioning pulse preceded the test pulse. The effect, mediated through corpus callosum pathways, is thought to prevent mirror movements with the opposite hand during volitional unilateral movements.17 Following stroke, however, IHI becomes unbalanced, with contralesional M1 exerting exaggerated inhibition onto the ipsilesional M1.18 The imbalance of IHI may be a manifestation of contralesional M1 reorganization in response to a reduced GABAA environment. Over time, repetitive voluntary effort with the nonparetic hand, in an effort to compensate for the paretic hand, reinforces the reorganization in the contralesional M1. Thus, a consequence of persisting overactivity in the contralesional M1 from reduced GABAA inhibition is an abnormally strong IHI exerted on the ipsilesional M1.19–21 In this regard, the ipsilesional M1 becomes “doubly disabled”20 by the stroke itself and by the exaggerated IHI imparted from contralesional M1. Figures 1 and 2 illustrate balanced IHI in a healthy brain and unbalanced IHI in a brain following stroke, respectively.
Figure 1.
Interhemispheric intervention (IHI): participant who is healthy. (A) Setup for measuring IHI with two 50-mm figure-8 coils over left and right primary motor areas (M1) of participant who is healthy and electromyography electrodes on bilateral first dorsal interosseous (FDI) muscles. (B) Mean (SD) values showing relatively balanced IHI in both directions, derived from multiple trials of left M1 inhibiting right M1 (left to right) (C–D) and right M1 inhibiting left M1 (right to left) (E–F). (C) Motor-evoked potential (MEP) in left FDI (upper trace) in response to unconditioned suprathreshold test stimulus to right M1. (D) MEP in right FDI (lower trace) in response to suprathreshold conditioning stimulus to left M1, followed by a MEP in left FDI in response to test stimulus to right M1 (interstimulus interval=10 ms). Interhemispheric intervention in the direction of left M1 inhibiting right M1 is demonstrated by reduction in conditioned test MEP (lower red circle) compared with unconditioned test MEP (upper red circle). (E–F) Interhemispheric intervention in the direction of right M1 to left M1 (compare yellow circles).
Figure 2.
Interhemispheric intervention (IHI): participant with stroke. (A) Magnetic resonance images of brain of participant with stroke (red arrows). (B) Mean (SD) values showing exaggerated IHI in direction of contralesional primary motor area (M1) inhibiting ipsilesional primary motor areas (M1) (contralesional to ipsilesional), derived from multiple trials of C–D, compared with direction of ipsilesional M1 inhibiting contralesional M1 (ipsilesional to contralesional), derived from multiple trials of E–F. (C) Motor-evoked potential (MEP) in paretic first dorsal interosseous muscle (FDI) (upper trace) in response to unconditioned suprathreshold test stimulus applied to the ipsilesional M1. (D) Motor-evoked potential in nonparetic FDI (lower trace) in response to suprathreshold conditioning stimulus applied to the contralesional M1, followed by an MEP in paretic FDI in response to test stimulus applied to the ipsilesional M1 (interstimulus interval=10 ms). Interhemispheric intervention in the direction of the contralesional M1 inhibiting the ipsilesional M1 is demonstrated by reduction in conditioned test MEP (lower red circle) compared with unconditioned test MEP (upper red circle). (E–F) Interhemispheric intervention in the direction of ipsilesional to contralesional M1 (compare yellow circles).
Interhemispheric inhibition represents an important example of diaschisis following stroke. Diaschisis involves a decline in excitability and function in brain areas that are spatially distinct but functionally related to the stroke site.22 Neurons killed from the stroke cannot be salvaged by rehabilitation. However, neurons in diaschisis are salvageable, as evidence shows in some people with stroke that forced use of the paretic hand combined with forced nonuse of the nonparetic hand (ie, constraint-induced movement therapy) can adjust the unbalanced IHI and improve ipsilesional M1 excitability and functional recovery.23,24 Behavioral training alone, however, may not be sufficiently potent to trigger optimal brain reorganization. Growing evidence suggests that neuromodulation of the brain using rTMS can serve as an adjunctive modality in certain people with stroke to make accompanying behavioral therapy more effective.25–27
rTMS and Synaptic Plasticity
Transcranial magnetic stimulation operates through Faraday's law of electromagnetic induction. In short, a rapidly changing electric current in the stimulating coil positioned over the intact scalp generates a rapidly changing magnetic field that traverses the skull and induces electric currents in underlying neural tissue.28 When stimulation is strategically delivered over M1 at a sufficient intensity, a contralateral involuntary muscle contraction, referred to as a motor-evoked potential (MEP), occurs. Collecting MEPs using electromyography and quantifying their peak-to-peak amplitude or other parameters provides a measurement of corticospinal excitability. Repetitive transcranial magnetic stimulation involves a continuous train or multiple intermittent trains of pulses that induce synaptic plasticity in the form of corticospinal excitability changes that persist even after stimulation has ceased (ie, aftereffects). High-frequency (≥3 Hz) rTMS, operating through long-term potentiation (LTP)–like mechanisms, produces aftereffects that transiently raise excitability,29 whereas low-frequency (≤1 Hz) rTMS, operating through long-term depression (LTD)–like mechanisms, produces aftereffects that transiently depress excitability.30
With rTMS treatment in stroke, the objective is to upregulate the excitability of surviving neurons in the ipsilesional hemisphere in order to make them more amenable to voluntary recruitment during subsequent behavioral therapy. To achieve this upregulation in ipsilesional M1, rTMS can be applied in 2 ways: (1) high-frequency rTMS over the ipsilesional M1 or (2) low-frequency rTMS over the contralesional M1. The latter approach involves “disinhibition” of the ipsilesional M1 by suppressing exaggerated IHI arising from the contralesional M1.31–34
Both of these rTMS approaches have been explored in stroke. As an example, Khedr et al20 directly compared 5 sessions of low- and high-frequency rTMS with sham rTMS and with each other in patients with stroke. One group received 1-Hz rTMS applied to the contralesional M1, another group received 3-Hz rTMS applied to the ipsilesional M1, and a third group received sham rTMS. All groups continued their customary therapy after the rTMS. Khedr et al20 found that both contralesional 1-Hz rTMS and ipsilesional 3-Hz rTMS were significantly more effective than sham rTMS in improving pegboard and keyboard tapping tasks. Furthermore, 1-Hz rTMS was significantly more effective than 3-Hz rTMS. For a thorough review of rTMS in stroke, including stimulation parameters, we recommend the meta-analysis by Hsu et al.35 Although 2 studies36,37 in Hsu and colleagues' analysis,35 along with 2 more recent studies,38,39 demonstrated no effect from rTMS, a large preponderance of studies did show an effect. Hsu et al35 concluded that rTMS has a positive influence on motor recovery in stroke and that the low-frequency approach in the contralesional M1 was more effective than the high-frequency approach in the ipsilesional M1.
As a prelude to discussing metaplasticity, synaptic plasticity must be characterized. Synaptic plasticity is a use-dependent increase (LTP) or decrease (LTD) in synaptic strength.40,41 The effects of rTMS on recovery have generally been attributed to synaptic plasticity, evidenced through changes in corticospinal excitability.20,21,25,31,34,42,43 As aforementioned by Khedr et al,20 application of 3-Hz rTMS to the ipsilesional M1 increased ipsilesional excitability, whereas application of 1-Hz rTMS to the contralesional M1 decreased contralesional excitability, with a further effect of increased excitability at the ipsilesional M1 through IHI. Khedr and colleagues' findings were consistent with the conventional biologic rules of use-dependent synaptic plasticity because high-frequency conditioning invoked an LTP-like increase in excitability, whereas low-frequency conditioning invoked an LTD-like decrease in excitability. Accordingly, LTP and LTD play a vital role in neurorehabilitation, as it is speculated that these processes are involved in neural upregulation with forced use44 or downregulation with nonuse.45
The activity-dependent nature of synaptic plasticity holds major implications for stroke rehabilitation. As a classic demonstration in the motor system, Classen et al46 applied TMS to a focal area of M1 in humans who were healthy to evoke reproducible thumb movements isolated to a certain direction at baseline. They then had participants practice thumb movements repeatedly in a different direction (forced use). Impressively, follow-up TMS applied to the same focal area produced thumb movements, not in the baseline direction but in the practiced direction transiently. Conversely, Wiesel and Hubel45 showed in kittens that temporary deprivation of visual stimulation to one eye resulted in a loss of visual cortex excitability serving that eye. Wiesel and Hubel concluded that monocular deprivation (nonuse) produces physiological defects in a system that was once capable of functioning. Constraint-induced movement therapy, with its emphasis on both using the paretic limb and not using the nonparetic limb to promote excitability and behavioral changes,23 is an application of these synaptic plasticity principles in neurorehabilitation. As the next section points out, by capitalizing on principles governing a higher order of synaptic plasticity known as metaplasticity, it may be possible to produce greater excitability changes in the desired direction.
Homeostatic Synaptic Plasticity and Metaplasticity
Although synaptic plasticity involves activity-dependent enhancement or reduction in synaptic strength, the possibility exists for the development of an unbridled positive-feedback loop,40,41 whereby use begets excessive use or nonuse begets no use. Thus, when unconstrained, LTP and LTD can be maladaptive. To counter this positive-feedback loop and maintain a functional, homeostatic range of synaptic plasticity, a negative-feedback loop is necessary.41 Homeostatic synaptic plasticity is a form of synaptic plasticity that encompasses a wide array of mechanisms aimed at stabilizing neuronal firing rates and neuronal network activity.47 Turrigiano eloquently defined homeostatic synaptic plasticity as “a homeostatic form of plasticity acting to stabilize the activity of a neuron or neuronal circuit in the face of perturbations.”47(p422) In this regard, homeostatic synaptic plasticity functions as a negative feedback loop to promote a “set-point” of synaptic activity within a functional range.40,41 Metaplasticity is one of several homeostatic mechanisms that involves modifying the plasticity-inducing capability of synapses to ensure that one type of synaptic plasticity (ie, LTP or LTD) does not predominate the system.3,40 The reader is referred to Abraham,40 Murphy and Corbett,41 and Huang et al.48 for greater detail on the cellular and molecular components underlying metaplasticity.
The Bienenstock-Cooper-Munro (BCM) theory of bidirectional synaptic plasticity highlights the changeability of synaptic plasticity inherent to metaplasticity.49 A basic tenet of this theory is that a synapse's recent history of activity influences its current level of reactivity. In a computational model of experience-dependent synaptic plasticity in the mammalian visual cortex, Bienenstock et al49 introduced a dynamic, sliding threshold for regulating the generation of future synaptic plasticity. Recent postsynaptic firing would activate cellular and molecular processes that elevate the threshold for future LTP induction while lowering the threshold for future LTD induction. Diminished postsynaptic firing would stage the opposite.
In a series of experiments, Huang et al48 demonstrated that the induction of LTP in the rat hippocampus depended on prior synaptic activity. When a weak bout of repeated stimulation (30 Hz, 0.1–0.2 seconds, capable of inducing short-term potentiation when given alone) preceded a stronger bout of stimulation (100 Hz, 0.5 seconds, capable of inducing LTP when given alone), subsequent LTP did not ensue. Huang and colleagues' work outlines the standard protocol for metaplasticity research that consists of 2 successive events: priming and conditioning. Priming results in the modification of various synaptic properties that persist long enough to alter the effects of the subsequent plasticity-inducing event.40 We operationally define priming as a session of neural conditioning that modifies the aftereffects of a subsequent conditioning session. Priming and conditioning can take the form of behavioral, environmental, pharmacological, or electrophysiological input; this article focuses on the last using rTMS.
High-Frequency Priming of Low-Frequency rTMS as Therapy
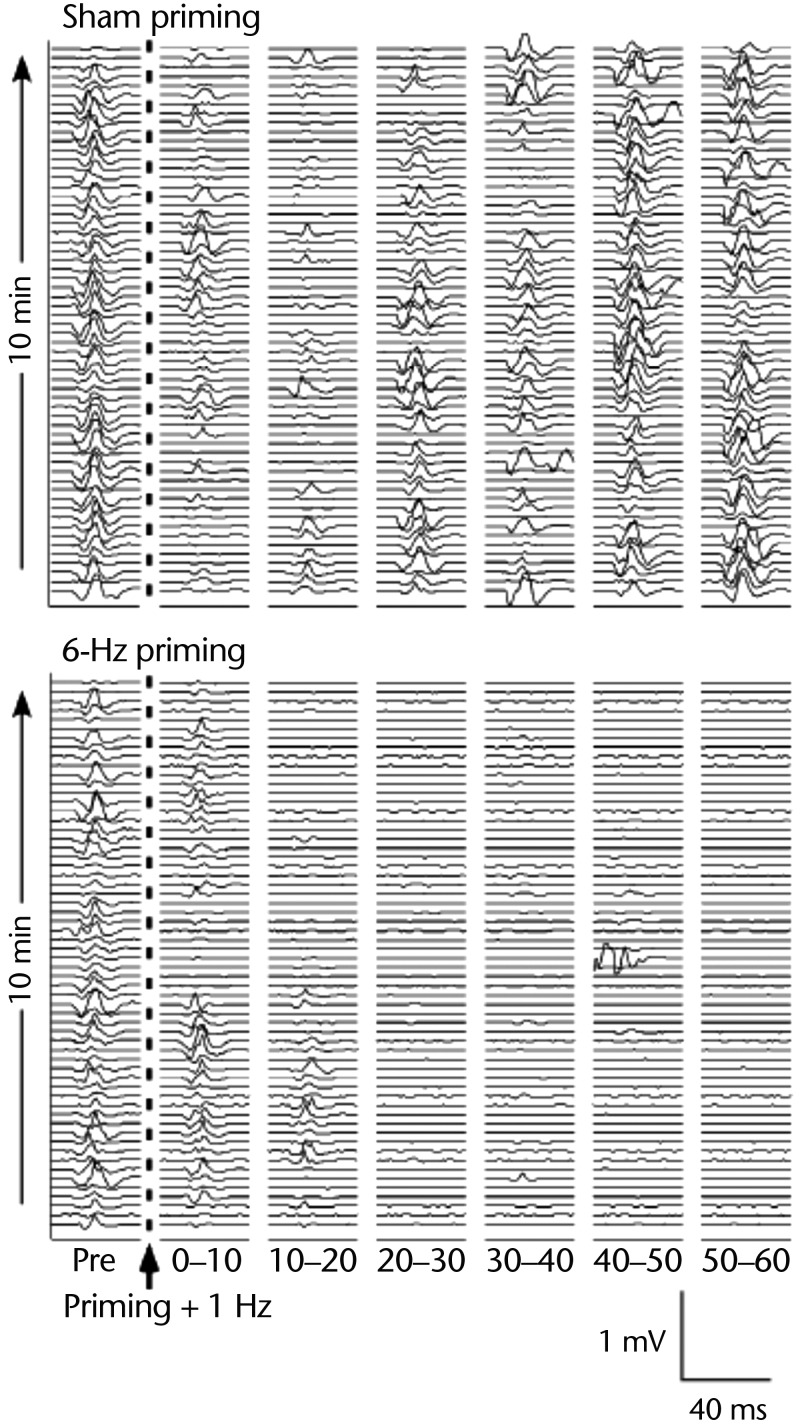
Principles of homeostatic synaptic plasticity and metaplasticity may augment the therapeutic effects of consequent rTMS conditioning. Iyer et al50 explored corticospinal excitability changes in adults who were healthy following high-frequency rTMS priming of low-frequency rTMS conditioning to the same M1. In different sessions, Iyer et al50 compared intermittent 6-Hz rTMS priming (10 minutes) of continuous 1-Hz rTMS conditioning (10 minutes) with sham rTMS priming (10 minutes) of continuous 1-Hz rTMS conditioning (10 minutes). Consistent with the BCM theory that recent high-frequency activity lowers the threshold for future LTD-like mechanisms, Iyer et al50 discovered that 6-Hz priming of 1-Hz rTMS conditioning resulted in significantly greater corticospinal depression, as demonstrated by reduced MEP amplitude in the stimulated M1 for a longer duration compared with sham priming of 1-Hz rTMS (Fig. 3).
Figure 3.
Metaplastic effects as predicted by Bienenstock-Cooper-Munro theory. (Top) Motor-evoked potentials (MEPs) from one participant's abductor pollicis brevis muscle collected for 10 minutes before (Pre) and up to 60 min (six 10-min bins) following delivery of sham 6-Hz priming stimulation + 1-Hz low-frequency repetitive transcranial magnetic stimulation (rTMS). (Bottom) Similar MEP collection taken from the same participant before and after active 6-Hz priming + 1-Hz low-frequency rTMS. Preceding low-frequency stimulation with active high-frequency priming resulted in an enhancement of corticospinal depression, as depicted by reduced MEP amplitude for a longer duration versus sham priming. Figure used with permission.50
The work by Iyer et al50 invites exploration of using primed low-frequency rTMS to the contralesional M1 in patients with stroke with the premise that greater depression of excitability in the contralesional M1 will more effectively diminish the IHI acting on the ipsilesional M1, thereby increasing excitability in the latter. A video demonstrating 6-Hz rTMS priming (part 1 of the video), followed by 1-Hz rTMS conditioning (part 2 of the video) to the contralesional M1 in a child with stroke is available below. An initial safety study in adults with stroke using 6-Hz rTMS priming of 1-Hz rTMS conditioning to the contralesional M1 resulted in no seizure or other major adverse events and no impairment of the nonparetic hand.51 A case study report of 2 adults with stroke also utilizing 6-Hz rTMS priming of 1-Hz rTMS conditioning to the contralesional M1 followed by behavioral training showed safety but mixed behavioral results.52 Additionally, Kakuda and colleagues26 demonstrated the safety of 6-Hz priming of low-frequency rTMS in adults with poststroke aphasia27 and hemiparesis. With the safety of 6-Hz primed low-frequency rTMS verified in stroke, further work addressing the efficacy of primed versus unprimed rTMS is necessary.
The presence or absence of homeostatic plasticity mechanisms in the brain of people with stroke may delineate the efficacy of priming in stroke. In participants who were healthy, Ragert et al53 applied depressive priming to the right M1 (thereby disinhibiting the left M1) followed by facilitatory conditioning to the left M1. Consistent with BCM theory and homeostatic plasticity, they found that the successive facilitatory influences combined to yield a decrease in excitability of the left M1.53 In contrast, in people with stroke, Sung et al54 applied inhibitory priming stimulation to the contralesional M1 and facilitatory conditioning stimulation to the ipsilesional M1. They observed an increase in excitability in the ipsilesional M1 and a decrease in excitability in the contralesional M1. These electrophysiological discrepancies between healthy53 and stroke54 populations justify the need for further experimentation, as homeostatic plasticity mechanisms (or lack thereof) may vary among populations. Additionally, the delivery method of priming and conditioning (ie, 2 hemispheres53,54 versus 1 hemisphere26,27,50–52) also may influence the efficacy of priming in stroke.
Importance of Timing
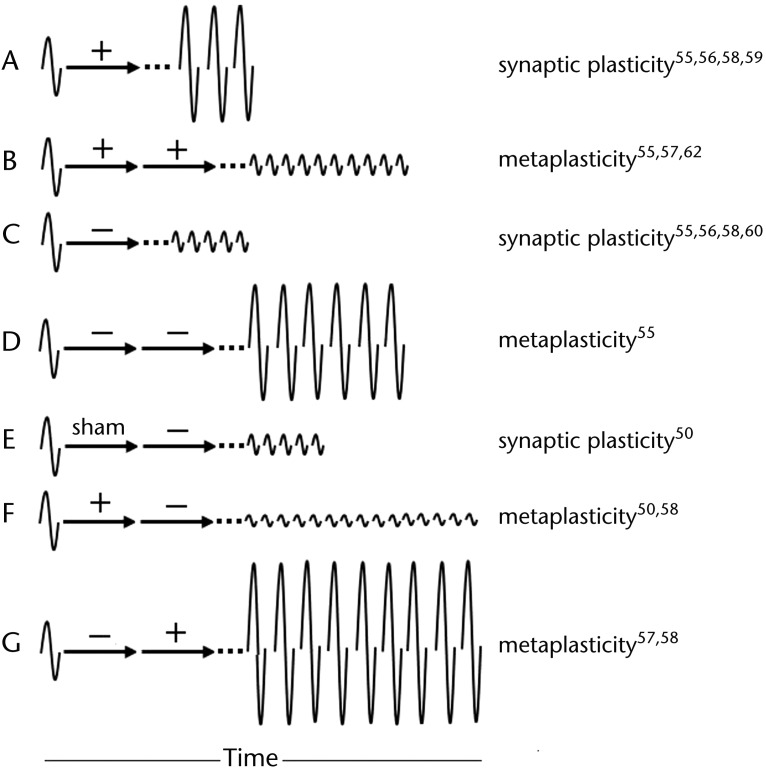
The following discussion introduces other forms of noninvasive neuromodulation used in a dual sequence of priming followed by conditioning to explore for more effective changes in brain excitability or behavior. Not surprisingly, each form of neuromodulation shows concordance with the history-dependent reactivity rule of the BCM theory. Changes in corticospinal excitability, expressed as a change in MEP amplitude, following priming and conditioning are summarized in Figure 4 along with the characterization of the aftereffects as synaptic plasticity or metaplasticity.
Figure 4.
Depiction of synaptic plasticity versus metaplasticity. Arrows represent conditioning. When 2 arrows are shown, the first is termed “priming.” Baseline excitability represented by amplitude of motor-evoked potential (MEP) to left of arrows. Aftereffects of conditioning represented by MEPs to right of arrows. Dotted lines reflect that aftereffects are not immediate. Illustration A shows normal aftereffects of increased excitability, compared with baseline, following facilitatory (+) conditioning, amounting to synaptic plasticity. Illustration B, however, shows aftereffects of decreased excitability even though both priming and conditioning were facilitatory. This alteration from normal response represents metaplasticity. Illustration C shows normal aftereffects of decreased excitability following depressive (−) conditioning. In contrast, illustration D shows opposite aftereffects when depressive priming precedes depressive conditioning. Illustration E shows normal aftereffects of decreased excitability following sham priming of depressive conditioning (identical to illustration C). Importantly, illustration F shows accentuation of aftereffects (ie, greater depression and longer duration compared with illustration E) following same depressive conditioning but preceded by opposite priming. Similarly, illustration G shows accentuated excitability aftereffects (compared with illustration A) following facilitatory conditioning but preceded by opposite priming.
Fricke et al55 evaluated excitability aftereffects in the M1 in individuals who were healthy in response to transcranial direct current stimulation (tDCS). This type of stimulation utilizes principles of conduction rather than induction to deliver continuous low-intensity direct current to the brain through strategically placed electrodes.56 Anodal tDCS applied over the M1 typically increases the excitability of that M1, whereas cathodal tDCS typically depresses it.56
Fricke et al55 first demonstrated that a single 5-minute control session of anodal tDCS increased excitability (Fig. 4A) and a 5-minute session of cathodal tDCS decreased excitability (Fig. 4C) for about the same duration as the stimulation time. They then compared aftereffects of a single 5-minute anodal/cathodal tDCS session with two 5-minute sessions of tDCS with breaks of 0, 3, or 30 minutes between sessions of either anodal priming followed by anodal conditioning (+/+) or cathodal priming followed by cathodal conditioning (−/−) (/ separates priming [first] and conditioning [second]; + represents facilitatory, − represents depressive). They found that 2 successive sessions with a 0-minute break (ie, 1 continuous stimulation session of 10 minutes) prolonged the aftereffects to be about as long as the total stimulation time but did not intensify them (not shown in Fig. 4). Successive sessions with a 30-minute break produced no difference compared with the corresponding single-session control (not shown in Fig. 4). Importantly, successive sessions with a 3-minute break resulted in aftereffects that were in the opposite direction of their corresponding single-session controls (ie, +/+ decreased excitability [Fig. 4B] and −/− increased it [Fig. 4D]). They deduced that, for metaplasticity to occur, there must be a break in the synaptic stimulation and that the second stimulation event (ie, conditioning) must commence within the time frame of the aftereffects of the first stimulation event (ie, priming). Clinically, they emphasized that this bidirectional nature of synaptic plasticity must be taken into account during modulation of the brain with successive forms of neurorehabilitation.
Lang et al57 performed similar work consisting of tDCS priming of 5-Hz rTMS conditioning to the M1 in humans who were healthy. They discovered that the direction of the aftereffects induced by the 5-Hz rTMS depended on the polarity of tDCS priming. Compared with the aftereffects of 5-Hz rTMS delivered alone, anodal tDCS priming of 5-Hz rTMS (+/+) significantly depressed corticospinal excitability (Fig. 4B), and cathodal tDCS priming of 5-Hz rTMS (−/+) significantly enhanced corticospinal excitability (Fig. 4G).
Murakami et al58 demonstrated homeostatic effects using theta-burst stimulation (TBS), a patterned-protocol of rTMS delivery consisting of 3 pulses of rTMS applied at 50 Hz repeated every 200 milliseconds. When delivered intermittently (iTBS), TBS typically exerts a facilitatory effect on corticospinal excitability (Fig. 4A).59 In contrast, continuous TBS (cTBS) typically decreases corticospinal excitability (Fig. 4C).59,60 Murakami et al58 used different combinations of iTBS and cTBS to prime and condition the M1 in humans who were healthy. They found that priming and conditioning with identical types of TBS (ie, +/+ or −/−) reduced but did not reverse the aftereffects relative to their unprimed controls (not shown in Fig. 4). However, priming and conditioning with opposing types of TBS (ie, +/− or −/+) magnified the conditioning aftereffects relative to their unprimed controls (Figs. 4F and 4G).
Quadripulse stimulation is another rTMS patterned-protocol consisting of 4 monophasic rTMS pulses per train, with each train delivered at 0.2 Hz.61 Similar to the other types of neuromodulation previously discussed, QPS also demonstrates depressive and facilitatory effects depending on its stimulation parameters. Quadripulse stimulation protocols with long interstimulus intervals (ISIs) between the pulses (≥30 milliseconds) of each train tend to depress corticospinal excitability, whereas shorter ISIs tend to increase corticospinal excitability.62 Hamada et al62 evaluated the effect of facilitatory QPS priming on subsequent facilitatory QPS conditioning (ie, +/+) in people who were healthy and found depressive, not facilitatory, aftereffects consistent with the BCM theory (Fig. 4B).
Jung and Ziemann63 studied the aftereffects following priming of behavioral conditioning. Specifically, in adults who were healthy, they evaluated the use of paired associative stimulation (PAS) followed by behavioral training, which involved rapid thumb movements, on motor learning. The PAS involved electrical stimulation of the median nerve to evoke pulses of proprioceptive feedback and sensory afferent information to the brain that were paired with precisely timed TMS pulses in the contralateral M1 so that the converging cortical inputs from these 2 sources caused either facilitatory (PAS-LTP) or depressive (PAS-LTD) aftereffects depending on the timing of pulses. Jung and Ziemann63 also included a PAS control condition. They evaluated 2 delay periods of either 0 (no delay) or 90 minutes between the PAS priming and the behavioral training, which, by itself, was known to be facilitatory. They found that with a 0-minute delay, PAS-LTD plus training (−/+) strongly facilitated motor learning, as evidenced by increased thumb acceleration relative to PAS control plus training. Their findings further suggest a metaplasticity mechanism because the depressive PAS priming interacted with the facilitatory behavioral conditioning to strengthen the effect of the latter. The PAS-LTP plus training (+/+) resulted in a brief nonsignificant trend toward improved motor learning. With the 90-minute delay between priming and training, homeostatic plasticity again prevailed for PAS-LTD plus training, as evidenced by significantly greater learning, but now it also prevailed for PAS-LTP plus training, as evidenced not by greater learning but by significantly depressed learning relative to the control.
Clinical Significance
The impediment to motor learning described above when facilitatory priming preceded training, as well as the enhancement of motor learning when depressive priming preceded training, is paradoxical but concordant with the BCM theory. A recent history (priming) of synaptic facilitation appears to deploy homeostatic processes that bias synapses toward reduced activity during subsequent conditioning, thus intercepting synaptic plasticity from achieving overexcitation. Accordingly, the facilitatory priming followed by facilitatory training actually diminished the aftereffects of the latter, which diminished learning while preserving the synapse (metaplasticity). Conversely, a recent history of synaptic depression appears to deploy homeostatic processes that produce the opposite effects, as facilitatory training superimposed on synapses already staged with prior depressive priming yielded an enhancement in motor learning. Although it is still premature to make sweeping changes to neurorehabilitation based on the BCM theory, therapists need to be cognizant of the theory as well as vigilant to patient responses (favorable and unfavorable) that may stem from these principles.
Compared with other areas of study in the neurosciences, metaplasticity is still in its infancy, at least in the clinical realm. We know that the brain is a dynamic organ that frequently rewires and recalibrates its complex array of neuronal networks to accommodate learning and the formation and storage of new memories. The studies discussed above support the notion that priming influences subsequent induction of plasticity in humans who are healthy. In stroke, with the reduction in GABAA inhibition,6 it is not known how this backdrop might influence the interaction of priming with conditioning. New research is paramount. Additionally, genetic polymorphisms affecting neural repair, specifically brain-derived neurotrophic factor (BDNF) val66met and apolipoprotein E (ApoE) ϵ4 polymorphisms, also may influence plasticity,64 stroke recovery,65 and response to rTMS.64
Although this article focused on several types of noninvasive brain stimulation methods to prime the brain in the study of metaplasticity, other forms of priming likely exist in visual imagery, cognitive training, sensory training, and physical movement.66 Interestingly, Avenanti et al25 demonstrated significant changes in corticospinal excitability and behavioral improvements in participants with stroke using physical therapy as a mode of priming for subsequent 1-Hz rTMS to the contralesional M1. However, they found the reverse order (ie, 1-Hz rTMS priming of subsequent physical therapy) to be optimal.25
The spectrum of therapeutic targets in neuromodulation is broad, extending beyond M1. Several studies have shown neuroplastic change in M1 by priming areas functionally connected to M1: somatosensory,67 supplementary,68 and premotor69 cortices along with the cerebellum.70 Therefore, addressing movement-related components such as motor planning, coordination, proprioception, and sensation prior to actual movement execution ultimately may enhance the latter.
Metaplasticity depends on a multitude of stimulation parameters: interstimulus interval, pattern or number of pulses per train, duration of stimulation, and frequency and intensity of stimulation. In this article, we focused on the factor of timing between priming and the subsequent conditioning. Beyond brain stimulation, however, a delicate balance also may exist between successive physical activity sessions and rest in patient recovery. Goedert and Miller71 found that distributed, not massed, practice72 led to significant improvement in visuomotor skill performance in individuals who were healthy. Participants undergoing massed practice performed significantly worse during subsequent testing. Metaplasticity could be a relevant factor in this example. Too much practice (ie, behavioral facilitatory conditioning) spaced closely together might invoke metaplasticity mechanisms to stabilize the excitability effects and hinder learning. Relatedly, a review on task-oriented training in people with stroke showed that distributed practice was 1 of 2 components (the other being feedback) associated with the greatest postintervention effect size.73 The authors explained the benefit of distributed practice over massed practice as the avoidance of fatigue with improved cognitive effort and memory consolidation. Yet, the homeostatic consequences of excessive excitation in a compressed period of time on synaptic activity, which distinguishes metaplasticity from more conventional forms of synaptic plasticity, also must be considered. This concept deserves attention as protocols for constraint-induced movement therapy, with its emphasis on massed practice,74 become refined.
In recent years, clinicians have embraced principles of neuroplasticity, emphasizing that repetitive activation can facilitate future activation44 and sustained inactivation can impede future activation45 (eg, “use it or lose it”). Similarly, we believe that with more research, principles of metaplasticity will eventually enter into clinical practice, emphasizing that the interaction of 2 successive facilitatory events over a certain time period can actually be depressive or facilitation followed by depression can actually accentuate the latter. Such metaplasticity principles, centered on the concept of preserving neural homeostasis, have important implications in the advancement of neurorehabilitation. Although numerous studies have demonstrated behavioral gains following the use of rTMS in stroke,21,31–33,43 the permanency of these gains, especially in response to rTMS given in isolation, is not yet clear. Recognizing the clinical utility of rTMS in producing long-standing functional improvements requires continued exploration, including investigation of the possible advantages of the priming-conditioning relationship.
Summary
Similar to “muscular wisdom,” in which intrinsic physiologic changes are instinctively deployed within muscle to avert undue fatigue,75 synaptic wisdom also appears to exist through deployment of metaplastic processes that alter synaptic plasticity to preserve synaptic function in the face of excessive excitation and depression.47 It seems possible that therapists could capitalize on such synaptic wisdom to achieve higher recovery of function. The robustness of homeostatic responses observed with priming and conditioning interactions across the varied forms of noninvasive brain stimulation strengthens the validity of metaplasticity in people who are healthy. Whether these characteristics apply equally, or perhaps even more so, in the injured nervous system is not yet known. Only with methodical experimentation and vigilant clinical observation will the priming and conditioning interactions of successive therapies be uncovered, possibly leading to improved stroke outcomes.
Supplementary Material
Footnotes
All authors provided concept/idea/project design, writing, and consultation (including review of manuscript before submission). Dr Carey provided project management and facilities/equipment.
This work was supported in part by the National Institutes of Health/National Institute of Child Health and Human Development (1 R01 HD 053153–01A2 and 1 RC1HD063838–01), the National Center for Research Resources to the University of Minnesota CTSI (1UL1RR033183), and a Promotion of Doctoral Studies scholarship from the Foundation for Physical Therapy.
References
- 1. Di Lazzaro V, Profice P, Pilato F, et al. Motor cortex plasticity predicts recovery in acute stroke. Cereb Cortex. 2010;20:1523–1528 [DOI] [PubMed] [Google Scholar]
- 2. Di Lazzaro V, Pilato F, Dileone M, et al. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol. 2008;119:715–723 [DOI] [PubMed] [Google Scholar]
- 3. Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130 [DOI] [PubMed] [Google Scholar]
- 4. Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012;69:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarkson AN, Huang BS, Macisaac SE, et al. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22:1463–1475 [DOI] [PubMed] [Google Scholar]
- 7. Lee S, Ueno M, Yamashita T. Axonal remodeling for motor recovery after traumatic brain injury requires downregulation of -aminobutyric acid signaling. Cell Death Dis. 2011;2:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiene K, Bruehl C, Zilles K, et al. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab. 1996;16:906–914 [DOI] [PubMed] [Google Scholar]
- 9. Cicinelli P, Pasqualetti P, Zaccagnini M, et al. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34:2653–2658 [DOI] [PubMed] [Google Scholar]
- 10. Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000;23:1761–1763 [DOI] [PubMed] [Google Scholar]
- 11. Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke. 1996;27:1105–1109 [DOI] [PubMed] [Google Scholar]
- 12. Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199 [DOI] [PubMed] [Google Scholar]
- 14. Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci. 2005;22:2069–2080 [DOI] [PubMed] [Google Scholar]
- 15. Allred RP, Maldonado MA, Hsu And JE, Jones TA. Training the “less-affected” forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restor Neurol Neurosci. 2005;23:297–302 [PubMed] [Google Scholar]
- 16. Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J Physiol (Lond). 1992;453:525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hinder MR, Schmidt MW, Garry MI, Summers JJ. Unilateral contractions modulate interhemispheric inhibition most strongly and most adaptively in the homologous muscle of the contralateral limb. Exp Brain Res. 2010;205:423–433 [DOI] [PubMed] [Google Scholar]
- 18. Murase N, Duque J, Mazzocchio R, Cohen L. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409 [DOI] [PubMed] [Google Scholar]
- 19. Duque J, Hummel F, Celnik P, et al. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946 [DOI] [PubMed] [Google Scholar]
- 20. Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009;16:1323–1330 [DOI] [PubMed] [Google Scholar]
- 21. Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121:1922–1929 [DOI] [PubMed] [Google Scholar]
- 22. Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830 [DOI] [PubMed] [Google Scholar]
- 23. Hamzei F, Liepert J, Dettmers C, et al. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31:710–720 [DOI] [PubMed] [Google Scholar]
- 24. Liepert J, Bauder H, Wolfgang HR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216 [DOI] [PubMed] [Google Scholar]
- 25. Avenanti A, Coccia M, Ladavas E, et al. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology. 2012;78:256–264 [DOI] [PubMed] [Google Scholar]
- 26. Kakuda W, Abo M, Kobayashi K, et al. Application of combined 6-hz primed low-frequency rTMS and intensive occupational therapy for upper limb hemiparesis after stroke. Neurorehabilitation. 2011;29:365–371 [DOI] [PubMed] [Google Scholar]
- 27. Kakuda W, Abo M, Momosaki R, Morooka A. Therapeutic application of 6-hz primed low-frequency rTMS combined with intensive speech therapy for post-stroke aphasia. Brain Inj. 2011;25:1242–1248 [DOI] [PubMed] [Google Scholar]
- 28. Wagner T, Rushmore J, Eden U, Valero-Cabre A. Biophysical foundations underlying TMS: setting the stage for an effective use of neurostimulation in the cognitive neurosciences. Cortex. 2009;45:1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(pt 4):847–858 [DOI] [PubMed] [Google Scholar]
- 30. Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403 [DOI] [PubMed] [Google Scholar]
- 31. Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122 [DOI] [PubMed] [Google Scholar]
- 32. Maeda F, Keenan JP, Tormos JM, et al. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805 [DOI] [PubMed] [Google Scholar]
- 33. Takeuchi N, Chuma T, Matsuo Y, et al. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686 [DOI] [PubMed] [Google Scholar]
- 34. Takeuchi N, Tada T, Toshima M, et al. Inhibition of the unaffected motor cortex by 1 hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40:298–303 [DOI] [PubMed] [Google Scholar]
- 35. Hsu WY, Cheng CH, Liao KK, et al. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857 [DOI] [PubMed] [Google Scholar]
- 36. Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86:707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Theilig S, Podubecka J, Bosl K, et al. Functional neuromuscular stimulation to improve severe hand dysfunction after stroke: does inhibitory rTMS enhance therapeutic efficiency? Exp Neurol. 2011;230:149–155 [DOI] [PubMed] [Google Scholar]
- 38. Talelli P, Wallace A, Dileone M, et al. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair. 2012;26:976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seniǒw J, Bilik M, Leśniak M, et al. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair. 2012;26:1072–1079 [DOI] [PubMed] [Google Scholar]
- 40. Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature Rev Neurosci. 2008;9:387. [DOI] [PubMed] [Google Scholar]
- 41. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872 [DOI] [PubMed] [Google Scholar]
- 42. Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476 [DOI] [PubMed] [Google Scholar]
- 43. Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke: a double-blind placebo-controlled crossover trial. Restor Neurol Neurosci. 2007;25:461–465 [PubMed] [Google Scholar]
- 44. Butefisch CM, Davis BC, Wise SP, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017 [DOI] [PubMed] [Google Scholar]
- 46. Classen J, Liepert J, Wise SP, et al. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123 [DOI] [PubMed] [Google Scholar]
- 47. Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733 [DOI] [PubMed] [Google Scholar]
- 49. Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carey JR, Evans CD, Anderson DC, et al. Safety of 6-hz primed low-frequency rTMS in stroke. Neurorehabil Neural Repair. 2008;22:185–192 [DOI] [PubMed] [Google Scholar]
- 52. Carey JR, Anderson DC, Gillick BT, et al. 6-hz primed low-frequency rTMS to contralesional M1 in two cases with middle cerebral artery stroke. Neurosci Lett. 2010;469:338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ragert P, Camus M, Vandermeeren Y, et al. Modulation of effects of intermittent theta burst stimulation applied over primary motor cortex (M1) by conditioning stimulation of the opposite M1. J Neurophysiol. 2009;102:766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sung W, Wang C, Chou C, et al. Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke. 2013;44:1375–1382 [DOI] [PubMed] [Google Scholar]
- 55. Fricke K, Seeber AA, Thirugnanasambandam N, et al. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–1149 [DOI] [PubMed] [Google Scholar]
- 56. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901 [DOI] [PubMed] [Google Scholar]
- 57. Lang N, Siebner HR, Ernst D, et al. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–639 [DOI] [PubMed] [Google Scholar]
- 58. Murakami T, Müller-Dahlhaus F, Lu M, Ziemann U. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol (Lond). 2012;590:5765–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206 [DOI] [PubMed] [Google Scholar]
- 60. Di Lazzaro V, Pilato F, Saturno E, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol (Lond). 2005;565(pt 3):945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hamada M, Hanajima R, Terao Y, et al. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin Neurophysiol. 2007;118:2672–2682 [DOI] [PubMed] [Google Scholar]
- 62. Hamada M, Terao Y, Hanajima R, et al. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol (Lond). 2008;586:3927–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 2009;29:5597–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol (Lond). 2008;586:5717–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cramer S, Procaccio V; GAIN Americas; GAIN International Study Investigators Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International Studies. Eur J Neurol. 2012;19:718–724 [DOI] [PubMed] [Google Scholar]
- 66. Stinear CM, Barber PA, Coxon JP, et al. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131:1381–1390 [DOI] [PubMed] [Google Scholar]
- 67. Bliem B, Müller-Dahlhaus JFM, Dinse HR, Ziemann U. Homeostatic metaplasticity in the human somatosensory cortex. J Cogn Neurosci. 2008;20:1517–1528 [DOI] [PubMed] [Google Scholar]
- 68. Hamada M, Hanajima R, Terao Y, et al. Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol (Lond). 2009;587(pt 20):4845–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pötter-Nerger M, Fischer S, Mastroeni C, et al. Inducing homeostatic-like plasticity in human motor cortex through converging corticocortical inputs. J Neurophysiol. 2009;102:3180–3190 [DOI] [PubMed] [Google Scholar]
- 70. Popa T, Velayudhan B, Hubsch C, et al. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb Cortex. 2013;23:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goedert KM, Miller J. Spacing practice sessions across days earlier rather than later in training improves performance of a visuomotor skill. Exp Brain Res. 2008;189:189–197 [DOI] [PubMed] [Google Scholar]
- 72. Magill RA. Motor Learning and Control: Concepts and Applications. 8th ed Boston, MA: McGraw-Hill Education; 2007:390–404 [Google Scholar]
- 73. Timmermans AA, Spooren AI, Kingma H, Seelen HA. Influence of task-oriented training content on skilled arm-hand performance in stroke: a systematic review. Neurorehabil Neural Repair. 2010;24:858–870 [DOI] [PubMed] [Google Scholar]
- 74. Mark VW, Taub E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restor Neurol Neurosci. 2004;22:317–336 [PubMed] [Google Scholar]
- 75. Marsden CD, Meadows JC, Merton PA. “Muscular wisdom” that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. Adv Neurol. 1983;39:169–211 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.