Abstract
Ginsenosides are the primary bioactive components of ginseng, which is a popular medicinal plant that exhibits diverse pharmacological activities. Protopanaxadiol, protopanaxatriol and oleanolic acid are three basic aglycons of ginsenosides. Producing aglycons of ginsenosides in Saccharomyces cerevisiae was realized in this work and provides an alternative route compared to traditional extraction methods. Synthetic pathways of these three aglycons were constructed in S. cerevisiae by introducing β-amyrin synthase, oleanolic acid synthase, dammarenediol-II synthase, protopanaxadiol synthase, protopanaxatriol synthase and NADPH-cytochrome P450 reductase from different plants. In addition, a truncated 3-hydroxy-3-methylglutaryl-CoA reductase, squalene synthase and 2,3-oxidosqualene synthase genes were overexpressed to increase the precursor supply for improving aglycon production. Strain GY-1 was obtained, which produced 17.2 mg/L protopanaxadiol, 15.9 mg/L protopanaxatriol and 21.4 mg/L oleanolic acid. The yeast strains engineered in this work can serve as the basis for creating an alternative way for producing ginsenosides in place of extractions from plant sources.
Ginsenosides are the primary bioactive components of ginseng, which is a popular medicinal plant. Ginsenosides are a group of triterpenoids that exhibit diverse pharmacological effects on the central nervous, endocrine, cardiovascular and immune systems1,2. Dammarane-type tetracyclic ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rh2 and Rg3) are the major constituents, while Ro, which belongs to oleanane-type pentacyclic ginsenosides, is a minor component of Panax ginseng3. These Dammarane-type ginsenosides are divided into two groups according to their aglycon structure: protopanaxadiol (Rb1, Rb2, Rc, Rd, Rh2 and Rg3) and protopanaxatriol (Re, Rf, and Rg1)3.
Currently, ginsenosides are mainly produced through their extraction from ginseng roots. Wild ginseng roots are scarce (i.e., they are endangered species in Asia and North America), but most commercial ginseng roots are collected from farms that cultivate ginseng in fields4. However, cultivating ginseng is time-consuming, labour-intensive and is influenced by many conditions such as soil, climate, pathogens and pests4.
Bakers' yeast (Saccharomyces cerevisiae) has been commonly used as a leavening agent in baking bread and bakery products, where it converts fermentable sugars that are present in dough into carbon dioxide and ethanol. In addition, because S. cerevisiae has been genetically and physiologically characterised and multiple genetic engineering tools exist, this microorganism is an ideal host for the heterologous production of valuable natural products and can provide an alternative and attractive route compared to traditional extraction methods5,6,7,8. With the development of metabolic engineering and synthetic biology tools, many natural products have been successfully synthesised in S. cerevisiae7,9,10,11,12.
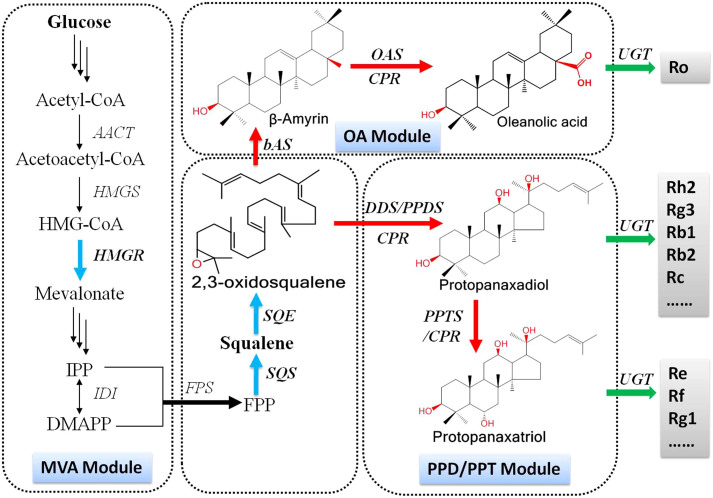
The first committed step in triterpenoid biosynthesis is the cyclisation of 2,3-oxidosqualene. This reaction is catalysed by specific oxidosqualene cyclases (OSCs), e.g., dammarenediol-II synthase (DDS), β-amyrin synthase (bAS), α-amyrin synthase (aAS), lansterol synthase (LAS) and lupeol synthase (LUS)13,14,15. In P. ginseng, cyclisation of 2,3-oxidosqualene to dammarenediol-II by DDS is the first reaction towards the biosynthesis of dammarane-type ginsenosides16,17. Dammarenediol-II is further converted to protopanaxadiol by protopanaxadiol synthase (PPDS), which is a cytochrome P450 enzyme that catalyses the hydroxylation of dammarenediol-II at the C-12 position (Fig. 1)3. Protopanaxadiol is further converted to protopanaxatriol by protopanaxatriol synthase (PPTS) (Fig. 1)16. In contrast, cyclisation of 2,3-oxidosqualene to β-amyrin by β-amyrin synthase (bAS) is the first reaction towards the biosynthesis of oleanane-type ginsenosides. β-amyrin is further converted to oleanolic acid by oleanolic acid synthase (OAS, a P450 enzyme), which catalyses a three-step, sequential oxidation at the C-28 position of β-amyrin (Fig. 1)14,15. These aglycons are further converted to ginsenoside compounds by uridine diphosphate glycosyltransferases (UGTs)18,19.
Figure 1. Biosynthetic pathways for protopanaxadiol, protopanaxatriol and oleanolic acid production in metabolically engineered S. cerevisiae.
Single arrows represent one-step conversions, while triple arrows represent multiple steps. Bold, blue arrows represent over-expressed yeast endogenous genes. Bold, red arrows represent exogenous plant genes that were introduced into S. cerevisiae. OA module includes of G. glabra bAS, M. truncatula OAS and A. thaliana CPR genes, while PPD/PPT module includes A. thaliana CPR and DDS, PPDS and PPTS of P. ginseng genes. Intermediates: HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; FPP, farnesyl diphosphate; OA, oleanolic acid; PPD, protopanaxadiol; and PPT, protopanaxatriol. Enzymes: HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; SQS, squalene synthase; SQE, squalene epoxidase; bAS, β-amyrin synthase; OAS, oleanolic acid synthase; CPR, cytochrome P450 reductase; DDS, dammarenediol-II synthase; PPDS, protopanaxadiol synthase; and PPTS, protopanaxatriol synthase.
In this work, S. cerevisiae was metabolically engineered for the efficient production of these three aglycons by introducing β-amyrin synthase, oleanolic acid synthase, dammarenediol-II synthase, protopanaxadiol synthase, protopanaxatriol synthase and NADPH-cytochrome P450 reductase from different plants. Genes coding for OAS from Medicago truncatula and DDS, PPDS and PPTS from P. ginseng have been previously demonstrated to be functional in yeast3,16,17. In addition, precursor supplies were increased to improve aglycon production. The yeast strains engineered in this work can serve as the basis for creating an alternative way for producing ginsenosides in place of extraction from plant sources.
Results
Increasing IPP and DMAPP supplies for terpenoid production in S. cerevisiae
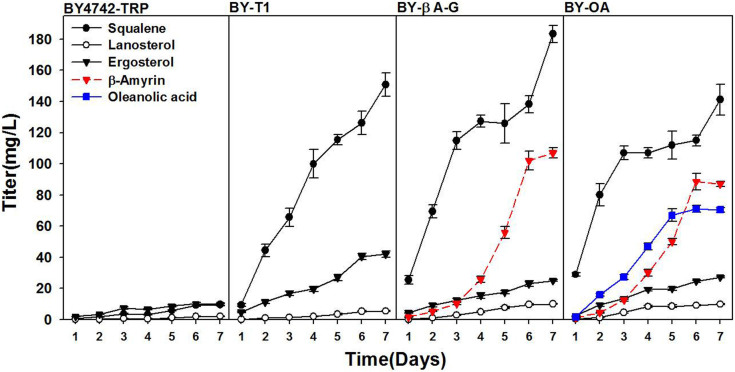
Triterpenoids are derived from two common building blocks, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are synthesised through the mevalonic acid (MVA) pathway in S. cerevisiae (Fig. 1). Most IPP and DMAPP precursors enter the ergosterol synthetic pathway20,21,22. When cultivated in YPD medium with 2% glucose for 7 days, strain BY4742-TRP produced 9.6 mg/L squalene, 2.1 mg/L lanosterol and 10.1 mg/L ergosterol (Fig. 2).
Figure 2. Production of squalene, lanosterol, ergosterol, β-amyrin and oleanolic acid by engineered S. cerevisiae strains BY4742-TRP, BY-T1, BY-βA-G and BY-OA.
All strains were cultivated in YPD medium with 2% glucose for 7 days. Three replicates were performed, and the error bars represented standard deviation. All these five chemicals were measured in the four strains. β-amyrin and oleanolic acid were not detected in strains BY4742-TRP and BY-T1. Oleanolic acid was not detected in strain BY-βA-G.
3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase is a key rate-limiting enzyme in the MVA pathway, and overexpression of a truncated HMG-CoA reductase gene (tHMG1) has commonly been used to increase the carbon flux through the MVA pathway11,25. This strategy had been used to successfully increase the production of many terpenoids, including sesquiterpenoids7,11,26,27, diterpenoids9,10,28, triterpenoids29,30 and carotenoids31.
The tHMG1 gene (controlled by the PGK1 promoter) and the LYS2 gene (controlled by the TEF1 promoter) were integrated into the chromosome of BY4742-TRP at the δDNA site to increase IPP and DMAPP supplies for better terpenoid production. The resulting strain, BY-T1 (Table 1), produced 150.9 mg/L squalene, 5.4 mg/L lanosterol and 42.0 mg/L ergosterol, which were 15.7-, 2.6- and 4.2-fold higher than that of the parent strain (Fig. 2). This result demonstrated that overexpressing tHMG1 significantly improved terpenoid production, and the engineered strain could be used for further production of aglycons of ginsenosides.
Table 1. Strains used in this study.
| Name | Description | Source |
|---|---|---|
| BY4742 | MATα, his3Δ1, leu2Δ0, lys2Δ0, MET15, ura3Δ0 | Brachmann et al.38 |
| BY4742-TRP | Deletion of the Trp1 gene of BY4742 | This study |
| BY-T1 | BY4742-TRP, δDNA;PPGK1-tHMG1-TADH1-PTEF1-LYS2-TCYC1 | This study |
| BY-βA-P | BY-T1, rDNA::PPGK1-PgbAS-TADH1-PTDH3-ERG1-TTPI1-PTEF1-ERG9-TCYC1 | This study |
| BY-βA-G | BY-T1, rDNA::PPGK1-GgbAS-TADH1-PTDH3-ERG1-TTPI1-PTEF1-ERG9-TCYC1 | This study |
| BY-βA-CK | BY4742-TRP, rDNA::PPGK1-GgbAS-TADH1-PTEF1-LYS2-TCYC1 | This study |
| BY-OA | BY-βA-G, Trp1::PPGK1-GgbAS-TADH1-PTDH3-AtCPR1-TTPI1-PTEF1-MtOAS-TCYC1 | This study |
| GY-1 | BY-OA, His3::PPGK1-PgDDS-TADH1-PFBA1-SynPgPPTS-TTDH2-PTDH3-AtCPR1- TTPI1-PTEF1-SynPPDS-TCYC1 | This study |
Construction of the β-amyrin synthetic pathway
β-amyrin serves as the basic precursor of oleanane-type triterpenoids18. β-amyrin is synthesised from 2,3-oxidosqualene in some medicinal plants by 2,3-oxidosqualene cyclases (OSCs)13,14,15,23. S. cerevisiae can produce 2,3-oxidosqualene from IPP and DMAPP through farnesyl diphosphate synthase (encoded by ERG20), squalene synthase (encoded by ERG9) and squalene epoxidase (encoded by ERG1) (Fig. 1).
Squalene synthase and squalene epoxidase are two key enzymes for triterpenoid synthesis. Squalene synthase is the first enzyme dedicated to synthesis of sterols in yeast, while squalene epoxidase catalyses the first oxygenation step in ergosterol biosynthesis and is suggested to represent one of the rate-limiting enzymes in this pathway32. Overexpressing the squalene synthase gene had been used to enhance the production of β-amyrin in yeast29,30 and the production of phytosterols and triterpenoids in plants33,34,35. Furthermore, overexpressing the squalene epoxidase gene had been used to increase sterol production21,25.
To construct the β-amyrin synthetic pathway and improve its production in S. cerevisiae, two different β-amyrin synthase genes of Glycyrrhiza glabra and P. ginseng, with the S. cerevisiae squalene synthase and squalene epoxidase genes, were integrated into the chromosome of strain BY-T1 at rDNA sites, resulting in strains BY-βA-G and BY-βA-P (Table 1), respectively. When cultivated in YPD medium with 2% glucose for 7 days, GC/MS analysis of cell extractions of both strains confirmed the production of β-amyrin (Fig. S1). Strain BY-βA-P produced 1.9 mg/L β-amyrin with a yield of 0.2 mg/g (data not shown), while strain BY-βA-G produced 107.0 mg/L β-amyrin with a yield of 9.3 mg/g DCW (Fig. 2). Strain BY-βA-G also produced 183.4 mg/L squalene, 10.0 mg/L lanosterol and 25.0 mg/L ergosterol (Fig. 2).
To verify that increasing 2,3-oxidosqualene supply did improve β-amyrin synthesis, the β-amyrin synthase gene of G. glabra (controlled by the PGK1 promoter) and the LYS2 gene (controlled by the TEF1 promoter) were integrated into the chromosome of strain BY4742-TRP at rDNA sites. The resulting strain, BY-βA-CK (Table 1), produced 77.7 mg/L β-amyrin, which was 73% of that produced by strain BY-βA-G (Fig. S2). In addition, strain BY-βA-CK produced 34.2 mg/L squalene, 1.4 mg/L lanosterol and 12.1 mg/L ergosterol, which were 19%, 14% and 48% of those produced by strain BY-βA-G (Fig. S2).
Construction of the oleanolic acid synthetic pathway
To construct the oleanolic acid synthetic pathway in S. cerevisiae, the oleanolic acid synthase gene (OAS) of Medicago truncatula13 (controlled by TEF1 promoter), with a cytochrome P450 reductase gene of Arabidopsis thaliana (AtCPR1)24(controlled by TDH3 promoter) and a new copy of the G. glabra β-amyrin synthase gene (controlled by PGK1 promoter) were integrated into the chromosome of strain BY-βA-G at the Trp1 site, resulting in strain BY-OA (Table 2). After cultivation in YPD medium with 2% glucose for 7 days, LC/MS analysis of the cell extraction of strain BY-OA confirmed the production of oleanolic acid (Fig. S3). Strain BY-OA produced 71.0 mg/L oleanolic acid with a yield of 6.1 mg/g DCW and 88.6 mg/L β-amyrin with a yield of 7.6 mg/g DCW (Fig. 2). This strain also produced 141.2 mg/L squalene, 9.8 mg/L lanosterol and 27.3 mg/L ergosterol (Fig. 2).
Construction of the synthetic pathways of all three aglycons
To construct the protopanaxadiol and protopanaxatriol synthetic pathways in strain BY-OA, the P. ginseng dammarenediol-II synthase gene (PgDDS, controlled by PGK1 promoter), P. ginseng protopanaxadiol synthase gene (PgPPDS, controlled by TEF1 promoter), P. ginseng protopanaxatriol synthase gene (PgPPTS, controlled by FBA1 promoter) and AtCPR1 (controlled by TDH3 promoter) genes were integrated into the chromosome of strain BY-OA at the His3 site, resulting in strain GY-1 (Table 1). After cultivation in YPD medium with 2% glucose for 5 days, LC/MS analysis of the cell extraction of strain GY-1 confirmed the production of protopanaxadiol and protopanaxatriol (Fig. S4 and S5). Strain GY-1 produced 17.2 mg/L protopanaxadiol with a yield of 1.4 mg/g DCW, and 15.9 mg/L protopanaxatriol with a yield of 1.3 mg/g DCW. The oleanolic acid titer decreased from 71.0 to 21.4 mg/L, and its yield decreased from 6.1 to 1.8 mg/g DCW (Fig. 3A). The proportion of protopanaxadiol, protopanaxatriol and oleanolic acid within the total aglycons was 31.5%, 29.2% and 39.3%, respectively (Fig. 3B). In addition, this strain produced 96.0 mg/l squalene, 11.1 mg/l lanosterol, 16.3 mg/l ergosterol, 24 mg/l β-amyrin and 7.9 mg/l dammarenediol-II (data not shown).
Figure 3. Production of protopanaxadiol, protopanaxatriol and oleanolic acid by engineered S. cerevisiae strain GY-1.
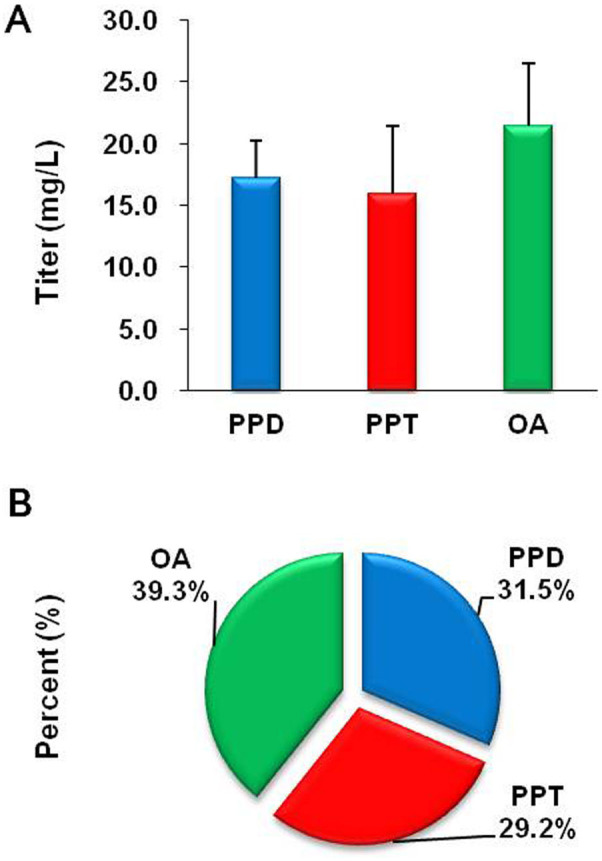
(A) Titers obtained after fermentation for 7 days; (B) Proportion of protopanaxadiol, protopanaxatriol and oleanolic acid within the total aglycons. Three replicates were performed, and the error bars represented standard deviation.
Discussion
S. cerevisiae is an ideal host for the heterologous production of valuable natural products. To be economically comparable to the extraction from plant sources, heterologous production of ginsenosides in yeast with high titer and productivity is required. Because 2,3-oxidosqualene is a basic precursor for ginsenoside synthesis, its sufficient supply is very important. In this work, three commonly used engineering strategies for increasing the 2,3-oxidosqualene supply were performed to improve β-amyrin production. After overexpressing the tHMG1, ERG9 and ERG1 genes, β-amyrin production increased 38% and all squalene-derived terpenoids increased 159% (Fig. S2), demonstrating that these three enzymes were essential for increasing the 2,3-oxidosqualene supply to improve triterpenoid production.
β-amyrin serves as the basic, oleanane-type triterpenoid precursor to variable downstream products18. Through the introduction of β-amyrin synthase of Artemisia annua, the overexpression of the tHMG1 gene and down-regulation of the lanosterol synthase gene (encoded by ERG7), an engineered S. cerevisiae was obtained that produced 6 mg/L β-amyrin29. By introducing β-amyrin synthase of Pisum sativum and overexpressing the phosphomevalonate kinase (encoded by ERG8), squalene synthase (encoded by ERG9) and acetyl-CoA carboxylase (encoded by HFA1) genes, an engineered S. cerevisiae was obtained that produced 3.93 mg/L β-amyrin30. To the best of our knowledge, strain BY-βA-G had the highest β-amyrin titer that was achievable by microbial fermentation. It was suggested that the β-amyrin synthase of G. glabra was better at producing β-amyrin than A. annua, P. sativum and P. ginseng. This suggestion was reasonable because G. glabra contains a large amount of glycyrrhizin (2–8% of the dry weight)23,36, while the other three plants contain minimal levels of oleanane-type triterpenoids16.
For strain BY-βA-G, squalene was produced with high productivity (1.60 mg/L·h), while β-amyrin had a relatively lower productivity (0.14 mg/L·h) during the first 72 hours (Fig. 2). From 72 to 144 h, squalene productivity decreased 80% to 0.32 mg/L·h, while β-amyrin productivity increased 9.1-fold to 1.27 mg/L·h. One possibility was that β-amyrin synthase activity was relatively lower for the first 72 h and limited β-amyrin production, while it was high from 72 to 144 h and led to high β-amyrin productivity. Another possibility was that 2,3-oxidosqualene supply was insufficient for the first 72 h, while it was enough from 72 to 144 h and led to high β-amyrin productivity.
β-amyrin and dammarenediol-II still accumulated in strain GY-1, suggesting that oleanolic acid synthase, protopanaxadiol synthase and protopanaxatriol synthase might be rate-limiting enzymes for aglycon production in this strain. These synthases are P450 enzymes. Poor coupling between P450 cytochromes and their reductases can result in the release of reactive oxygen species37, and the CPR levels would thus affect cell health7. The CPR gene of A. thaliana (AtCPR1) was used in this study; however, more CPRs from other organisms, such as M. truncatula and G. glabra, must be further investigated to identify the best enzyme for aglycon production.
The artemisinic acid synthetic pathway had been constructed in yeast by using cytochrome P450 CYP71AV1, which can catalyse a three-step, sequential oxidation that can convert amorphadiene to artemisinic acid11. However, the product titer was relatively low, only 100 mg/L. Recently, two plant dehydrogenases and a second cytochrome were discovered that could provide a more efficient biosynthetic route toward artemisinic acid. Cytochrome b5 (CYB5) facilitated the oxidation of amorphadiene to artemisinic alcohol, alcohol dehydrogenase (ADH1) oxidised artemisinic alcohol to artemisinic aldehyde, while artemisinic aldehyde dehydrogenase (ALDH1) oxidised artemisinic aldehyde to artemisinic acid. After reconstituting the entire, heterologous biosynthetic pathway in yeast, the engineered strain produced 25 g/L artemisinic acid7. Because oleanolic acid synthase also catalyses a three-step sequential oxidation, it was suggested that identification of other cytochromes and dehydrogenases from plants (such as G. glabra, P. ginseng and M. truncatula) and reconstituting them into the engineered yeast would improve oleanolic acid production.
In ginseng, aglycons (protopanaxadiol, protopanaxatriol and oleanolic acid) are further converted to ginsenoside compounds by uridine diphosphate glycosyltransferases (UGTs)18,19. Although protopanaxadiol, protopanaxatriol and oleanolic acid have diverse pharmacological effects, ginsenosides (such as Rh2 and Rg3) have more applications in pharmaceutical industries. After functional genomic analysis of P. ginseng and other plants, candidate UGTs can be introduced into strain GY-1, which can be used as the host strain for the identification of appropriate UGTs for ginsenoside production.
Methods
Strains and medium
S. cerevisiae BY4742, a derivative of S288C38, was obtained from EUROSCARF and used as the parent strain for all yeast strains. Engineered yeast strains were grown either in SD medium9,39 lacking leucine, uracil, tryptophan and histidine, where appropriate, or in YPD medium39. Escherichia coli Trans-10 (TransGen Biotech, Beijing, China) was used for transformation and plasmid DNA extraction. Strains were cultivated at 37°C in LB medium with 100 mg/L ampicillin.
Plasmid construction
Panax ginseng cells were induced with 0.1 mg/L MeJA for 24 h. Total RNA was isolated by the Trizol method40, and cDNA was synthesised using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). The P. ginseng β-amyrin synthase (PgbAS) were PCR-amplified from the cDNA of P. ginseng (using primer sets SexA1-PgPNY/PgPNY-Asc1). The amplified DNA fragments were cloned into pEASY-Blunt, resulting in p-PgbAS.
The Glycyrrhiza glabra β-amyrin synthase gene (GgbAS) (GenBank: AB037203), P. ginseng protopanaxatriol synthase gene (PgPPTS) (GenBank: JX036031) and Medicago truncatula oleanolic acid synthase gene (MtOAS) (GenBank:FN995113), together with the SexA1 and Asc1 restriction sites at their 5′- and 3′- ends, were synthesised by GenScript (Nanjing, China) with codon optimisation for improved expression in S. cerevisiae. The synthesised DNA fragments were cloned into pUC57, resulting in p-GgbAS, p-SynPgPPTS and p-MtOAS, respectively.
The plasmids of p-ΔTrp1, pδ-tHMG1, pM13-LYS2, pM3-ERG9, pM11-ERG1, pM11-AtCPR1, pM2-PgbAS, pM2-GgbAS, pM3-MtOAS, pM3-synPgPPDS, pM14-PgDDS, pM8-synPgPPTS, prDNA-LEU, pTrp-HIS and pHis-TRP are described in the supplementary data.
All the plasmids are summarised in Table S1, and the primers used during plasmid construction are summarised in Table S2.
Strain construction
Transformation of S. cerevisiae strains was performed by the standard lithium acetate method39. Strain BY4742-TRP was constructed by deleting the TRP1 gene of strain BY4742, thus leading to tryptophan auxotrophy and was accomplished as follows. The homologous recombination region of the partial TRP1 gene with the Loxp-HIS3-Loxp cassette was amplified from p-ΔTrp1 using primer sets ZD-TRP1-int-up(400)/ZD-TRP1-int-down(450). The amplified DNA fragment was then transformed into strain BY4742 followed by selection on SD-HIS plates. Strains were verified by PCR analysis, and the correct colony was transformed with plasmid pSH47. The transformant was cultivated at 30°C in SD-HIS-URA medium with 2% galactose as its sole carbon source for 24 h followed by selection on YPD medium with 1 g/L 5-FOA as a selection for the loss of plasmid pSH47, resulting in strain BY4742-TRP. This strain could not grow on SD-HIS and SD-TRP plates.
Strain BY-T1 was constructed by integrating the tHMG1 and LYS2 genes into the δDNA sites of BY4742-TRP using the DNA assembler method as described previously41,42. Three DNA fragments were amplified from pδ-tHMG1 (using primer sets 1-M-HisG-PGK1-F/1-M-ADHt-TEF1-R and 3-M-CYC1t-δ2-F/3-δ2-R) and pM13-LYS2 (using primer set 2-M-ADHt-TEF1-F/2-M-CYC1t-δ2-R), and another DNA fragment was obtained by BamHI and XhoI digestion of plasmid pδ-UB. These four DNA fragments were transformed into strain BY4742-TRP followed by selection on SD-URA plates. All strains were verified by PCR analysis, and twelve colonies were screened by GC-MS for the selection of the best squalene-producing transformant.
Strain BY-βA-P was constructed by integrating the ERG9, ERG1 and PgbAS genes into the rDNA sites of strain BY-T1. Five DNA fragments were amplified from pM2-PgbAS (using primer set 1-M-pEASY-PGK1-F/3G-1-M-ADHt-TDH3-R), pM3-ERG9 (using primer set 3G-2-M-TPI1t-TEF1-F/M-CYC1t-pEASY-R), pM11-ERG1 (using primer set 3G-3-M-ADHt -TDH3-F/3G-3-M-TPI1t-TEF1-R) and prDNA-LEU (using primer sets X1-M-pEASY-r-t-F/X1-r-t-R-rDNA and X2-r-t-F-rDNA/X2-M-pEASY-r-t-R). These fragments were then transformed into strain BY-T1 followed by selection on SD-URA-LEU plates. All strains were verified by PCR analysis, and twelve colonies were screened by GC/MS for the selection of the best-producing transformant.
BY-βA-G was constructed by integrating the ERG9, ERG1 and GgbAS genes into the rDNA sites of strain BY-T1 to construct strain BY-βA-P.
BY-βA-CK was constructed by integrating the LYS2 and GgbAS genes into the rDNA sites of strain BY4742-TRP. Four DNA fragments were amplified from pM2-GgbAS (using primer set 1-M-pEASY-PGK1-F/1-M-ADHt-TEF1-R), pM13-LYS2 (using primer set 2-M-ADHt-TEF1 -F/M-CYC1t-pEASY-R) and prDNA-LEU (using primer sets X1-M-pEASY-r-t-F/X1-r-t-R-rDNA and X2-r-t-F-rDNA/X2-M-pEASY-r-t-R) and were then transformed into strain BY4742-TRP followed by selection on SD-LEU plates. All strains were verified by PCR analysis, and 12 colonies were screened by GC/MS for the selection of the best-producing transformant.
Strain BY-OA was constructed by integrating the GgbAS, MtOAS and AtCPR1 genes into the Trp1 site of BY-βA-G. Five DNA fragments were amplified from pM2-GgbAS (using primer set 1-M-pEASY-PGK1-F/3G-1-M-ADHt-TDH3-R), pM3-MtOAS (using primer set 3G-2-M-TPI1t-TEF1-F/M-CYC1t-pEASY-R), pM11-AtCPR1 (using primer set 3G-3-M-ADHt-TDH3-F/3G-3-M-TPI1t-TEF1-R) and pTrp-HIS (using primer sets X1-M-pEASY-r-t-F/ZD-TRP1interg.-1 and ZD-TRP1interg.-2/X2-M-pEASY-r-t-R) and were then transformed into strain BY-βA-G followed by selection on SD-URA-LEU-HIS plates. All strains were verified by PCR analysis, and 12 colonies were screened by GC/MS and HPLC for selection of the best-producing transformant.
Strain GY-1 was constructed by integrating the PgDDS, SynPgPPDS, SynPPTS and AtCPR1 genes into the His3 site of BY-OA. Six DNA fragments were amplified from pM14-PgDDS (using primer set 1-M-pEASY-PGK1-F/4G-1-M-ADHt-FBA1-R), pM8-SynPgPPTS (using primer set 4G-4-M-ADHt-FBA1-F/4G-4-M-TDH2t-TDH3-R), pM11-AtCPR1 (using primer set 4G-3-M-TDH2t-TDH3-F/3G-3-M-TPI1t-TEF1-R), pM3-SynPgPPDS (using primer set 3G-2-M-TPI1t-TEF1-F/M-CYC1t-pEASY-R) and pHis-TRP (using primer sets X1-M-pEASY-r-t-F/ZD-His3 interg.-1 and ZD-His3 interg.-2/X2-M-pEASY-r-t-R) and were transformed into strain BY-OA followed by selection on SD-URA-LEU-HIS-TRP plates. All strains were verified by PCR analysis, and 12 colonies were screened by GC/MS and HPLC for the selection of the best-producing transformant.
Primers used in the DNA assembly are summarised in Table S3.
Yeast cultivation
YPD medium was used for cultivating strains BY4742, BY-T1, BY-βA-P, BY-βA-G, BY-βA-CK, BY-OA and GY-1. All strains were first inoculated into 15 ml culture tubes containing 2 ml medium and grown at 30°C and 250 rpm to an OD600 of approximately 1.0. Flasks (250 ml) containing 100 ml medium were then inoculated to an OD600 0.05 with the seed cultures. Strains were grown at 30°C and 250 rpm for 7 days, and all optical densities at 600 nm (OD600) were measured using a Shimadzu UV-2550 spectrophotometer.
Chemical analysis
Cells were collected from fermentation culture via centrifugation. The mixed-solution (600 μl; acetone: methanol = 1:1) was added to the tube and crushed by a BeadBeater (BioSpec, USA) 3 times. The samples were then centrifuged at 10,000×g for 1 min, and 1 μl of supernatant was analysed by GC/MS using an Agilent technologies 5975C insert xl MSD with triple-Axis Detector equipped with a HP-5ms (30 m×0.25 mm×0.5 μm) GC column. Compound separation was achieved with an injector temperature of 300°C and a 30 min temperature gradient program for GC-separation starting at 80°C for 1 min followed by heating the column to 300°C at 20°C min−1 and a final constant hold at 300°C for 15 min. Mass detection was achieved with electric ionisation using SIM-scan mode with diagnostic ions monitored as followed: m/z 69, m/z 218, m/z 363, m/z 411 and m/z 437. A crystallised β-amyrin sample was used as the standard for quantification (purchased from BioBioPha, China), and squalene, lanosterol and ergosterol standards (purchased from Sigma Aldrich) were also used for quantification.
For the determination of oleanolic acid, acetone and methanol (1:1) extracts (20 μl) were analysed by LC/MS using an Agilent 1200 HPLC system coupled to a Bruker-micrOTOF-II with an electrospray ionisation (ESI) interface. Data acquisition and processing were performed with the MicrOTOF control version 3.0/Data Analysis Version 4.0 software. For chromatographic separation, a Waters Symmetry C18® column (250 mm×4.6 mm, 5 μm) was used. The mobile phase consisted of 0.1% ammonium acetate in water (A) and acetonitrile (B), and a program of A:B = 15:85 for 30 minutes was used. The solvent flow rate was 1.0 mL/min and the column temperature was set at 30°C. Optimised MS operating conditions were as follows: all spectra were obtained in the negative ion mode over an m/z range of 100–1200; dry gas flow, 6.0 L/min; dry temperature, 180°C; nebuliser pressure, 1 bar; and probe voltage, −4.5 kV. Oleanolic acid was purchased from Sigma Aldrich and was used as the standard for analysis.
For the determination of protopanaxadiol and protopanaxatriol, acetone and methanol (1:1) extracts (20 μl) were analysed by LC/MS using an Agilent 1200 HPLC system coupled to a Bruker-micrOTOF-II with an electrospray ionisation (ESI) interface. Data acquisition and processing were performed with the MicrOTOF control version 3.0/Data Analysis Version 4.0 software. For chromatographic separation, a Waters Symmetry C18® column (250 mm×4.6 mm, 5 μm) was used. The mobile phase consisted of 0.1% formic acid and 10% methanol in water (A) and acetonitrile (B), and a program of A:B = 15:85 for 30 minutes was used. The solvent flow rate was 1.0 mL/min, and the column temperature was set at 30°C. The optimised MS operating conditions were as follows: all spectra were obtained in the positive ion mode over an m/z range of 100–1200; dry gas flow, 6.0 L/min; dry temperature, 180°C; nebuliser pressure, 1 bar; and probe voltage +4.5 kV. Protopanaxadiol and protopanaxatriol was purchased from Sigma Aldrich and was used as the standard for analysis.
For quantitative analysis of protopanaxadiol, protopanaxatriol and oleanolic acid, acetone and methanol (1:1) extracts (20 μl) were injected into an Agilent 1200 HPLC apparatus with UV detection at 203 nm. For chromatographic separation, a Waters Symmetry C18® column (250 mm×4.6 mm, 5 μm) was used. The mobile phase consisted of 0.1% Formic acid and 10% methanol in water (A) and acetonitrile (B), and a program of A:B = 15:85 for 30 minutes was used. The solvent flow rate was 1.0 mL/min, and the column was held at 30°C during the separation. Protopanaxadiol, Protopanaxatriol and oleanolic acid was purchased from Sigma Aldrich and was used for quantification.
Author Contributions
Z.D., L.H., T.L. and X.Z. designed the experiments; Z.D., Y.L., B.W., M.S., D.W. and X.N.Z. performed the experiments; Y.L., Z.D. and X.Z. analysed the data; Z.D. and X.Z. wrote the paper; and all authors reviewed the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
This research was supported by grants from the National Basic Research Program of China (2011CBA00800), National High Technology Research and Development Program (2012AA02A704) and the National Natural Science Foundation of China (81202864, 81130070 and 81072989). Xueli Zhang was supported by the Hundred Talent Program of the Chinese Academy of Sciences. We thank Dr. N.A. Da Silva (University of California) for providing the plasmid pδ-UB, and Dr. Qinhong Wang (Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences) for providing the yeast vectors pRS313, pRS314.
Footnotes
This work has been included in a patent application by the Tianjin Institute of Industrial Biotechnology.
References
- Qi L. W., Wang C. Z. & Yuan C. S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 72, 689–699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radad K., Moldzio R. & Rausch W. D. Ginsenosides and their CNS targets. CNS Neurosci Ther 17, 761–768 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. Y., Kim H. J., Kwon Y. S. & Choi Y. E. The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 52, 2062–2073 (2011). [DOI] [PubMed] [Google Scholar]
- Paek K. Y., Murthy H. N., Hahn E. J. & Zhong J. J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv Biochem Eng Biotechnol 113, 151–176 (2009). [DOI] [PubMed] [Google Scholar]
- Ajikumar P. K. et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm 5, 167–190 (2008). [DOI] [PubMed] [Google Scholar]
- Chang M. C. & Keasling J. D. Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol 2, 674–681 (2006). [DOI] [PubMed] [Google Scholar]
- Paddon C. J. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496, 528–532 (2013). [DOI] [PubMed] [Google Scholar]
- Dejong J. M. et al. Genetic engineering of taxol biosynthetic genes in Saccharomyces cerevisiae. Biotechnol Bioeng 93, 212–224 (2006). [DOI] [PubMed] [Google Scholar]
- Dai Z., Liu Y., Huang L. & Zhang X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol Bioeng 109, 2845–2853 (2012). [DOI] [PubMed] [Google Scholar]
- Engels B., Dahm P. & Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab Eng 10, 201–206 (2008). [DOI] [PubMed] [Google Scholar]
- Ro D. K. et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943 (2006). [DOI] [PubMed] [Google Scholar]
- Westfall P. J. et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. P Natl Acad Sci USA 109, E111–118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli M. et al. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell 23, 3070–3081 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima E. O. et al. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant cell physiology 52, 2050–2061 (2011). [DOI] [PubMed] [Google Scholar]
- Huang L. et al. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta 236, 1571–1581 (2012). [DOI] [PubMed] [Google Scholar]
- Han J. Y. et al. Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 53, 1535–1545 (2012). [DOI] [PubMed] [Google Scholar]
- Tansakul P., Shibuya M., Kushiro T. & Ebizuka Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett 580, 5143–5149 (2006). [DOI] [PubMed] [Google Scholar]
- Augustin J. M., Kuzina V., Andersen S. B. & Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 72, 435–457 (2011). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. 454 EST analysis detects genes putatively involved in ginsenoside biosynthesis in Panax ginseng. Plant Cell Rep 30, 1593–1601 (2011). [DOI] [PubMed] [Google Scholar]
- Kennedy M. A., Barbuch R. & Bard M. Transcriptional regulation of the squalene synthase gene (ERG9) in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1445, 110–122 (1999). [DOI] [PubMed] [Google Scholar]
- Leber R. et al. A novel sequence element is involved in the transcriptional regulation of expression of the ERG1 (squalene epoxidase) gene in Saccharomyces cerevisiae. European journal of biochemistry/FEBS 268, 914–924 (2001). [DOI] [PubMed] [Google Scholar]
- Ma S. M. et al. Optimization of a heterologous mevalonate pathway through the use of variant HMG-CoA reductases. Metab Eng 13, 588–597 (2011). [DOI] [PubMed] [Google Scholar]
- Seki H. et al. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. The Plant cell 23, 4112–4123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P., Mignotte C., Kazmaier M., Delorme F. & Pompon D. Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J BIOL CHEM 272, 19176–19186 (1997). [DOI] [PubMed] [Google Scholar]
- Veen M., Stahl U. & Lang C. Combined overexpression of genes of the ergosterol biosynthetic pathway leads to accumulation of sterols in Saccharomyces cerevisiae. FEMS Yeast Res 4, 87–95 (2003). [DOI] [PubMed] [Google Scholar]
- Albertsen L. et al. Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl Environ Microbiol 77, 1033–1040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadollahi M. A., Maury J., Schalk M., Clark A. & Nielsen J. Enhancement of farnesyl diphosphate pool as direct precursor of sesquiterpenes through metabolic engineering of the mevalonate pathway in Saccharomyces cerevisiae. Biotechnol Bioeng 106, 86–96 (2010). [DOI] [PubMed] [Google Scholar]
- Zhou Y. J. et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J Am Chem Soc 134, 3234–3241 (2012). [DOI] [PubMed] [Google Scholar]
- Kirby J., Romanini D. W., Paradise E. M. & Keasling J. D. Engineering triterpene production in Saccharomyces cerevisiae beta-amyrin synthase from Artemisia annua. FEBS J 275, 1852–1859 (2008). [DOI] [PubMed] [Google Scholar]
- Madsen K. M. et al. Linking genotype and phenotype of Saccharomyces cerevisiae strains reveals metabolic engineering targets and leads to triterpene hyper-producers. PLoS One 6, e14763 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwaal R. et al. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73, 4342–4350 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber R. et al. A novel sequence element is involved in the transcriptional regulation of expression of the ERG1 (squalene epoxidase) gene in Saccharomyces cerevisiae. Eur J Biochem 268, 914–924 (2001). [DOI] [PubMed] [Google Scholar]
- Kim Y. S. et al. Gene regulation patterns in triterpene biosynthetic pathway driven by overexpression of squalene synthase and methyl jasmonate elicitation in Bupleurum falcatum. Planta 233, 343–355 (2011). [DOI] [PubMed] [Google Scholar]
- Lee M. H. et al. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant cell physiology 45, 976–984 (2004). [DOI] [PubMed] [Google Scholar]
- Seo J. W. et al. Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry 66, 869–877 (2005). [DOI] [PubMed] [Google Scholar]
- Seki H. et al. Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci USA 105, 14204–14209 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar R. C., Davydov D. R. & Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199, 316–331 (2004). [DOI] [PubMed] [Google Scholar]
- Brachmann C. B. et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 (1998). [DOI] [PubMed] [Google Scholar]
- Burke D., Dawson D. & Stearns T. Methods in yeast genetics: a cold spring harbor laboratory course manual (Cold Spring Harbor Laboratory Press, New York, 2000). [Google Scholar]
- Dai Z., Cui G., Zhou S. F., Zhang X. & Huang L. Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J Plant Physiol 168, 148–157 (2011). [DOI] [PubMed] [Google Scholar]
- Shao Z. & Zhao H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res 37, e16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z. et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng 20, 146–156 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information