Abstract
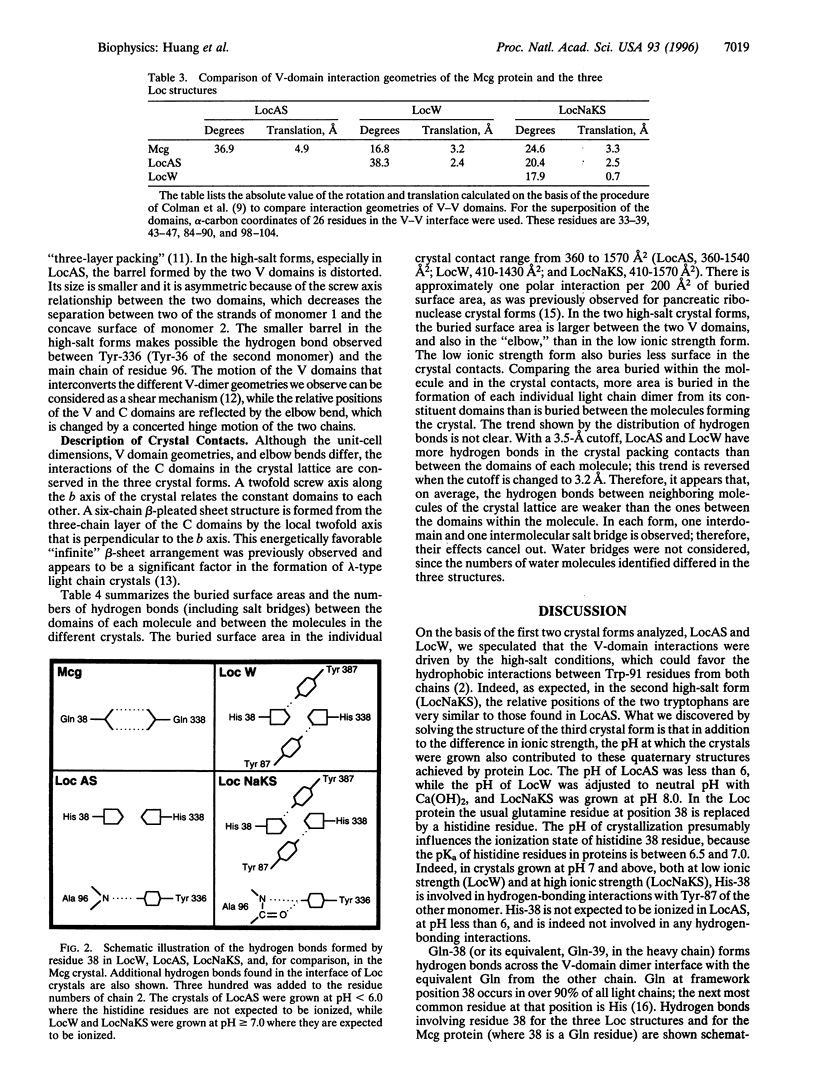
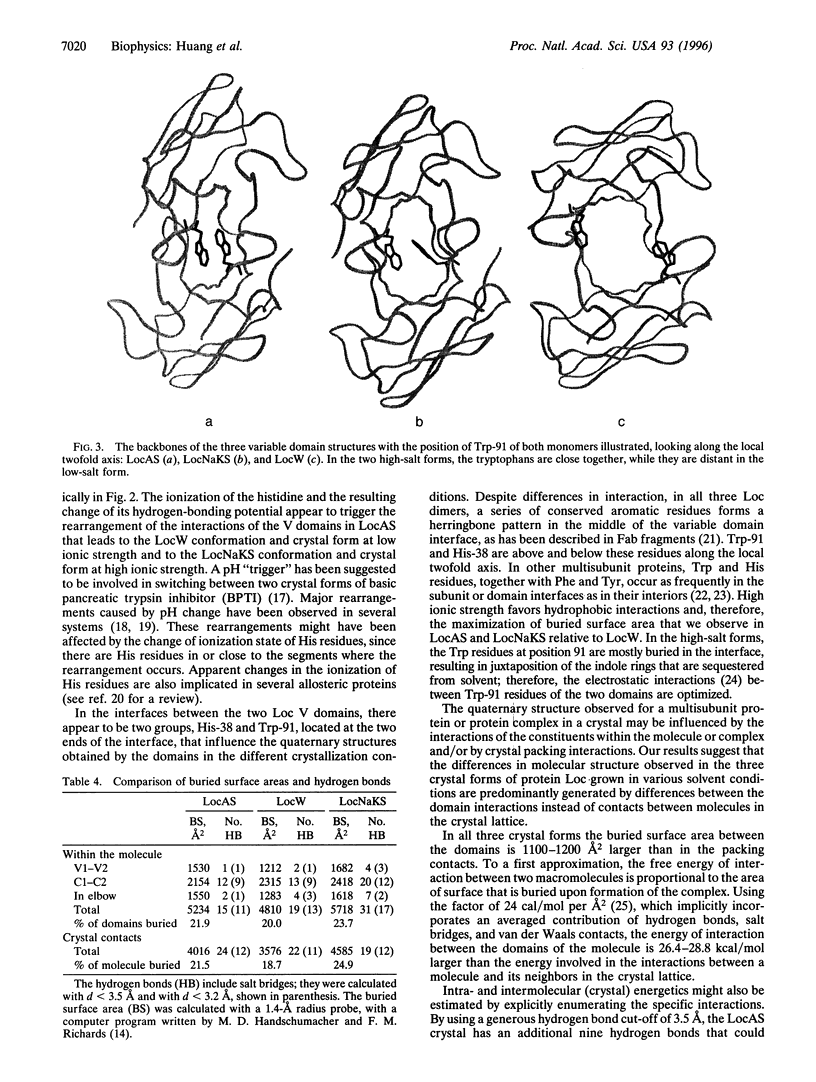
The structure of a multisubunit protein (immunoglobulin light chain) was solved in three crystal forms, differing only in the solvent of crystallization. The three structures were obtained at high ionic strength and low pH, high ionic strength and high pH, and low ionic strength and neutral pH. The three resulting "snapshots" of possible structures show that their variable-domain interactions differ, reflecting their stabilities under specific solvent conditions. In the three crystal forms, the variable domains had different rotational and translational relationships, whereas no alteration of the constant domains was found. The critical residues involved in the observed effect of the solvent are tryptophans and histidines located between the two variable domains in the dimeric structure. Tryptophan residues are commonly found in interfaces between proteins and their subunits, and histidines have been implicated in pH-dependent conformation changes. The quaternary structure observed for a multisubunit protein or protein complex in a crystal may be influenced by the interactions of the constituents within the molecule or complex and/or by crystal packing interactions. The comparison of buried surface areas and hydrogen bonds between the domains forming the molecule and between the molecules forming the crystals suggest that, for this system, the interactions within the molecule are most likely the determining factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. An investigation of protein subunit and domain interfaces. Protein Eng. 1988 Jul;2(2):101–113. doi: 10.1093/protein/2.2.101. [DOI] [PubMed] [Google Scholar]
- Bennett M. J., Choe S., Eisenberg D. Domain swapping: entangling alliances between proteins. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3127–3131. doi: 10.1073/pnas.91.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat T. N., Bentley G. A., Fischmann T. O., Boulot G., Poljak R. J. Small rearrangements in structures of Fv and Fab fragments of antibody D1.3 on antigen binding. Nature. 1990 Oct 4;347(6292):483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994 Sep 1;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Burgess K. R., Evans J. A., Whitelaw W. A. No evidence for hypothalamic cooling during nasal cold air breathing in man. Clin Invest Med. 1988 Apr;11(2):134–138. [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Weakly polar interactions in proteins. Adv Protein Chem. 1988;39:125–189. doi: 10.1016/s0065-3233(08)60376-9. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Short M. T., Westholm F. A., Stevens F. J., Wang B. C., Furey W., Jr, Solomon A., Schiffer M. Novel arrangement of immunoglobulin variable domains: X-ray crystallographic analysis of the lambda-chain dimer Bence-Jones protein Loc. Biochemistry. 1985 Aug 27;24(18):4890–4897. doi: 10.1021/bi00339a025. [DOI] [PubMed] [Google Scholar]
- Chothia C. Hydrophobic bonding and accessible surface area in proteins. Nature. 1974 Mar 22;248(446):338–339. doi: 10.1038/248338a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Novotný J., Bruccoleri R., Karplus M. Domain association in immunoglobulin molecules. The packing of variable domains. J Mol Biol. 1985 Dec 5;186(3):651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Crosio M. P., Janin J., Jullien M. Crystal packing in six crystal forms of pancreatic ribonuclease. J Mol Biol. 1992 Nov 5;228(1):243–251. doi: 10.1016/0022-2836(92)90503-c. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- Foote J., Milstein C. Conformational isomerism and the diversity of antibodies. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10370–10374. doi: 10.1073/pnas.91.22.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher W. H., Croker K. M. Identification of a molecular switch that selects between two crystals forms of bovine pancreatic trypsin inhibitor. Protein Sci. 1994 Sep;3(9):1602–1604. doi: 10.1002/pro.5560030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein M., Lesk A. M., Chothia C. Structural mechanisms for domain movements in proteins. Biochemistry. 1994 Jun 7;33(22):6739–6749. doi: 10.1021/bi00188a001. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 1985;115:252–270. doi: 10.1016/0076-6879(85)15021-4. [DOI] [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. Elbow motion in the immunoglobulins involves a molecular ball-and-socket joint. Nature. 1988 Sep 8;335(6186):188–190. doi: 10.1038/335188a0. [DOI] [PubMed] [Google Scholar]
- Novotný J., Haber E. Structural invariants of antigen binding: comparison of immunoglobulin VL-VH and VL-VL domain dimers. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4592–4596. doi: 10.1073/pnas.82.14.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Mechanisms of cooperativity and allosteric regulation in proteins. Q Rev Biophys. 1989 May;22(2):139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Ainsworth C., Xu Z. B., Carperos W., Olsen K., Solomon A., Stevens F. J., Chang C. H. Structure of a second crystal form of Bence-Jones protein Loc: strikingly different domain associations in two crystal forms of a single protein. Biochemistry. 1989 May 2;28(9):4066–4072. doi: 10.1021/bi00435a065. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Chang C. H., Naik V. M., Stevens F. J. Analysis of immunoglobulin domain interactions. Evidence for a dominant role of salt bridges. J Mol Biol. 1988 Oct 5;203(3):799–802. doi: 10.1016/0022-2836(88)90210-0. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Chang C. H., Stevens F. J. Formation of an infinite beta-sheet arrangement dominates the crystallization behavior of lambda-type antibody light chains. J Mol Biol. 1985 Nov 20;186(2):475–478. doi: 10.1016/0022-2836(85)90120-2. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Girling R. L., Ely K. R., Edmundson A. B. Structure of a lambda-type Bence-Jones protein at 3.5-A resolution. Biochemistry. 1973 Nov 6;12(23):4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Takimoto-Kamimura M., Rini J. M., Profy A. T., Wilson I. A. Major antigen-induced domain rearrangements in an antibody. Structure. 1993 Oct 15;1(2):83–93. doi: 10.1016/0969-2126(93)90024-b. [DOI] [PubMed] [Google Scholar]
- Stevens F. J., Chang C. H., Schiffer M. Dual conformations of an immunoglobulin light-chain dimer: heterogeneity of antigen specificity and idiotope profile may result from multiple variable-domain interaction mechanisms. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6895–6899. doi: 10.1073/pnas.85.18.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]