Abstract
Background
The study aim was to determine the accuracy of axillary ultrasound (AUS) and fine needle aspiration biopsy (FNAB)/needle core biopsy in axillary breast cancer staging.
Methods
We reviewed 256 patients with clinically node-negative breast cancer who underwent AUS +/− FNAB/needle core biopsy. AUS-guided FNAB/needle core biopsy was compared to histopathology to determine sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV).
Results
AUS-guided FNAB/needle core biopsy and final pathology were positive in 72/256 patients (28%). In 125/256 cases (49%), the AUS and final pathology were negative. Two of 110 patients had a false positive FNAB (1.8%); both received neoadjuvant chemotherapy. Nine patients (8%) had a false negative FNAB/needle core biopsy; the median size of lymph node metastasis was 3 mm. The sensitivity and specificity of AUS-guided FNAB/needle core biopsy was 71% and 99%, with a NPV of 84% and PPV of 97%.
Conclusions
AUS-guided FNAB/needle core biopsy is accurate in predicting the status of the axilla in 70% of clinically node-negative breast cancer patients. This technique is minimally invasive with a low complication rate and can obviate the need for staged lymph node procedures.
Keywords: Breast cancer, Axillary ultrasound (AUS), Fine needle aspiration biopsy (FNAB)
INTRODUCTION
Lymph node status is an important factor in the medical and surgical management of women with breast cancer (1). The presence of metastatic disease in the axillary lymph nodes is considered the single most important prognostic factor for patients with breast cancer, whereby patients have a poorer prognosis with increasing numbers of metastatic lymph nodes (2).
Evaluation of the axilla by sentinel lymph node biopsy (SLNB) is an accurate, less invasive alternative to axillary lymph node dissection (ALND), and it has become the standard of care in patients with clinically node-negative breast cancer (3, 4). Although SLNB is clearly less invasive than ALND, SLNB is not without morbidity and anesthetic risk. A recent randomized, prospective trial of SLNB versus ALND confirms that complications of SLNB include seroma formation, lymphedema, sensory nerve injury, and limitation in range of motion (5). In addition, SLNB is often performed as a staged procedure, requiring that breast cancer patients undergo two or more operations for definitive staging and treatment of the axilla. Such patients include those who have node-positive disease by SLNB and require completion ALND, those who require axillary staging prior to breast reconstruction, and those undergoing neoadjuvant chemotherapy (6). These clinical scenarios represent up to 40–50% of patients treated for breast cancer. Finally, staged SLNB/ALND may result in greater surgical morbidity (7).
Multiple reports in the literature suggest that axillary ultrasound (AUS) is a potentially valuable technique for identifying axillary metastases (8–10). AUS permits the visualization of lymph node size, shape, contour, and changes in cortical morphology and texture that appear to be associated with the presence of axillary metastases. However, sonographic signs of metastatic disease sometimes overlap with those of benign reactive changes limiting the ability of this modality alone to accurately stage the axilla (11). The addition of fine needle aspiration biopsy (FNAB) has been shown to increase the specificity of nodal staging (12–17).
The aim of the current study was to evaluate our experience with AUS and FNAB to determine the feasibility and accuracy of these techniques for staging the axilla. We also sought to identify factors that may result in discordance between the pre-operative imaging and cytopathological assessment compared to final histopathological staging.
PATIENTS AND METHODS
Institutional review board approval was obtained prior to the commencement of this retrospective study. Written informed consent of patients was not required. The surgical, radiology, and pathology databases at Washington University/Barnes Jewish Hospital were queried from January 1, 2004 to December 31, 2006 to identify all patients with a diagnosis of Stage I–III invasive breast cancer who underwent surgical treatment of their cancer at our institution. Patients who were referred from other centers following surgical excisional biopsy of their breast cancer were excluded from the study. A total of 311 patients underwent AUS prior to their surgical treatment; 55 had clinically positive axillas and were excluded from the final analysis. Charts of 256 consecutive patients with clinically node-negative operable breast cancer who underwent AUS were retrospectively reviewed. This represented approximately 40% of the total number of patients eligible for axillary ultrasound during the study period. Demographic and tumor characteristics evaluated included patient age, tumor histology, tumor size, tumor grade, overall pathologic stage, use of neoadjuvant chemotherapy prior to definitive surgical therapy, type of surgical therapy, estrogen receptor (ER) status, progesterone receptor (PR) status, Her-2-neu status, and final pathology findings.
AUS was performed with either a Siemens Sonoline Antares or Siemens Acuson Antares (Siemens Medical Solutions, Inc; Malvern, PA, USA) using a standard 5–13 MHz linear array transducer. AUS was performed prospectively by dedicated breast imaging radiologists, most often at the time of ultrasound interrogation of the primary tumor, before a tissue diagnosis of the primary lesion was performed. Axillary lymph nodes were determined to be either normal in appearance or suspicious in appearance. Suspicious lymph nodes were identified based on standard criteria, including generalized or focal thickening of the cortex, disparity in size of one or more lymph nodes compared with others, rounded appearance, and effacement of the lymph node fatty hilum (8–10).
Only suspicious-appearing lymph nodes were sampled with FNAB or needle core biopsy. The decision to perform FNAB versus core needle biopsy was at the discretion of the attending radiologist. Of the 256 patients, 13 (5%) had a needle core biopsy and 243 (95%) had FNAB. FNAB was performed manually using a 25 gauge needle attached to a 10 mL syringe, following administration of superficial local anesthesia with 1% Xylocaine. Needle core biopsy was performed with the Achieve 14 gauge Programmable Automatic Biopsy System (Cardinal Health Inc., Dublin, Ohio) following local anesthesia with 1% Xylocaine. Both techniques were performed under ultrasound-guidance with direct visualization of the needle entering the cortex of the lymph node to confirm position of the needle tip in the appropriate location. On average, three passes were made during FNAB and one or two passes were made during needle core biopsy.
Aspirates were prepared with standard Giemsa and Papanicolaou staining and examined by a dedicated cytopathologist. Cytology was classified as benign, malignant, suspicious (but not definitive for malignancy), or quantity not sufficient for diagnosis. Malignancy was defined by the presence of cells with enlarged, irregular nuclei and prominent nucleoli based on standard cytopathological criteria (15, 16). Needle core specimens were submitted for standard pathological analysis.
Patients with a malignant FNAB or needle core result underwent complete ALND at the time of definitive surgery. Patients with benign, suspicious, or quantity not sufficient for diagnosis results underwent standard SLNB using radiocolloid and/or blue dye injection. For patients undergoing neoadjuvant chemotherapy, standard institutional practice is to perform SLNB prior to chemotherapy initiation. The types and number of cycles of neoadjuvant therapies utilized varied, though anthracycline-based regimens were most common.
The performance of AUS and FNAB/needle core biopsy for staging the axilla was summarized using sensitivities and specificities, treating the pathologic findings as the “true” status. Similar summary statistics were also calculated within subgroups determined by patient and tumor characteristics. However, all of the data analyses were descriptive in nature and no formal statistical inference was performed.
RESULTS
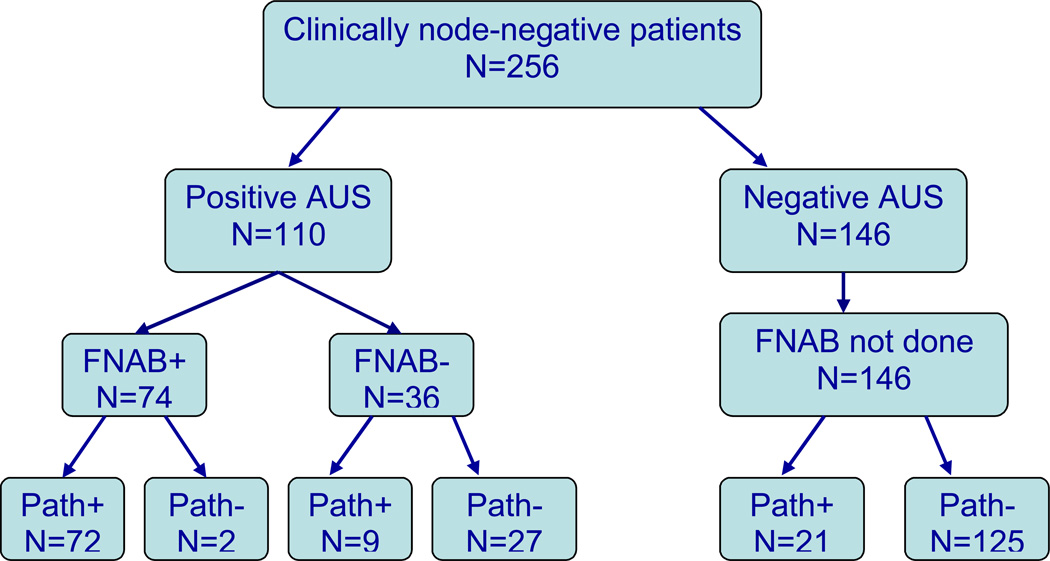
Between January 1, 2004 and December 31, 2006, 256 clinically node-negative patients underwent AUS followed by definitive surgical intervention at our institution. Representative ultrasounds of both normal and suspicious lymph nodes are illustrated in Figure 1. Demographics and tumor characteristics are shown in Table 1. Approximately 66% patients had T1 lesions, and the majority of patients had Stage I or II disease. Breast conservation therapy was the preferred method of treatment for 66% of patients, while 34% underwent mastectomy. Most of the tumors were ER and PR positive (65% and 50%, respectively); 17% of the cancers were amplified for Her-2-neu. Of the total study population, 34% received neoadjuvant chemotherapy. The outcomes of the 256 clinically node negative patients that completed AUS and surgical axillary staging are illustrated in Figure 2.
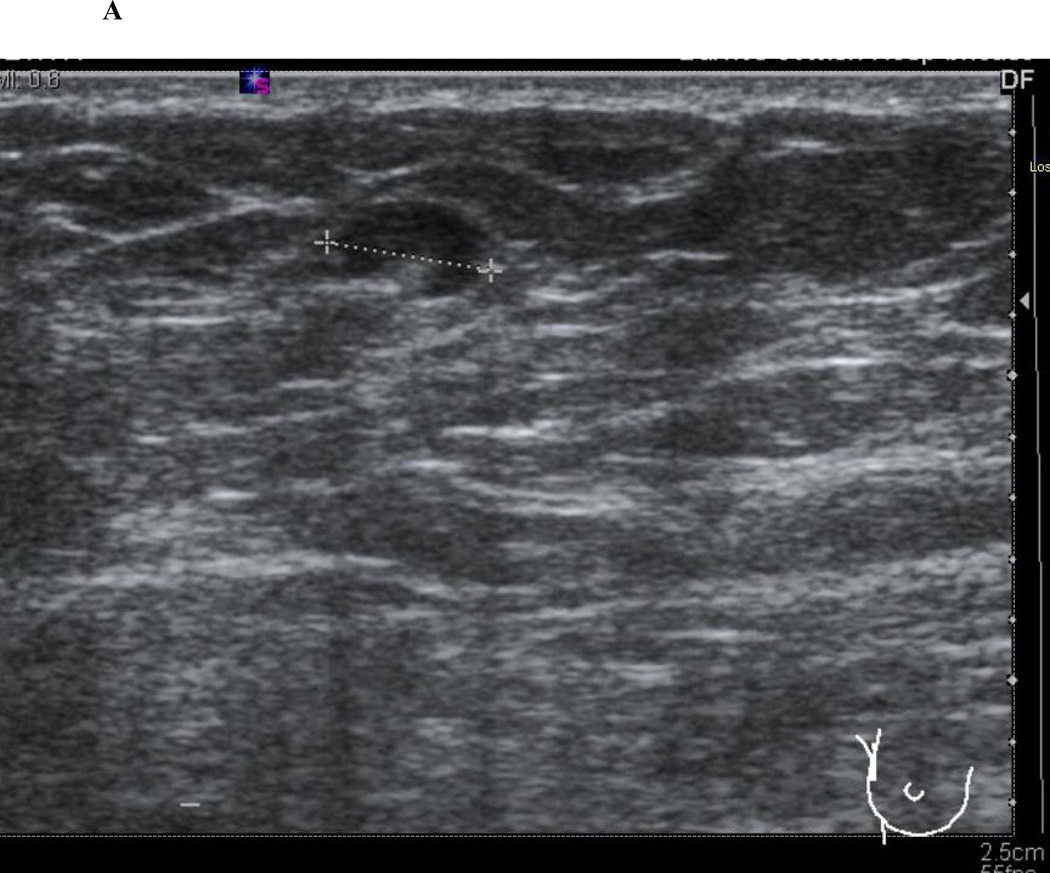
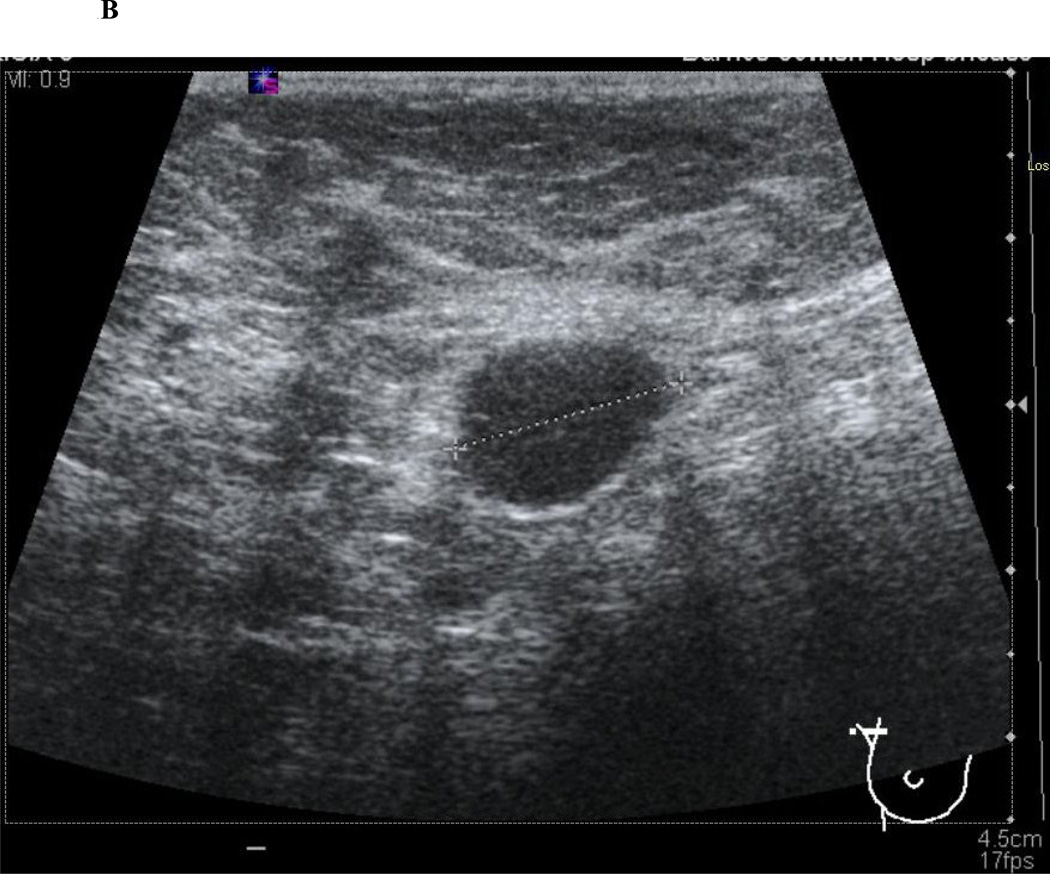
Figure 1.
Axillary ultrasound characteristics of normal and abnormal lymph nodes. Normal lymph nodes have a smooth, homogenous cortex with a centrally located, preserved fatty hilum (1A). Abnormal, or suspicious for metastatic involvement, lymph nodes have a rounded appearance with an eccentrically thickened, heterogenous cortex and effacement of the fatty hilum (1B).
Table 1.
Patient and tumor characteristics of 256 clinically node-negative invasive breast cancer patients undergoing AUS +/− FNAB/needle core biopsy
| Characteristic | Number of patients (%) |
|---|---|
| Mean age (years) | 50.4 |
| T stage (%) | |
| T1 | 168 (65.6) |
| T2 | 70 (27.3) |
| T3 | 11 (4.3) |
| T4 | 7 (2.7) |
| Clinical Stage (%) | |
| I | 125 (48.8) |
| II | 109 (42.6) |
| III | 22 (8.6) |
| Pathologic Stage (%) | |
| I | 118 (46.1) |
| II | 102 (39.8) |
| III | 36 (14.1) |
| Type of Surgery (%) | |
| BCT | 169 (66.0) |
| Mastectomy | 87 (34.0) |
| Pathological node stage (%) | |
| N0 | 155 (60.5) |
| N1 | 74 (28.7) |
| N2 | 21 (8.2) |
| N3 | 6 (2.3) |
| ER status (%) | |
| Positive | 167 (65.0) |
| Negative | 89 (35.0) |
| PR status (%) | |
| Positive | 128 (50.0) |
| Negative | 126 (50.0) |
| Her2-neu status (%) | |
| Positive | 43 (17.0) |
| Negative | 213 (83.0) |
| Neoadjuvant chemotherapy (%) | 87 (34.0) |
AUS = axillary ultrasound; FNAB = fine needle aspiration biopsy; BCT= breast conserving therapy; ER = estrogen receptor; PR = progesterone receptor
Figure 2.
Flow chart representing the axillary ultrasound (AUS), fine needle aspiration biopsy/needle core biopsy (shown as FNAB on diagram for simplicity), and final lymph node pathology for patients with clinically negative axillary lymph nodes.
There were 110 patients (43%) who had suspicious lymph nodes on AUS and underwent FNAB or needle core biopsy. Of these, 74 had a positive FNAB or needle core biopsy and underwent complete ALND at the time of definitive breast surgical therapy; 72 (97%) were confirmed to have axillary disease on final pathological assessment. Two of the 74 patients (3%) who had a positive AUS-guided FNAB/needle core biopsy, had N0 pathological disease on their final specimens. Review of the cytology slides by two independent pathologists confirmed the presence of malignant cells. Both patients had adequate ALNDs with 11 and 14 nodes removed, respectively. Both of these patients had undergone neoadjuvant chemotherapy prior to definitive surgical intervention. Thirty-six patients had an abnormal AUS with a benign or normal cytopathology/pathology result following FNAB or needle core biopsy. Of those 36 patients, 27 (75%) were confirmed to be node-negative, representing false positive results of the AUS alone and likely attributable to reactive lymph node morphology. Nine patients had an abnormal AUS, a benign FNAB/needle core biopsy, but a positive final pathology, representing a false negative rate for FNAB/needle core biopsy of 8% (4% for the total analysis group). In those 9 patients, the median size of the lymph node metastasis was 3 mm (range 1.5 mm–2.2 cm).
There were 146 patients (57%) who had clearly benign appearing lymph nodes or lymph nodes that did not meet criteria for suspicion on AUS. For patients with a negative AUS who did not have pre-operative FNAB/needle core biopsy, the final pathology was negative for nodal disease in 125 (86%) patients and positive in 21 (14%) patients. Retrospectively, there were no particular patterns on AUS in the 21 false negative patients that could be identified as suspicious.
Overall, the sensitivity and specificity of AUS alone were 79% and 81%, respectively. The sensitivity and specificity of FNAB/needle core biopsy alone were 89% and 93%, respectively. The overall combined sensitivity and specificity for AUS-guided FNAB/needle core biopsy were 71% and 99%, respectively, with a NPV of 84% and PPV of 97%. There were no identifiable clinical factors that significantly altered the sensitivity and specificity of this minimally invasive technique (data not shown). The routine use of AUS and FNAB/needle core biopsy spared 74 (29%) patients an additional, staged axillary procedure.
DISCUSSION
Staging of the axilla plays a vital role in determining treatment pathways for patients newly diagnosed with breast cancer. For those patients with small breast cancers (T1 and T2 lesions) without clinical evidence of lymph node involvement, SLNB at the time of operation for the primary lesion has become the preferred method of axillary sampling, replacing complete ALND (3, 4) . The overall accuracy and false negative rate of SLNB has been validated by several large studies and ranges from 95–99% and 8–12%, respectively (3–6). Furthermore, SLNB has been shown to be associated with less morbidity than complete ALND. A recent randomized controlled trial by Purushotham et al. (5) compared morbidity of ALND versus SLNB alone. The SLNB group was found to have significantly less arm lymphedema, seroma formation, and sensory deficit than the ALND group.
Despite the obvious decreases in morbidity with the use of SLNB, there are new challenges that have arisen in these patients who are no longer undergoing complete ALND at the time of the definitive breast procedure and who are found to have a positive SLNB. Although more robust intraoperative SLN assessments are emerging, many patients who undergo SLNB and have a positive node, require delayed ALND at a separate, staged operation. Scar tissue and edema may obscure identification of neurovascular structures in patients undergoing a second, staged axillary surgery compared to a single-stage axillary procedure, leading to higher complication rates. Although these concerns persist, a recent study by Goyal et al. (7), comparing morbidity of staged ALND after positive SLNB versus ALND alone, found that rates of lymphedema, seroma formation, sensory deficit, and shoulder dysfunction were similar between the two groups. Wound infection rates and return to work time were also similar. On the other hand, patients undergoing a staged ALND post-SLNB were found to have significantly increased operative times and longer overall hospital stays (7).
AUS combined with FNAB/needle core biopsy represents a minimally invasive procedure that can accurately stage the axilla, avoid staged operative procedures, and allow for definitive treatment planning. In the current study, the use of AUS-guided FNAB spared 74 patients (73% of the total node-positive population) a staged axillary procedure. Based upon estimates provided by Goyal et al. (7), immediate ALND saves approximately 7.5 minutes of operative time per patient (mean operative time 32.5 minutes for two-step, staged ALND vs. 25 minutes for one-step ALND) as well as approximately 4 days of hospital stay as compared to patients who have two separate axillary procedures (10.3 days for two step ALND (first and second surgery) vs. 6.2 days for one step ALND). The decrease in hospital time is unlikely to be significantly altered in the current study, as most patients spend only one night in the hospital following initial or staged ALND in the United States compared to the 6–10 days in the British National Health System as documented by Goyal et al. (7). However, the routine use of AUS-guided FNAB likely resulted in approximately 555 minutes less operative time for our 74 patients. Although we have not performed a comprehensive cost analysis, this would suggest a cost benefit for AUS-FNAB as well, as previously shown (18). Avoidance of staged axillary procedures also may result in less delay in initiating neoadjuvant and/or adjuvant treatments.
Physical examination of the axilla alone can be inaccurate in identifying lymph nodes involved with metastatic disease. The false negative rate of physical examination alone has been reported to be as high as 30–45% (19–20). In fact, a recent study by Specht et al. (21) found that clinical axillary examination is also subject to false positive results and is, by itself, insufficient justification for ALND. In the current study, 55 patients who had clinically positive axillas were excluded from our analysis. Of these patients, 48 (87%) had a positive, or suspicious AUS, and 45 (82%) of these patients had axillary disease on final pathology (data not shown). Therefore, there may be a role for routine AUS even in patients who have clinically positive lymph nodes to increase the accuracy of detection of involved lymph nodes.
For patients with clinically negative axillas, the use of ultrasound surveillance of the axilla allows the assessment of lymph node morphology. Several criteria have been identified that allow lymph nodes to be categorized as suspicious or highly suggestive of metastatic lymph node involvement, including thickening or eccentric lobulation of the cortical tissue, change in lymph node shape to a more rounded structure, compression or displacement of the hilum, and replacement of the normally hyperechoic fatty hilum by hypoechoic tissue (8–17, 19, 20). Bonnema et al. (19) studied 150 axillas and reported 36% sensitivity and 95% specificity for AUS alone, utilizing abnormal echo patterns (echo-poor lymph node, inhomogenous pattern within the lymph node cortex, loss of fatty hilum) as criteria for malignancy. The reported sensitivity and specificity of AUS alone in other series using similar criteria ranges from 56–72% and 70–90%, respectively (8–20, 22). The sensitivity increased to 87% and specificity decreased to 56% when lymph node size greater than 5mm was added to criteria for identifying malignancy on AUS (22). The relatively lower sensitivity and higher specificity of AUS observed in most series suggests that while AUS is unlikely to lead to a high false positive rate, it does not have the diagnostic power to identify all involved metastatic lymph nodes as a screening tool. In the current study, we observed a high sensitivity and specificity for AUS alone of 79% and 81%, respectively. This is likely due to the experience of our dedicated breast radiologists, and for this reason, AUS is an extremely valuable screening tool to predict the axillary status at our institution. Whether similar results can be generalized across all institutions treating patients with breast cancer is unclear.
AUS alone is not definitive for axillary staging and a tissue diagnosis is essential to determine the presence of metastatic lymph node disease. When AUS was combined with FNAB/needle core biopsy, we observed a sensitivity of 71% and a specificity of 99%. The false negative rate for FNAB/needle core biopsy was 8% which is consistent with that reported in other series, which range from 10–12% (8–20, 22–24). There are several reasons likely accounting for false negative results of cytopathology when compared to final pathology. The main reason for this finding is likely attributed to sampling error. Krishnamurthy et al. (10) found that 66% of the false negative cases following AUS and FNAB had a metastatic deposit that was < 5 mm. A recent study by Hinson et al. (22) evaluated the use of AUS and FNAB in patients with clinically negative axillas. They stratified patients as high or low risk for axillary lymph node involvement using specific criteria: high risk patients included those with tumors ≥1 cm that were grade III or tumors ≥1.5 cm regardless of tumor grade. The overall sensitivity and specificity of AUS and FNAB were 82% and 100%, respectively (22). For patients with metastatic deposits ≥ 5 mm, the sensitivity and specificity approached 100%, while 18 patients with metastatic lymph node deposits <5mm were incorrectly staged by AUS and FNAB even though 8 of 18 AUS were considered abnormal; all 18 patients had single node involvement (22). In the current study, 86% of the false negative cases had metastatic deposits between 1.5–4.0 mm, and all had single node involvement. This highlights the observation that AUS-guided FNAB/needle core biopsy is less likely to make a positive diagnosis in lymph nodes with small metastatic deposits <5mm.
We also observed 2 false positive results with AUS and FNAB/needle core biopsy. Interestingly, both of these patients received neoadjuvant chemotherapy prior to axillary node dissection. On review of the cytology, both specimens had unequivocal presence of malignant cells. The final negative pathology more than likely represents clearance of the lymph nodes by the administration of chemotherapy. Rates of complete axillary conversion post neoadjuvant chemotherapy are reported to be between 23–38% (25–29). We believe that both of our false positives are secondary to clearance by chemotherapy, and therefore, we did not have any “true” false positive results with the technique. The “true” false positive rate has been estimated to be 1.4–1.6% for AUS-guided FNAB (30, 31). “True” false positive results are likely due to misinterpretation of the cytologic specimens, thus stressing the importance of an experienced cytopathologist for accurate lymph node staging.
In conclusion, AUS combined with FNAB/needle core biopsy for suspicious-appearing lymph nodes represents a minimally invasive method for accurate staging of the axilla in patients with invasive breast cancer and clinically negative physical exams. Using this combined technique, we were able to accurately predict the final pathologic status of the axilla in over 70% of our patients. Patients with positive FNAB can be spared a SLNB procedure and staged treatment of the axilla which may result in decreased time to adjuvant therapies. Factors that contribute to discordance between pre-operative FNAB and final histopathology include sampling error in lymph nodes with <5mm metastatic deposits and the administration of neoadjuvant chemotherapy. AUS and lymph node sampling of suspicious lymph nodes is an excellent adjunct to the pre-operative work-up of all patients with clinically node-negative invasive breast cancer.
SUMMARY.
Axillary ultrasound combined with fine needle aspiration biopsy of suspicious lymph nodes represents a minimally invasive method for detecting metastatic disease in lymph nodes of women with breast cancer, thereby avoiding multiple, staged axillary procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Joint Committee on Cancer. Manual for staging of cancer. Philadelphia: AJCC/JB Lippincott; 1992. [Google Scholar]
- 2.Valgussa PBG, Veronesi U. Patterns of relapse and survival following radical mastectomy. Cancer. 1978;41:1170–1178. doi: 10.1002/1097-0142(197803)41:3<1170::aid-cncr2820410355>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer – a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 4.McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–2566. doi: 10.1200/JCO.2000.18.13.2560. [DOI] [PubMed] [Google Scholar]
- 5.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 6.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel lymph node resection and conventional axillary lymph node dissection in patients with clinically node negative breast cancer: results from the NSABP B-32 randomized phase III trial. Lancet. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 7.Goyal A, Newcombe R, Chhabra A, et al. Morbidity in Breast Cancer Patients with Sentinel Node Metastases Undergoing Delayed Axillary Lymph Node Dissection (ALND) Compared with Immediate ALND. Ann Surg Oncol. 2008;15(1):262–267. doi: 10.1245/s10434-007-9593-3. [DOI] [PubMed] [Google Scholar]
- 8.Brancato B, Zappa M, Bricolo D, et al. Role of ultrasound-guided fine needle cytology of axillary lymph nodes in breast carcinoma staging. Radiol Med (Torino) 2004;108:345–355. [PubMed] [Google Scholar]
- 9.Deurloo EE, Tanis PJ, Gilhuijs KG, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39:1068–1073. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy SN, Sneige DG, Bedi BS, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 11.Verbanck J, Vandewiele I, DeWinter HD, et al. Value of axillary ultrasonography and sonographically guided puncture of axillary nodes. A prospective study in 144 consecutive patients. J Clin Ultrasound. 1997;25:53–56. doi: 10.1002/(sici)1097-0096(199702)25:2<53::aid-jcu1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Popil MB, Sahoo M, Mehotra N, et al. Preoperative ultrasound guided fine needle aspiration cytology for axillary staging in breast carcinoma. Australasian Radiology. 2006;50:122–126. doi: 10.1111/j.1440-1673.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 13.Oruwari J, Chung M, Koelliker S, et al. Axillary staging using ultrasound guided fine needle aspiration biopsy in locally advanced breast cancer. Am J Surg. 2002;184:307–309. doi: 10.1016/s0002-9610(02)00957-1. [DOI] [PubMed] [Google Scholar]
- 14.Davis J, Brill Y, Simmons S, et al. Ultrasound guided fine needle aspiration of clinically negative lymph node versus sentinel node mapping in patients at high risk for axillary metastasis. Ann Surg Oncol. 2006;13(12):1545–1552. doi: 10.1245/s10434-006-9095-8. [DOI] [PubMed] [Google Scholar]
- 15.van Rijk M, Deurloo E, Nieweg O, et al. Ultrasonography and fine needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol. 2005;13(1):31–35. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Jain A, Haisfield-Wolfe ME, Lange J, et al. The Role of Ultrasound-Guided Fine-Needle Aspiration of Axillary Nodes in the Staging of Breast Cancer. Ann Surg Oncol. 2007;15(2):462–471. doi: 10.1245/s10434-007-9623-1. [DOI] [PubMed] [Google Scholar]
- 17.Kanter AT, Van Eijck CHJ, VanGeel AN, et al. Multicenter study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg. 1999;86:1459–1462. doi: 10.1046/j.1365-2168.1999.01243.x. [DOI] [PubMed] [Google Scholar]
- 18.Genta F, Zanon E, Camanni M. Cost/Accuracy ratio analysis in breast cancer patients undergoing ultrasound guided fine needle aspiration cytology, sentinel node biopsy and frozen section of node. World J Surg. 2007;31:1155–1163. doi: 10.1007/s00268-007-9009-3. [DOI] [PubMed] [Google Scholar]
- 19.Bonnema J, VanGeel AN, Ooijen BV, et al. Ultrasound guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients. New Diagnostic Method. World J Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 20.Feu J, Tressera F, Fabregas R, et al. Metastatic breast carcinoma in axillary lymph nodes: in vitro US detection. Radiology. 1997;205:831–835. doi: 10.1148/radiology.205.3.9393544. [DOI] [PubMed] [Google Scholar]
- 21.Specht MC, Fey JV, Borgen PI, Cody HS. Is the clinically positive axilla in breast cancer really a contraindication to sentinel lymph node biopsy? J Am Coll Surg. 2005;200:10–14. doi: 10.1016/j.jamcollsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Hinson JL, McGrath P, Moore A, et al. The Critical Role of Axillary Ultrasound and Aspiration Biopsy in the Management of Breast Cancer Patients with Clinically Negative Axilla. Ann Surg Oncol. 2007;15(1):250–255. doi: 10.1245/s10434-007-9524-3. [DOI] [PubMed] [Google Scholar]
- 23.Gilissen F, Oostenbroek R, Storm R, Westenend P, Plaisier P. Prevention of futile sentinel node procedures in breast cancer: Ultrasonography of the axilla and fine-needle aspiration cytology are obligatory. Eur J Surg Oncol. 2007 doi: 10.1016/j.ejso.2007.07.198. [DOI] [PubMed] [Google Scholar]
- 24.Koelliker SL, Chung MA, Mainiero MB, Steinhoff MM, Cady B. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer – correlation with primary tumor size. Radiology. 2008;246:81–89. doi: 10.1148/radiol.2463061463. [DOI] [PubMed] [Google Scholar]
- 25.Kuerer H, Sahin A, Hunt K, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230(1):72–78. doi: 10.1097/00000658-199907000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamounas EP. Overview of National Surgical Adjuvant Breast Project neoadjuvant chemotherapy studies. Semin Oncol. 1998;25:31–35. [PubMed] [Google Scholar]
- 27.Sapino A, Cassoni P, Zanon E, et al. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: role in breast cancer management. Br J Cancer. 2003;88:702–706. doi: 10.1038/sj.bjc.6600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedrosian I, Bedi D, Kuerer HM, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann Surg Oncol. 2003;10:1025–1030. doi: 10.1245/aso.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Alkuwari E, Auger M. Accuracy of fine-needle aspiration cytology of axillary lymph nodes in breast cancer patients: a study of 115 cases with cytologic-histologic correlation. Cancer. 2008 Feb 19; doi: 10.1002/cncr.23344. Online publication. [DOI] [PubMed] [Google Scholar]
- 30.Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn IM, Urich D, Van Der Kwast TH. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer. 2003;39:170–174. doi: 10.1016/s0959-8049(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 31.Ciatto S, Brancato B, Risso G, et al. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res Treat. 2007;103:85–91. doi: 10.1007/s10549-006-9355-0. [DOI] [PubMed] [Google Scholar]