Abstract
Introduction: The dysregulation of pH by cancerous cells of solid tumors is able to create a unique milieu that is in favor of progression, invasion and metastasis as well as chemo-/immuno-resistance traits of solid tumors. Bioelements involved in pH dysregulation provide new set of oncotargets, inhibition of which may result in better clinical outcome. Methods: To study the impacts of pH dysregulation, we investigated the tumor development and progression in relation with Warburg effect, glycolysis and formation of aberrant tumor microenvironment. Results: The upregulation of glucose transporter GLUT-1 and several enzymes involve in glycolysis exacerbates this phenomenon. The accumulation of lactic acids in cancer cells provokes upregulation of several transport machineries (MCT-1, NHE-1, CA IX and H+ pump V-ATPase) resulting in reinforced efflux of proton into extracellular fluid. This deviant event makes pH to be settled at 7.4 and 6.6 respectively in cancer cells cytoplasm and extracellular fluid within the tumor microenvironment, which in return triggers secretion of lysosomal components (various enzymes in acidic milieu with pH 5) into cytoplasm. All these anomalous phenomena make tumor microenvironment (TME) to be exposed to cocktail of various enzymes with acidic pH, upon which extracellular matrix (ECM) can be remodeled and even deformed, resulting in emergence of a complex viscose TME with high interstitial fluid pressure. Conclusion: It seems that pH dysregulation is able to remodel various physiologic functions and make solid tumors to become much more invasive and metastatic. It also can cause undesired resistance to chemotherapy and immunotherapy. Hence, cancer therapy needs to be reinforced using specific inhibitors of bioelements involved in pH dysregulation of TME in solid tumors.
Keywords: Tumor microenvironment, Warburg effect, pH dysregulation, Glycolysis
Introduction
Fundamentally, at the early stage of tumor development, two main types of signals resonate the initiation of biological damage(s) leading to malignancy. Such biological micro-damages appear to be initiated through inadvertent enhanced hydrophobicity and undesired expression of nucleic acids. Hydrophobic domains (Hyppos) of biostructures are intrinsically concealed with hydrophilic molecules. While there exists a hydrophobic and hydrophilic balance within all cellular bioelements in normal condition,1 any precipitously exposed Hyppos aggregates may be a sign for initiation of an undesired molecular injury. It seems to be the same for nucleic acids because the unmethylated CPG sequences can be a sign for molecular/cellular damage(s).2 Though the mechanisms, by which cancerous cells regulate gene expression, are often altered in various tumors, the nuclear packaging of DNA is deemed to modulate transcription through remodeling and/or modifying DNA, resulting in blockage of access to specific sites of transcription. Of DNA modifications, methylation of cytosine molecules as well as histone acetylation have been shown to regulate the gene expression, in which the expressed genes are located in highly acetylated chromatin while methylation seems to direct gene repression through a histone deacetylase complex.3 The concept for damage-associated molecular pattern (DAMP) of solid tumors appears to be the reflection of danger signals, causing unique genotypic and/or phenotypic alterations—as if the damaged cells form some kind of recalcitrant biostructure!
Hanahan and Weinberg have well discussed that most, if not all, cancers have inherently acquired almost the same set of functionalities during initiation and development exploiting various mechanisms.4 All solid tumors’ hallmarks show (a) self-sufficiency in growth signals, (b) insensitivity to anti-growth signals, (c) evading immunosurveillance and apoptosis, (d) sustained angiogenesis, (e) tissue invasiveness and metastasis, and (f) limitless replicative potential.4 Hence, solid tumors appear to be self-organizing complex adaptive systems.
The question is how do cancerous cells maintain their unique traits? And, why does the immunosurveillance system fail to recognize the rebellious biostructures? Further, chemotherapy of cancer often fails due to initiation of drug resistance and/or severe side effects. Based upon the Goldie-Coldmanmodel, the formation of chemotherapy-resistant clones (CRCs) can result in the failure of the conventional chemotherapy. While the main question would be: what is the Achilles’ heel of these cells? Having considered the Warburg effect and aberrant metabolism of glucose towards significantly high amount of lactic acid, cancer cells must maintain the intercellular pH (pHi) by pumping out the H+ through functional expression of pH regulating transport machineries such as Na+/H+ exchanger isoform 1 (NHE1).5 Such pH change at very early stage of carcinogenesis seems to be one of the main pathophysiological traits of solid tumors, resulting in alkalinized pHi and acidified extracellular pH (pHe), which is a driving force for various aspects of solid tumors’ behaviors.5 In the current review, we discuss the developmental process of solid tumors related to energetic pathway(s) and initiation of altered TME on the basis of pH dysregulation within cancerous cells.
Tumor microenvironment
The progression and invasion of solid tumors appear to be the consequence of series of unique biological events within TME. Important elements of such orchestrated phenomena are discussed in the following sections.
Epithelial and mesenchymal transition
As a rule, the tightness of cell-to-cell junctions is considered as determinant of epithelial organization, in which such junctional adhesion is largely dependent upon transmembrane glycoproteins such as E-cadherin as a typical epithelial marker. Epithelial cellular characteristics include (a) cohesive interactions among cells forming continuous cell layers, (b) membrane architectures (as apical, lateral and basal membranes), (c) tight junctional interactions, (d) polarized membranes with asymmetric dissemination of cellular machineries, and (e) proximate immobility of cells in the local epithelial microenvironment. Unlike the epithelial architecture, mesenchymal cells characteristically show different properties such as (a) motility and in some cases invasiveness, (b) no interaction between cells, (c) no apical and lateral membranes, and (d) no polarization.6
In tumor generation, transition of the epithelial cells to the mesenchymal cells emerge malignant cells with enhanced motility and increased capability to obliterate and penetrate. The epithelial-mesenchymal transition (EMT) process, which occurs normally in embryo during organogenesis (type 1), enables migration of cells, which forms the germ layers such as endoderm, ectoderm, and mesoderm. Similarly, upon an incidence of an injury within epithelial cells, such transition activates myofibroblasts and induces the process leading to re-epithelialization (type 2).7 Aberrant process of the EMT may trigger initiation and progression of tumor (type 3). While the type 1 and type 2 EMTs occur respectively during the organogenesis and tissue regeneration, the type 3 EMT appears to be the dominant phenomena in the case of aberrant solid tumors development and/or progression. Fig. 1 schematically represents the tumor development process (TDP).
Fig. 1 .
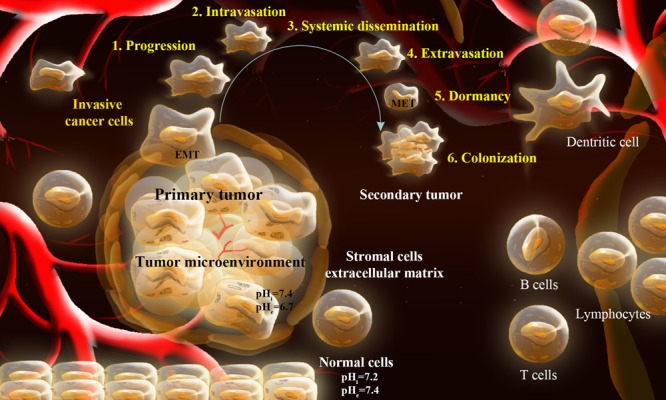
Schematic representation of tumor development process (TDP). During TDP, the cancer cells within the primary tumor achieve some sort of capabilities to strike the neighboring tissue (1: progression). The cancerous cells after destructing the ECM get into the lymphatic and blood vessels (2: intravasation), traverse through the vessels (3: dissemination), leave the traveling vessels (4: extravasation), negotiate with the new microenvironment, survive and proliferate (5: dormancy) forming micrometastatic secondary tumor (colonization). EMT: epithelial–mesenchymal transition. MET: mesenchymal–epithelial transition.
In type 3 EMT, various molecular events are deemed to happen including activation of several transcription factors (TFs) that coordinate the EMT program and some important molecular remodeling and signal transductions. Important molecular signaling pathways of SMAD and mitogen-activated protein kinase (MAPK) are activated through TGF-β, while aberrant activity of E-cadherin can also occur. Thus, the molecular machineries of this transitional step may provide potential target for inhibition of type 3 EMT. In a study, the expressions of E-cadherin and ZO-1 were investigated in a total of 48 cases (24 primary colorectal cancers with liver metastasis and 24 without). It was found that E-cadherin and ZO-1 were markedly down-regulated in the cancer cells of tumors with liver metastasis, indicating that dedifferentiation and decreased expression of E-cadherin and ZO-1 are closely related to liver metastasis.8 The miR-200 and miR-205 families of microRNAs were evinced to regulate the EMT as well as cancer cell migration through direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2,9,10 in which enforced expression of the miR-200 family alone was sufficient to prevent EMT induced by transforming growth factor-α (TGF-α).10 In pancreatic cancer, caveolin-1 (Cav-1) happens to promote cell differentiation and restore membranous E-cadherin through suppression of the EMT.11 Besides, mutations of SMAD correlate with loss of response to TGF-β, and thus blockage of the interaction of TGF-β with the SMAD signaling pathway. Some studies revealed that MAPKs (e.g., ERK1, ERK2) and isoforms of p38 are involved in cancer progression. While it is possible to inhibit elements of these signaling pathways, it is not clear that it can prevent occurrence of type 3 EMT.12 This clearly implies that EMT is intrinsically orchestrated by a set of pleiotropically acting transcription factors (TFs). The functions of these EMT-based TFs are deemed to enable the early steps of metastasis through local invasion and subsequent spreading to other tissues. Nonetheless, the subsequent outgrowth of micrometastatic cancerous rebellions toward macroscopic colonization reveals their adaptation and self-renewal capacity within the new microenvironment that is mediated by cancer stem cells (CSCs). It is deemed that series of genetic and epigenetic mechanisms regulate the activation of such process.7 Fig. 2 schematically epitomizes the transitional molecular events from normal cells to adenocarcinoma of colorectal cancer.
Fig. 2 .
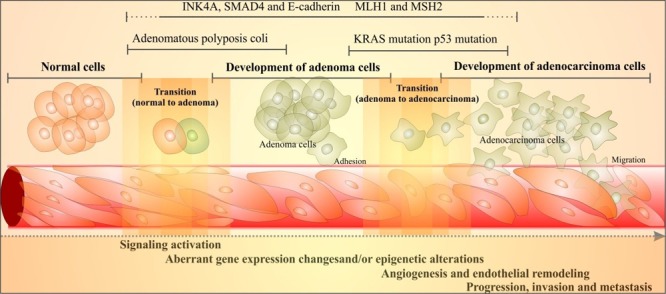
Schematic illustration of molecular events of the transition of normal cells to adenocarcinoma in colorectal cancer (CRC). Transitional stages (from normal colon epithelium to premalignant adenoma then to an invasive adenocarcinoma) are characterized through well-described sequence of mutations. Cell adhesion is compromised through loss of function of the adenomatous polyposis coli (APC) gene in up to 85% of all cases of CRC. KRAS is mutated in 50–60% of cases of CRC. Cell adhesion transmembrane glycoprotein E-cadherin is downregulated. MLH1 and MSH2 gene are mutated. SMAD4, which is involved in the transforming growth factor-β (TGF-β) signaling pathway, plays a key role in suppressing the epithelial-cell growth. While INK4A gene is involved in tumor-suppressor pathway, the p53 mutation appears to be the late phenomenon that makes cancer cells resistant to apoptosis. There exist overlaps between different stages. For detailed information, reader is referred to an excellent review by Kerr.13
Morphological changes during epithelial–mesenchymal transition
During EMT as a major developmental process, epithelial cells undergo some unique changes through (a) developing fibroblast-like properties, (b) enhanced motility and (c) diminished intercellular adhesion leading to mesenchymal cells’ formation. Inherently, similar alterations occur in terms of tumor initiation, progression and transformation. Such changes can markedly provoke the invasiveness of the incipient cancer cells. As mentioned previously, several emergent oncogenic factors/pathways (e.g., peptide growth factors, Src, Ras, Ets, integrin, Wnt/β-catenin, and Notch) are involved in EMT. Of these, downregulation of the cell adhesion molecules and abnormal activity of epithelial marker E-cadherin seem to be the center of EMT, even though some other TFs need to activate different cell signaling pathways.6 We will briefly discuss some of the biological changes that manifest formation of TME during tumor development.
Warburg effect and reverse Warburg effect
Cells produce their energy needs in the mitochondria through an oxygen-mediated oxidative phosphorylation (OMOP) that exploits oxidation of NADH and FADH2 along with phosphorylation of ADP to form ATP. Alternatively, in contrast to OMOP, ATP can also be produced through glycolysis within the cytosol in the absence of oxygen, in which one molecule of glucose is broken into two molecules of pyruvate generating ATP by means of NAD+and conversion of pyruvate to lactate (Fig. 3). In cancer cells, despite the presence of sufficient levels of O2, the rate of glycolysis is increased – a phenomenon called aerobic glycolysis or “Warburg effect”.14 As a “self-governing” disease, cancer cells and the related stromal cells are closely connected in terms of bioenergetic metabolism standpoint. During this scenario, stromal cells, which are subjected to oxidative stress and switch to a glycolysis-based metabolism, can also generate high levels of lactate and ketone bodies that initiate the oxidative phosphorylation process. This metabolic circuitry crosstalk, which closely resembles the well-known lactate shuttle bridging in cancer cells and stroma, is called “reverse Warburg effect”. Cancer cells have been shown to secrete high levels of hydrogen peroxide (H2O2),15 by which they can attain a local oxidative microenvironment. Stromal cells tend to react to such oxidative conditions through enhancing their autophagic flux as well as activation of hypoxia-inducible factor 1 (HIF-l), which can in return trigger degradation of Cav-1 and also activate several important genes (e.g., MCT4) related to glycolysis and result in production of lactic acids and ketone bodies. These byproducts can be taken up by cancer cells through MCT1 to fuel oxidative phosphorylation.16
Fig. 3 .
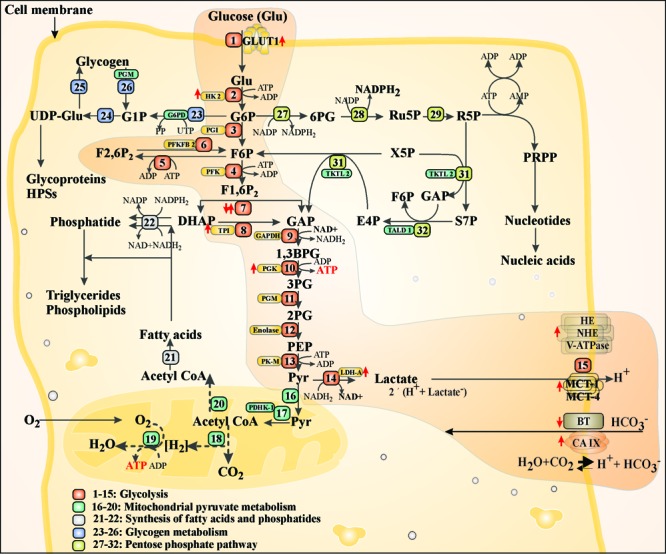
Glucose metabolism path in normal cells and cancer cells. Cells transport glucose through GLUT1 and phosphorylate it to G6P through hexokinases (HK2). G6P is further metabolized through glycolysis path (1–15), glycogen metabolism (23–26) and pentose phosphate pathway (27–32). Cancer cells exploit the glycolysis path, which results in production of lactic acid that is pumped out of the dells by monocarboxylate transporter (MCT1). Pyruvate can also undergo mitochondrial pyruvate metabolism (16-20) toward synthesis of fatty acids and phosphatides (21-22). Highlighted path shows glycolysis in cancer cells. GLUT1: glucose transporter 1. G6P: glucose 6-phosphate. HK2: hexokinase 2. F6P: fructose 6-phosphate. F1,6P2: fructose 1,6-phosphate. GAP: glyceraldehyde phosphate. DHAP: dihydroxyacetone phosphate. TPI: triose phosphate isomerase, 1,3BPG: glycerate 1,3-bisphosphate. 3PG: glycerate 3-phosphate. 2PG: glycerate 2-phosphate. Pyr: pyruvate. G1P: glucose 1-phosphate. UDPGlu: uracil-diphosphate glucose. F2,6P2: fructose 2,6-phosphate. NAD: nicotinamide adenine dinucleotide. NADP: nicotinamide adenine dinucleotide phosphate. 6PG: 6-phosphate gluconate. Ru5P: ribulose 5-phosphate. R5P: ribose 5-phosphate. X5P: xylose 5-phosphate. S7P: sedoheptulose 7-phosphae. E4P: erythrose 4-phosphate. TKTL2: transketolase-like 2. TALD1: transaldolaselike 1. MCT1: monocarboxylate transporter 1. BT: bicarbonate transporter. CAIX: carbonic anhydrase 9. HE: H+ exchanger. NHE: Na-H+ exchanger. V-ATPase: Vacuolar-type H+-ATPase. PDHK-1: pyruvate dehydrogenase kinase 1.
One of the key elements of Warburg effect was shown to be HIF-l, whose activation in malignancies can increase the transcription of many genes involved in glucose metabolism, apoptosis resistance, invasion, metastasis and angiogenesis. It seems that the activation of glycolysis can favor the biosynthesis process in conformity with cancer, supporting cell proliferation. For example, glycolytic metabolites such as glucose 6-phosphate, 3- phosphoglycerate, phosphoenolpyruvate and pyruvate are key precursors in the biosynthesis of several amino acids while dihydroxyacetone phosphate is the key precursor of glycerol, a necessary metabolite for the synthesis of lipids.17 Hence, the hypoxia and HIF-1 will be briefly discussed in the following sections.
Hypoxia and HIF-1
Hypoxia epitomizes the unique characteristic of all solid tumors, in which an imbalance between enhanced oxygen consumption by cancer cells and insufficient oxygen delivery from the aberrant tumor vasculature appear to be the main reason for such phenomenon though it happens even in the presence of oxygen. Given the fact that the metabolism of glucose in cancer cells is mainly through glycolysis, the acidic byproducts are pumped out of the cytoplasm resulting in pH dysregulation within the TME; that is, alkalinized intracellular fluid and acidified extracellular fluid. Such transformed metabolism in cancer cells is reported to enhance the resistance of the cancer cells to radiation and chemotherapy.18
The Warburg effect and the hypoxia are associated with activation of HIF-1 that is a transcription factor critically involved in cellular responses to hypoxia and tumor progression and modulate the expression of a large number of genes within TME under hypoxic conditions. As a heterodimeric protein comprised of an aryl hydrocarbon receptor and a nuclear translocator, the α subunit of HIF-1 is regulated by oxygen concentrations. Accordingly, there exist evidences showing that the HIF-1α is over-expressed in various malignancies. It is associated with a range of genetic alterations such as loss of function of tumor suppressor genes (VHL, PTEN and CDKN2A) along with increased activity of some oncogenes (RAS, SRC, BCR-ABL, TWIST1 and MET).19-21 HIF-l has been being considered as one of the attractive oncomarkers for the development of novel cancer therapeutics. Table 1 represents some selected inhibitors of HIF-1.
Table 1 . Selected inhibitors of HIF-1 .
| Inhibitor | Mechanism of action | Reference |
| FK228, a histone deacetylase inhibitor | Inhibition of angiogenesis through suppression of HIF-1α in Lewis lung carcinoma induced by hypoxia | 22 |
| R59949, a diacylglycerol kinase inhibitor | Blockage of accumulation of HIF-1α in the presence of von Hippel-Lindau (VHL) by activation of HIF prolyl hydroxylases | 23 |
| Vitexin, a natural flavonoid compound identified as apigenin-8-C-b-D-glucopyranoside | Inhibition of HIF-1α in PC12 cells by blockage of c-jun N-terminal kinase (JNK) and diminished levels VEGF, smad3, aldolase A, enolase 1, and collagen type III | 24 |
| YC-1, a HIF-1αsiRNA | Inhibition of tumor invasion or metastasis in nude mice through suppression of HIF-1α | 25 |
| RX-0047, a HIF-1α antisense | Inhibition of metastasis of human lung in xenograft mice | 26 |
| KC7F2, a lead compound with a central structure of cystamine | Down-regulation of HIF-1α protein synthesis along with suppression of phosphorylation of factor 4E binding protein 1 and p70 S6 kinase (key regulators of HIF-1α) | 27 |
| Caffeic acid phenethyl ester (CAPE), an active component of honeybee’s propolis | Inhibition of HIF prolyl hydroxylase (the key enzyme for von Hippel-Lindau-dependent HIF-1α degradation) | 28 |
| PX-478 (S-2-amino-3-[4′-N,N,-bis(chloroethyl)amino]phenyl propionic acid N-oxide dihydrochloride) | Reduction of HIF-1α mRNA and inhibition of its translation | 29,30 |
| Pantoprazole, a proton pump inhibitor | Inhibition of HIF-1α in human gastric adenocarcinoma sgc-7901 cells | 31 |
Moreover, in invasive breast cancer, HIF-1α was shown to be associated with angiogenesis and expression of growth factors such as basic fibroblast growth factor (bFGF) and platelet derived growth factor-BB (PDGF-BB), and epidermal growth factor receptor (EGFR). Hence, it is possible to control the breast cancer growth (both angiogenesis and growth factors) through inhibition of HIF-1.32 Gefitinib, an EGFR inhibitor, was also shown to decrease the expression of vascular endothelial growth factor (VEGF) through downregulation of HIF-1α33 Some other known target genes affecting activity of HIF-1 are carbonic anhydrase IX (CA IX), matrix metalloproteinase 2 (MMP2), endothelin 1, and enolase 1.27
Aberrant metabolism: some selected paradigms
Aberrant metabolism of glucose and hypoxia are the main characteristics of TME. Tumor cells possess specific enzyme-regulatory mechanisms, by which they can direct the main flux of glucose carbons towards the signaling elements that are instantly required for their survival/progression under challenging external conditions such as varying substrate availability, presence of anti-cancer drugs or different phases of the cell cycle.34 Inherently, glucose trafficking by cancerous cells is in favor of the generation of biomass and regulation of the cellular signaling that are critical for oncogenic progression. Such specialized trafficking, demands specific transporters such as glucose transporters (GLUTs), which are key rate-limiting transport machineries. Of various GLUTs, the expressions of GLUT1, insulin-responsive GLUT4 and GLUT9 happen to be enhanced as enigmatic class of proteins.35 Once in the cytoplasm, glucose is subjected to metabolism, and the glycolytic intermediates fuel several biosynthetic pathways necessary for duplication of biomass during cellular proliferation (Fig. 3). Glucose is first phosphorylated to glucose-6-phosphate through hexokinases (HKs), and then it can be subjected to glycolysis, glycogen metabolism and pentose phosphate pathway. In the case of solid tumors, the dominant pathway by cancerous cells for glucose metabolism is glycolysis (Fig. 3), where nicotinamide adenine dinucleotide (NAD+) is involved in redox reactions that are essential for conversion of pyruvate to lactic acid produced by cancer cells. Unlike the metabolic redox reactions that reversibly oxidize/reduce pyridine nucleotides, NAD+-dependent signaling processes continuously use NAD+. Thus, maintenance of the cellular NAD content seems to be a crucial homeostatic process.36
As shown in Fig. 3, the ratio of NAD+ to NADH is balanced in normal cells, while in cancer cells lactate dehydrogenase isoform A (LDHA) favorably converts accumulating pyruvate to lactate, thus regenerating NAD+ from NADH in support of glycolysis.36 In fact, certain bioelements are involved in the enzymatic breakdown of glucose to pyruvate while some other enzymes appear to play central roles in the metabolism of pyruvate. In cancer cells, pyruvate is converted to lactate by lactate dehydrogenase (LDH), which is then released into the extracellular space where HIF-l regulates cell adaptation to hypoxia through pyruvate dehydrogenase kinase 1 (PDK1) that inhibits pyruvate dehydrogenase (PDH), and accordingly the conversion of pyruvate to acetyl-CoA and mitochondrial respiration.
NAD+ as an essential part of bioenergetic processes is routinely synthesized from the vitamin B3 (niacin) and the related nucleosides. Given that nicotinamide (Nam), the main energy source, arises from NAD+-dependent signaling paths, the enzymatic function of the nicotinamide phosphoribosyl transferase (NamPRT) is pivotal for conversion of salvages Nam into NAD+. Thus, to block the synthesis of NAD+, NamPRT and nicotinamide mononucleotide Adenylyl transferases (NMNATs) as potential oncotargets must be inhibited. Further, NAD+dependent protein deacetylases (Sirtuins) such as SIRT1, SIRT3, SIRT6 and SIRT7 appear to provide another possibility for cancer therapy.36 For example, thiazolidinediones (also known as glitazones used in the treatment of diabetes mellitus type 2, have been introduced as a novel class of energy restriction-mimetic agents. They are able to induce hallmark cellular responses that are characteristics of energy restriction such as (a) induction of silent information regulator 1 (sirtuin-1 or SIRT1), (b) activation of the intracellular fuel sensor AMP-activated protein kinase, and (c) endoplasmic reticulum stress, resulting in activation of autophagic and apoptotic death.37 Table 2 represents some selected inhibitors of NAD biosynthesis/function.
Table 2 . Selected inhibitors involved in inhibition of NAD biosynthesis/function.
| Inhibitor | Pharmacological effect(s) | Reference |
| FK866/K22.175, inhibitor of nicotinamide phosphoribosyltransferase (NamPRT) | Potent antiangiogenic effects in murine renal cell carcinoma | 38 |
| APO866, inhibitor of NamPRT | Potent inhibition of most cancer cells (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], mantle cell lymphoma [MCL], chronic lymphocytic leukemia [CLL], and T-cell lymphoma) | 39 |
| GMX1778, inhibitor of NamPRT | Neuroblastoma regression and vessel maturation without inducing drug resistance in engrafted mice | 40 |
| Beta-lapachone, an orthonaphthoquinone | Induction of cell death by activation of a futile cycling of the drug by the cytoplasmic two-electron reductaseNAD(P) H: quinone oxidoreductase (NQO1), DT-diaphorase and Xip3. Production of reactive oxygen species (ROS) at higher drug concentrations | 41 |
| Retinoic acid | Induction of apoptosis by all-trans-retinoic acid and N-(4-hydroxyphenyl)retinamide in MCF7 cancer cells through transient increase in NADH level and mitochondrial oxidative turnover and a slow decline in reduced thiol level and mitochondrial membrane potential | 42 |
| Mycophenolic acid, a inhibitor of inosine 5'-monophosphate dehydrogenase (IMPDH) | Enhanced cytotoxicity in tumor cell lines by binding to IMPDH at the nicotinamide sub-site of the NAD cofactor binding domain | 43 |
| Dicumarol, a NQO1 inhibitor | Inhibition of NADPH: quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism | 44 |
| Sirtinol, a SIRT1 inhibitor | Induction of senescence-like growth arrest, increased expression of plasminogen activator inhibitor, and impaired activation of mitogen-activated protein kinase (MAPK) pathways in human breast cancer MCF-7 cells and lung cancer H1299 cells. | 45 |
| Sulindac | Induction of carcinogen metabolizing enzymes in human colon cancer cells | 46 |
| Salermide, a Sirtuin inhibitor | Induction of cancer-specific proapoptotic effect in cancer cell s and mice by inhibiting SIRT1 and SIRT2 | 47 |
Dysregulation of pH in cancer cells
For transitional stages to malignancy, the rate of glycolysis within cancerous cells is substantially increased, which results in high generation of lactic acid. Cancer cells pump out the acidic byproducts through upregulation of some special transporters. In fact, during Warburg phenomenon, many molecular elements are consistently upregulated by HIF-l and hypoxia in association with glucose metabolism. It seems that Warburg effect is able to upregulate the expression of crucial transporters for glucose uptake and to induce expression of transporters involved in transportation of byproducts of glucose metabolism, upon which cancer cells can control intracellular pH at 7.4 whilst acidifying the extracellular fluid within the TME (Fig. 4).
Fig. 4 .
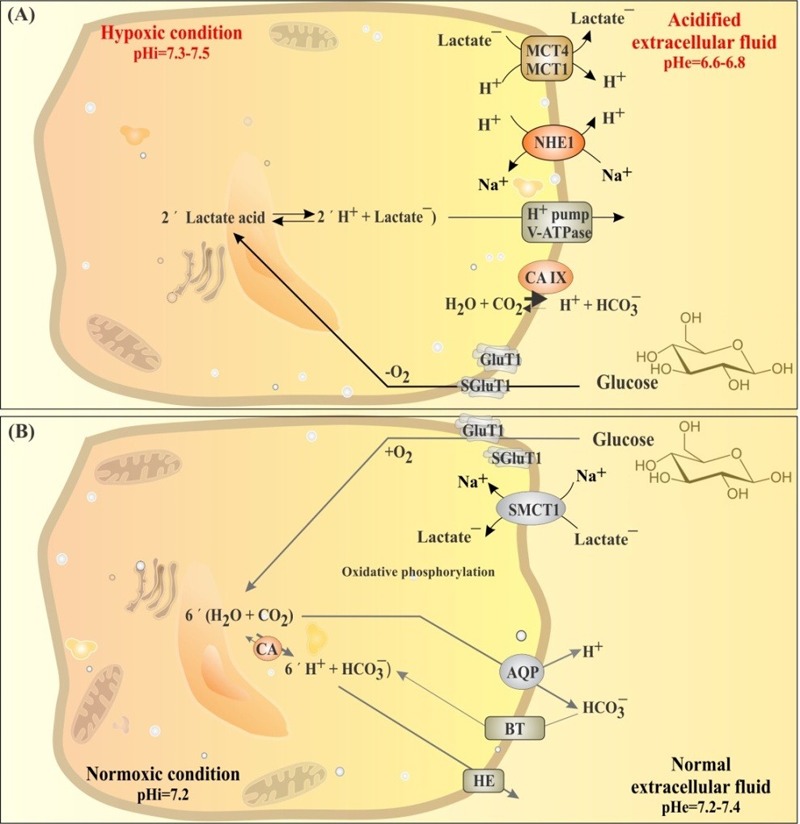
Regulation of pH in hypoxic and normoxic conditions in solid tumors. A) Glucose metabolism in hypoxic condition through glycolysis produces lactic acid, dysregulating pH in cancer cells. B) Glucose metabolism in normoxic condition through oxidative phosphorylation uptake lactic acid trough MCT1 transporter and consume it via oxidative phosphorylation. Expression of various transporters (MCT4, Glut1, CA-IX, V-ATPase, NHE1) at plasma membrane and mitochondria of hypoxic cancer cells favor alkalization of intracellular fluid (pHi=7.4) in cancer cells and acidification of extracellular fluid (pHe=6.6-6.9) in the tumor microenvironment. Dysregulated pH support cancer cells chemoresistance and immune system escape. MCT4: monocaroxylic acid transporter 4. Glut1: glucose transporter 1. CA-IX: carbonic anhydrase IX. V-ATPase: vacuolar-type H+-ATPase. NHE1: Na+/H+ exchanger. BT: bicarbonate transporter. HE: H+ exchanger. CA: carbonic anhydrase.
During glycolysis, tumor cells enhance their enzymatic activities and/or increase transcriptomes of glycolytic enzymes (e.g., HKII, PFKs, LDH-A) and GLUTs. Of the elevated enzymes, some were found to have anti-apoptotic function, resulting in promotion of chemoresistance in cancer cells. Glucose transportation is mediated via GLUTs that are an important family of membrane-associated proteins with isoform-specific tissue distribution. While novel engagement of the insulin-responsive GLUT4 in myeloma and identification of GLUT9 as a urate transporter open new avenues on this large class of proteins,35 GLUTl and GLUT5 have been shown to be overexpressed in most solid tumors such as human small intestine and colon,48 cervical and ovarian cancers,49, 50 and kidney cancer.51 Overexpression of GLUT1 in stage I-III colorectal cancer was independently associated with poor prognosis in colorectal cancer.52 In breast cancer, GLUT1 overexpression has been shown to correlate with carbonic anhydrase IX (CAIX) and monocarboxylate transporters 1 (MCT1) and 4 (MCT4) as well as their chaperone CD147.53 Fig. 4 represents pH regulation in hypoxic and normoxic cells.
Physiologically, mammalian solute carrier (SLC) proteins control trans-membranous inward and/or outward traverse of nutrients, electrolytes, vitamins, endogenous metabolites and some drugs. As epitomized in Fig. 4, a number of plasma membrane transporters expressed by cancerous cells are H+-coupled transporters that control the local H+-electrochemical gradients and play a key role in trafficking of some drugs.54 Table 3 represents some of the transporters expressed in plasma membrane of cancer cells.
Table 3 . Selected transporters involved in pH dysregulation of cancer cells.
| Transporter | PDescription | Function | Cancer type |
| MCT1 (SLC16A1) MCT3 (SLC16A3) MCT4 (SLC16A4) |
Proton-linked monocarboxylate transporter (MCT) characterized by 12 predicted transmembrane domains | Plasma membrane rapid transportation of monocarboxylates (lactate, pyruvate, branched-chain oxo acids derived from leucine, valine and isoleucine, ketone bodies acetoacetate, beta-hydroxybutyrate and acetate) | Colorectal 55; breast 56; prostate 57; lung 58; gastric 59 |
| SMCT1 (SLC5A8) | An electrogenic Na+and Cl‾-dependent Na+-coupled (SMCT) solute transporter | Transportation of monocarboxylates such as short-chain fatty acids including L-lactate, D-lactate, pyruvate, acetate, propionate, valerate and butyrate, lactate, mocarboxylate drugs (nicotinate, benzoate, salicylate and 5-minosalicylate) | Acute myeloid leukemia 60 |
| NHE1 (SLC9A1) NHE2 (SLC9A2) NHE4 (SLC9A4) |
A Na+/H+ exchanger (NHE) as an antiporter that is a member of the solute carrier family | Involved in pH regulation to eliminate acids generated by active metabolism in cancer cells as a major proton extruding system driven by the inward sodium ion chemical gradient | Cervical 61; breast 62; colon 63; prostate 64; gastric 65 |
| CA IX (CA9) | Carbonic anhydrases (CAs), as a large family of zinc metalloenzymes, catalyze the reversible hydration of carbon dioxide | Reversible hydration of carbon dioxide | Most cancers 66-70 |
| GLUT1 (SLC2A1) | Glucose transporter (GLUT) | Transportation of glucose as an essential source of energy for mammalian cells, involved in an aerobic glycolysis - often observed in tumor cells | Most solid tumors 35, 71 |
Having enhanced glucose uptake via induction of GLUT1 and SGLT1, to prevent intracellular acidification, tumor cells upregulate MCT4, a member of solute carrier family, which is an H+-coupled lactate transporter (Table 3). Nevertheless, the Na+-coupled lactate transporter SMCT1 (SLC5A8), which transports butyrate and pyruvate, functions as a tumor suppressor factor.60,72
It should be highlighted that most solid tumors contain well-oxygenated (normoxic) and poorly oxygenated (hypoxic) regions that respectively utilize glucose for oxidative and glycolytic metabolism (Fig. 4). While hypoxic cancer cells convert glucose to lactate and extrude it into the TME, the normoxic cancer cells take up lactate via SMCT1 and consume it via oxidative phosphorylation. It is deemed that, upon inhibition of SMCT1, normoxic cancer cells take up glucose rather than lactate and hypoxic cancer cells die due to glucose deprivation. Treatment of tumor-bearing mice with an inhibitor of SMCT1 was shown to retard the tumor growth.73
Fascinatingly, a genome-wide haploid genetic screening has recently been conducted to identify the resistance mechanisms to 3-bromopyruvate (3-BrPA), a small molecule that inhibits glycolysis in a poorly understood fashion. It has been evinced that MCT1 is the main transporter for the uptake of 3-BrPA, and its elevation in cancer cells results in sensitivity of in vivo tumor xenograft to 3-BrPA treatment. As H+-coupled transporter, MCT1 catalyzes the movement of many monocarboxylatesand therefore its mRNA level (SLC16A1 mRNA) as the most elevated bioelement in glycolytic cancer cells can be considered as the best predictor of cancer response to 3-BrPA therapy.74 Presumably, this is the first report on identification of a potential biomarker for 3-BrPA sensitivity, which provides proof of concept that the selectivity of cancer-expressed transporters can be exploited for delivering toxic molecules to tumors.
Of the pH dysregulating transporters, perhaps, the Na+/H+ exchanger 1 (NHE1) is one of the best studied bioelements. NHE1 regulates acidity of both intracellular fluid (pHi) and extracellular fluid (pHe). As an integral membrane transport protein, it is activated during oncogene-dependent transformation by various stimulants such as growth factors, hormones, enzymes, the metabolic bioelements in microenvironment (low serum, acidic pH and hypoxia).75,76 The exocytosis of lysosomes to the cell periphery can substantially be induced at pHe 6.4-6.8, which depends on PI3K pathway, GTPase RhoA and sodium-proton exchange activity. Accordingly, NHE1 and NHE3 play a key role on the trafficking of lysosomes.64 Taken all together, dysregulated pH in cancer cells can be reverted via inhibition of NHE1/NHE3 by specific inhibitors such as amiloride, troglitazone and cariporide.64,77,78
As demonstrated in Fig. 4, another pH dysregulator of cancer cells is carbonic anhydrase (CA) that is mainly involved in intracellular catalysis. The catalytically-active intracellular CA isoforms are CAl, CA2, CA3, CA7 and CAl3, while there exist a number of extracellular-facing catalytic sites such as CA4, CA9, CA12, and CA14. Of these, CA9 and CA12 are hypoxically induced and correlate positively with various malignancies.66-70 Therefore, targeting enzymes and transporters involved in pH dysregulation may be considered as logical therapeutic strategy as shown in Fig. 5.
Fig. 5 .
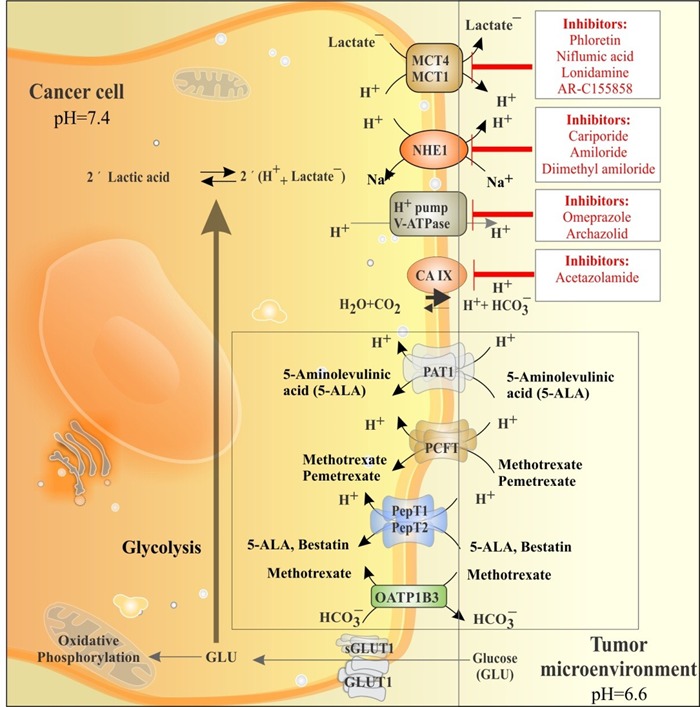
The key players of the pH dysregulation and main transport machineries involved in cellular trafficking of chemotherapeutics in solid tumors. Glucose is mainly taken up by glucose transporter 1 (GLUT1) and metabolized through glycolysis mechanism producing lactic acid (LA) and acidifying cytoplasm. LA is extruded by transporters such as the Na+/H+ exchanger 1 (NHE1), monocarboxylate transporters (MCT1 and 4), carbonic anhydrase IX (CA IX) and H+ pump V-ATPase. These transport machineries can be inhibited with specific/delective inhibitors (red boxes). Cancer cells maintain intracellular pH (pHi) and extracellular pH (pHe) at 7.4 and 6.6, respectively. Transportation of some chemotherapeutics is via cell surface transporters (inset black box).
Further, together with other transport machineries, the V-ATPase appears to be also responsible for development of TME through proton extrusion to the extracellular fluids,which results in activation of destructive enzymes and thereby remodeling of the extracellular matrix.79 Hence, specific inhibition of this transporter may reduce tumor metastasis and prevent the resistance of cancerous cells to chemotherapy and immunotherapy such as the HER2 targeting trastuzumab.80
Final remarks and expert opinions
The dysregulation of pH within TME has been shown to provoke a series of intrinsic functions which triggers progression and invasion of the solid tumors. Warburg effect induces HIF-1 which can in return elicit upregulation of series of TFs, enzymes and transporters. As a result, even in the presence of oxygen, glucose metabolism is shifted toward glycolysis that produces lactic acids as byproducts of such aberrant energetic metabolism by cancerous cells. The upregulation of glucose transporter GLUT-1 and several enzymes involved in glycolysis exacerbates this phenomenon. The accumulation of lactic acids in cancer cells provokes upregulation of several transport machineries (MCT-1, NHE-1, CA IX and H+ pump V-ATPase) and results in reinforced efflux of proton into extracellular fluid. This deviant event makes pH to be settled at 7.4 and 6.6 respectively in cancer cells cytoplasm and extracellular fluid within the tumor microenvironment, which in return triggers secretion of lysosomes’ components into cytoplasm. The exocytosis of lysosomes to the cell periphery can substantially be induced at acidic pH of extracellular fluid, which is also in close relation with PI3K pathway as a GTPase RhoA and sodium-proton exchange activity. Further, some proton exchangers, specifically NHE1 and NHE3 have been reported to play a key role in the trafficking of lysosomes.64 It is well-defined fact that immunotherapy through targeting of various oncomarkers such as epidermal growth factor receptors,81,82 G-protein coupled receptors83 and many others84 is one the most effective approaches for cancer therapy.85 However, targeted therapy of cancer may result in better clinical outcome if the pH dysregulation is synchronically inhibited. All these anomalous happenings make TME to be exposed to cocktail of various enzymes with acidic pH, upon which ECM can be remodeled and even deformed resulting in emergence of a viscose TME with high interstitial fluid pressure that also acts as an obstacle to cancer therapy.86
In conclusion, it appears that pH dysregulation within TME remodels many normal physiologic functions and makes solid tumors to become invasive and metastatic, while induces chemoresistance and immunoresistance in cancerous cells. Therefore, the next step for cancer therapy seems to be the combination therapy using specific inhibitors of bioelements involved in pH dysregulation along with conventional anticancer agents. To have a successful combination therapy, it is crucial to deliver the agents into TME using appropriate drug/gene delivery systems (DDSs/GDSs) ideally through passive and/or active targeting approaches. However, it should be pointed out that there exist intrinsic bio-signature for many DDSs/GDSs,87 which need to be considered during delivery of the active agents into TME to avoid any inadvertent impacts as previously shown for several non-viral GDSs.88-94 Biodegradable polymers such as poly (lactic-co-glycolic acid) (PLGA) and some other biodegradable block copolymers (e.g., poly(ethylene glycol)-block-poly(ε−caprolactone) methyl ether) may provide safer path for delivery of the active agents into TME.95 Therefore, we envision that targeted nanoparticles, engineered using biodegradable polymers/lipids encapsulating designated drug(s), should be employed for specific delivery of the components of combination therapy into TME to circumvent undesired sides effects of such cocktail within the normal tissues.
Acknowledgements
The authors express their sincere gratitude to Prof. George Coukos (Ovarian Cancer Research Center at University of Pennsylvania and Ludwig Center for Cancer Research at the University of Lausanne) for his valuable comments.
Ethical issues
The authors declare no ethical issues.
Competing interests
The authors declare no conflict of interests.
References
- Omidi Y, Gumbleton M. Biological Membranes and Barriers. In: Mahato RI, editor. Biomaterials for Delivery and Targeting of Proteins Nucleic Acids. New York: CRC Press; 2005. p. 232-74.
- Matzinger P. Friendly and dangerous signals: is the tissue in control? . Nat Immunol . 2007; 8: 11– 3. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- Gray SG, Eriksson T, Ekstrom TJ. Methylation, gene expression and the chromatin connection in cancer (review) Int J Mol Med . 1999; 4:333–50. doi: 10.3892/ijmm.4.4.333. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell . 2000; 100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Cardone RA, Harguindey S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov . 2013; 8:85–99. doi: 10.2174/15748928130108. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Larue L. PI3K/AKT Pathway and the Epithelial–Mesenchymal Transition. In: Thomas-Tikhonenko A, editor. Cancer Genome and Tumor Microenvironment. New York: Springer Science; 2010. p. 11-32.
- Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: Concepts and molecular links. Semin Cancer Biol . 2012; 22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaihara T, Kusaka T, Nishi M, Kawamata H, Imura J, Kitajima K. et al. Dedifferentiation and decreased expression of adhesion molecules, E-cadherin and ZO-1, in colorectal cancer are closely related to liver metastasis. J Exp Clin Cancer Res . 2003; 22:117–23. [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem . 2008; 283:14910–4. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol . 2008; 10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Salem AF, Bonuccelli G, Bevilacqua G, Arafat H, Pestell RG, Sotgia F. et al. Caveolin-1 promotes pancreatic cancer cell differentiation and restores membranous E-cadherin via suppression of the epithelial-mesenchymal transition. Cell Cycle . 2011; 10:3692–700. doi: 10.4161/cc.10.21.17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CE, Kuo PC. The tumor microenvironment. Surg Oncol . 2012; 21:172–7. doi: 10.1016/j.suronc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Kerr D. Clinical development of gene therapy for colorectal cancer. Nat Rev Cancer . 2003; 3:615–22. doi: 10.1038/nrc1147. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? . Nat Rev Cancer . 2004; 4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Balliet RM, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F. et al. Hereditary ovarian cancer and two-compartment tumor metabolism: Epithelial loss of BRCA1 induces hydrogen peroxide production, driving oxidative stress and NFkappaB activation in the tumor stroma. Cell Cycle . 2012; 11:4152–66. doi: 10.4161/cc.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. Reverse Warburg: straight to cancer. Cell Cycle . 2012; 11: 1059. doi: 10.4161/cc.11.6.19746. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? . Anticancer Agents Med Chem . 2008; 8: 305– 12. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- Weljie AM, Jirik FR. Hypoxia-induced metabolic shifts in cancer cells: moving beyond the Warburg effect. Int J Biochem Cell Biol . 2011; 43:981–9. doi: 10.1016/j.biocel.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer . 2003; 3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Gort EH, van Haaften G, Verlaan I, Groot AJ, Plasterk RH, Shvarts A. et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene . 2008; 27:1501–10. doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- Corso S, Migliore C, Ghiso E, De Rosa G, Comoglio PM, Giordano S. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene . 2008; 27:684–93. doi: 10.1038/sj.onc.1210697. [DOI] [PubMed] [Google Scholar]
- Mie Lee Y, Kim SH, Kim HS, Jin Son M, Nakajima H, Jeong Kwon H. et al. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochem Biophys Res Commun . 2003; 300:241–6. doi: 10.1016/s0006-291x(02)02787-0. [DOI] [PubMed] [Google Scholar]
- Temes E, Martin-Puig S, Acosta-Iborra B, Castellanos MC, Feijoo-Cuaresma M, Olmos G. et al. Activation of HIF-prolyl hydroxylases by R59949, an inhibitor of the diacylglycerol kinase. J Biol Chem . 2005; 280:24238–44. doi: 10.1074/jbc.M414694200. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Eun JS, Kim BG, Kim SY, Jeon H, Soh Y. Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells. Molecules and cells . 2006; 22:291–9. [PubMed] [Google Scholar]
- Shin DH, Kim JH, Jung YJ, Kim KE, Jeong JM, Chun YS. et al. Preclinical evaluation of YC-1, a HIF inhibitor, for the prevention of tumor spreading. Cancer Lett . 2007; 255:107–16. doi: 10.1016/j.canlet.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Dikmen ZG, Gellert GC, Dogan P, Yoon H, Lee YB, Ahn CH. et al. In vivo and in vitro effects of a HIF-1alpha inhibitor, RX-0047. J Cell Biochem . 2008; 104:985–94. doi: 10.1002/jcb.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T, Yin S, Gelin CF, Moreno CS, Yepes M, Nicolaou KC. et al. Identification of a novel small molecule HIF-1alpha translation inhibitor. Clin Cancer Res . 2009; 15:6128–36. doi: 10.1158/1078-0432.CCR-08-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Han J, Lee Y, Choi J, Han S, Hong S. et al. Caffeic acid phenethyl ester is a potent inhibitor of HIF prolyl hydroxylase: structural analysis and pharmacological implication. J Nutr Biochem . 2010; 21:809–17. doi: 10.1016/j.jnutbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL. et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther . 2008; 7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- Lee K, Kim HM. A novel approach to cancer therapy using PX-478 as a HIF-1alpha inhibitor. Arch Pharm Res . 2011; 34:1583–5. doi: 10.1007/s12272-011-1021-3. [DOI] [PubMed] [Google Scholar]
- Shen Y, Wu Y, Chen M, Shen W, Huang S, Zhang L. et al. Effects of pantoprazole as a HIF-1alpha inhibitor on human gastric adenocarcinoma sgc-7901 cells. Neoplasma . 2012; 59:142–9. doi: 10.4149/neo_2012_019. [DOI] [PubMed] [Google Scholar]
- Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, van der Wall E. Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology . 2005; 46:31–6. doi: 10.1111/j.1365-2559.2005.02045.x. [DOI] [PubMed] [Google Scholar]
- Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res . 2006; 66:3197–204. doi: 10.1158/0008-5472.CAN-05-3090. [DOI] [PubMed] [Google Scholar]
- Herling A, Konig M, Bulik S, Holzhutter HG. Enzymatic features of the glucose metabolism in tumor cells. The FEBS journal . 2011; 278:2436–59. doi: 10.1111/j.1742-4658.2011.08174.x. [DOI] [PubMed] [Google Scholar]
- Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol . 2012; 24:650–4. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A, Dolle C, Felici R, Ziegler M. The NAD metabolome--a key determinant of cancer cell biology. Nat Rev Cancer . 2012; 12:741–52. doi: 10.1038/nrc3340. [DOI] [PubMed] [Google Scholar]
- Wei S, Kulp SK, Chen CS. Energy restriction as an antitumor target of thiazolidinediones. J Biol Chem . 2010; 285:9780–91. doi: 10.1074/jbc.M109.065466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevs J, Loser R, Rattel B, Esser N. Antiangiogenic potency of FK866/K22. 175, a new inhibitor of intracellular NAD biosynthesis, in murine renal cell carcinoma. Anticancer Res . 2003; 23:4853–8. [PubMed] [Google Scholar]
- Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV. et al. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood . 2009; 113:3276–86. doi: 10.1182/blood-2008-08-173369. [DOI] [PubMed] [Google Scholar]
- Fuchs D, Rodriguez A, Eriksson S, Christofferson R, Sundberg C, Azarbayjani F. Metronomic administration of the drug GMX1777, a cellular NAD synthesis inhibitor, results in neuroblastoma regression and vessel maturation without inducing drug resistance. Int J Cancer . 2010; 126:2773–89. doi: 10.1002/ijc.25206. [DOI] [PubMed] [Google Scholar]
- Pardee AB, Li YZ, Li CJ. Cancer therapy with beta-lapachone. Curr Cancer Drug Targets . 2002; 2:227–42. doi: 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- Poot M, Hosier S, Swisshelm K. Distinct patterns of mitochondrial changes precede induction of apoptosis by all-trans-retinoic acid and N-(4-hydroxyphenyl)retinamide in MCF7 breast cancer cells. Exp Cell Res . 2002; 279:128–40. doi: 10.1006/excr.2002.5582. [DOI] [PubMed] [Google Scholar]
- Yalowitz JA, Pankiewicz K, Patterson SE, Jayaram HN. Cytotoxicity and cellular differentiation activity of methylenebis(phosphonate) analogs of tiazofurin and mycophenolic acid adenine dinucleotide in human cancer cell lines. Cancer Lett . 2002; 181:31–8. doi: 10.1016/s0304-3835(02)00045-9. [DOI] [PubMed] [Google Scholar]
- Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu J, Zhang YP. et al. Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res . 2003; 63:5513–20. [PubMed] [Google Scholar]
- Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M. et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene . 2006; 25:176–85. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Bass SE, MacDonald CJ, Cheng RY, Yeh GC. Sulindac and its metabolites induce carcinogen metabolizing enzymes in human colon cancer cells. Int J Cancer . 2008; 122:990–8. doi: 10.1002/ijc.23218. [DOI] [PubMed] [Google Scholar]
- Lara E, Mai A, Calvanese V, Altucci L, Lopez-Nieva P, Martinez-Chantar ML. et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene . 2009; 28:781–91. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- Mahraoui L, Rousset M, Dussaulx E, Darmoul D, Zweibaum A, Brot-Laroche E. Expression and localization of GLUT-5 in Caco-2 cells, human small intestine, and colon. Am J Physiol . 1992; 263:G312–8. doi: 10.1152/ajpgi.1992.263.3.G312. [DOI] [PubMed] [Google Scholar]
- Rudlowski C, Moser M, Becker AJ, Rath W, Buttner R, Schroder W. et al. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology . 2004; 66:404–10. doi: 10.1159/000079489. [DOI] [PubMed] [Google Scholar]
- Rudlowski C, Becker AJ, Schroder W, Rath W, Buttner R, Moser M. GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am J Clin Pathol . 2003; 120:691–8. doi: 10.1309/4KYN-QM58-62JW-2GD7. [DOI] [PubMed] [Google Scholar]
- Ozerlat I. Kidney cancer: targeted therapy of glucose uptake via GLUT1 kills RCC cells. Nat Rev Urol . 2011; 8: 471. doi: 10.1038/nrurol.2011.124. [DOI] [PubMed] [Google Scholar]
- Shen YM, Arbman G, Olsson B, Sun XF. Overexpression of GLUT1 in colorectal cancer is independently associated with poor prognosis. Int J Biol Markers . 2011; 26:166–72. doi: 10.5301/JBM.2011.8550. [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D. et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol . 2011; 26:1279–86. doi: 10.14670/HH-26.1279. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology . 2010; 25:364–77. doi: 10.1152/physiol.00027.2010. [DOI] [PubMed] [Google Scholar]
- Verstraete M, Debucquoy A, Devos E, Sagaert X, Penninckx F, Begg A. et al. Investigation of possible endogenous hypoxia markers in colorectal cancer. Int J Radiat Biol . 2013; 89:9–15. doi: 10.3109/09553002.2012.715789. [DOI] [PubMed] [Google Scholar]
- Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R. et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle . 2012; 11:1108–17. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Masko EM, Poulton SL, Kennedy KM, Pizzo SV, Dewhirst MW. et al. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU Int . 2012; 110:1062–9. doi: 10.1111/j.1464-410X.2012.10971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Takahashi M, Uramoto H, Nakayama Y, Oyama T, Wang KY. et al. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci . 2011; 102:1007–13. doi: 10.1111/j.1349-7006.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Longatto-Filho A, Simoes K, Jacob CE, Bresciani CJ, Zilberstein B. et al. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. Eur J Cancer . 2009; 45:2418–24. doi: 10.1016/j.ejca.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Whitman SP, Hackanson B, Liyanarachchi S, Liu S, Rush LJ, Maharry K. et al. DNA hypermethylation and epigenetic silencing of the tumor suppressor gene, SLC5A8, in acute myeloid leukemia with the MLL partial tandem duplication. Blood . 2008; 112:2013–6. doi: 10.1182/blood-2008-01-128595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MR, Wilkins RJ, Chou CY, Ellory JC. Anion exchanger isoform 2 operates in parallel with Na(+)/H(+) exchanger isoform 1 during regulatory volume decrease of human cervical cancer cells. FEBS letters . 2002; 512:52–8. doi: 10.1016/s0014-5793(01)03317-8. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Cardone RA, Tommasino M, Casavola V, Paradiso A. Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells. Clin Cancer Res . 2003; 9:2366–73. [PubMed] [Google Scholar]
- Beltran AR, Ramirez MA, Carraro-Lacroix LR, Hiraki Y, Reboucas NA, Malnic G. NHE1, NHE2, and NHE4 contribute to regulation of cell pH in T84 colon cancer cells. Pflugers Arch . 2008; 455:799–810. doi: 10.1007/s00424-007-0333-0. [DOI] [PubMed] [Google Scholar]
- Steffan JJ, Snider JL, Skalli O, Welbourne T, Cardelli JA. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic . 2009; 10:737–53. doi: 10.1111/j.1600-0854.2009.00904.x. [DOI] [PubMed] [Google Scholar]
- Hosogi S, Miyazaki H, Nakajima KI, Ashihara E, Niisato N, Kusuzaki K. et al. An Inhibitor of Na/H(+) Exchanger (NHE), Ethyl-Isopropyl Amiloride (EIPA), Diminishes Proliferation of MKN28 Human Gastric Cancer Cells by Decreasing the Cytosolic Cl(-) Concentration via DIDS-Sensitive Pathways. Cell Physiol Biochem . 2012; 30:1241–53. doi: 10.1159/000343315. [DOI] [PubMed] [Google Scholar]
- Salmon AJ, Williams ML, Wu QK, Morizzi J, Gregg D, Charman SA. et al. Metallocene-based inhibitors of cancer-associated carbonic anhydrase enzymes IX and XII. J Med Chem . 2012; 55:5506–17. doi: 10.1021/jm300427m. [DOI] [PubMed] [Google Scholar]
- Smyth LG, O’Hurley G, O’Grady A, Fitzpatrick JM, Kay E, Watson RW. Carbonic anhydrase IX expression in prostate cancer. Prostate Cancer Prostatic Dis . 2010; 13:178–81. doi: 10.1038/pcan.2009.58. [DOI] [PubMed] [Google Scholar]
- Said HM, Supuran CT, Hageman C, Staab A, Polat B, Katzer A. et al. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr Pharm Des . 2010; 16:3288–99. doi: 10.2174/138161210793429788. [DOI] [PubMed] [Google Scholar]
- Nakao M, Ishii G, Nagai K, Kawase A, Kenmotsu H, Kon-No H. et al. Prognostic significance of carbonic anhydrase IX expression by cancer-associated fibroblasts in lung adenocarcinoma. Cancer . 2009; 115:2732–43. doi: 10.1002/cncr.24303. [DOI] [PubMed] [Google Scholar]
- Pastorekova S, Kopacek J, Pastorek J. Carbonic anhydrase inhibitors and the management of cancer. Curr Top Med Chem . 2007; 7:865–78. doi: 10.2174/156802607780636708. [DOI] [PubMed] [Google Scholar]
- Zawacka-Pankau J, Grinkevich VV, Hunten S, Nikulenkov F, Gluch A, Li H. et al. Inhibition of glycolytic enzymes mediated by pharmacologically activated p53: targeting Warburg effect to fight cancer. J Biol Chem . 2011; 286:41600–15. doi: 10.1074/jbc.M111.240812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life sciences . 2006; 78:2419–25. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest . 2008; 118:3835–7. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Wang T, Possemato R, Yilmaz OH, Koch CE, Chen WW. et al. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nature genetics . 2012; 45:104–8. doi: 10.1038/ng.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Bunch L, Pedersen SF. Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences, and implications for cancer therapy. Curr Pharm Des . 2012; 18:1345–71. doi: 10.2174/138161212799504830. [DOI] [PubMed] [Google Scholar]
- Loo SY, Chang MK, Chua CS, Kumar AP, Pervaiz S, Clement MV. NHE-1: a promising target for novel anti-cancer therapeutics. Curr Pharm Des . 2012; 18:1372–82. doi: 10.2174/138161212799504885. [DOI] [PubMed] [Google Scholar]
- Friday E, Oliver R 3rd, Welbourne T, Turturro F. Glutaminolysis and glycolysis regulation by troglitazone in breast cancer cells: Relationship to mitochondrial membrane potential. J Cell Physiol . 2011; 226:511–9. doi: 10.1002/jcp.22360. [DOI] [PubMed] [Google Scholar]
- Wong P, Lee C, Tannock IF. Reduction of intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin Cancer Res . 2005; 11:3553–7. doi: 10.1158/1078-0432.CCR-04-2472. [DOI] [PubMed] [Google Scholar]
- Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Rey JM, Garcia-Garcia A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev . 2009; 35:707–13. doi: 10.1016/j.ctrv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- von Schwarzenberg K, Lajtos T, Simon L, Muller R, Vereb G, Vollmar AM. V-ATPase inhibition overcomes trastuzumab resistance in breast cancer. Mol Oncol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran B, Hosseini AZ, Majidi J, Farajnia S, Barar J, Saraf ZH. et al. Development and characterization of monoclonal antibodies against human epidermal growth factor receptor in Balb/c mice. Hum Antibodies . 2009; 18:11–6. doi: 10.3233/HAB-2009-0195. [DOI] [PubMed] [Google Scholar]
- Rezaiemanesh A, Majidi J, Baradaran B, Movasaghpour A, Nakhlband A, Barar J. et al. Impacts of anti-EGFR monoclonal antibody in prostate cancer PC3 cells. Hum Antibodies . 2010; 19:63–70. doi: 10.3233/HAB-2010-0229. [DOI] [PubMed] [Google Scholar]
- Tohidkia MR, Asadi F, Barar J, Omidi Y. Selection of potential therapeutic human single-chain Fv antibodies against cholecystokinin-B/gastrin receptor by phage display technology. BioDrugs . 2013; 27:55–67. doi: 10.1007/s40259-012-0007-0. [DOI] [PubMed] [Google Scholar]
- Barkholt L, Bregni M. Current immunotherapy for solid tumors. Immunotherapy . 2009; 1:483–93. doi: 10.2217/imt.09.13. [DOI] [PubMed] [Google Scholar]
- Majidi J, Barar J, Baradaran B, Abdolalizadeh J, Omidi Y. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies . 2009; 18:81–100. doi: 10.3233/HAB-2009-0204. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nature reviews Cancer . 2004; 4:806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- Barar J, Omidi Y. Intrinsic bio-signature of gene delivery nanocarriers may impair gene therapy goals. Bioimpacts . 2013; 3:105–9. doi: 10.5681/bi.2013.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barar J, Hamzeiy H, Mortazavi-Tabatabaei SA, Hashemi-Aghdam SE, Omidi Y. Genomic signature and toxicogenomics comparison of polycationic gene delivery nanosystems in human alveolar epithelial A549 cells. Daru . 2009; 17:139–47. [Google Scholar]
- Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods . 2008; 18:369–78. doi: 10.1080/15376510801891286. [DOI] [PubMed] [Google Scholar]
- Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. JDrug Target . 2007; 15:83–8. doi: 10.1080/10611860601151860. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv . 2005; 2:429–41. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target . 2003; 11:311–23. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. BI . 2011; 1: BI 2011; 1. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol . 2009; 28:113–22. doi: 10.1177/1091581809335177. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Davaran S. Impacts of biodegradable polymers: Towards biomedical applications. In: Sharma S, A Mudhoo, editors. Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications. Cambridge: Royal Society of Chemistry; 2011. p. 388-418.


