Abstract
Introduction: At present, the polymer gel dosimeter is considered to be the best possible dosimeter for measuring 3-dimesional radiation dose distribution in radiotherapy. These gels are normally toxic; therefore, manufacturing, handling and discarding them require special attention. In order to find less toxic recipe, N-isopropyle acrylamide polymer gel (NIPAM) was introduced. In this study, the reproducibility and stability of NIPAM polymer gel dose response together with some influencing factors related to MR imaging were studied. Methods: The NIPAM gel was prepared according to a method, described by senden et al in 2006. The gels were irradiated approximately 2 h after manufacturing and MR images of the gel were made 24 h after irradiation. The effects of different batches, post-irradiation time and the MRI room temperature on reproducibility and stability of polymer gel dose response were explored by analyzing the NMR response (R2) of the gel. Results:: In a fixed temperature, the response of the gel was found to be stable 24 h after irradiation. The results showed that the dose response of the NIPAM polymer gel is highly reproducible in the same and different batches of chemical. No inhomogeneity was observed for magnetic fields in the specified position of measurements and 5°C fluctuation was recorded for MRI room temperature. Conclusion: Fluctuation in MRI room temperature necessitates that stringent attention to be paid to controlling the gel temperature at the time of imaging. The new formulation of polymer gel ensures stability of the gels’ spatial resolution and makes it a suitable dosimeter for distant or remote measurements.
Keywords: NIPAM, MRI, NMR
Introduction
Polymer gel dosimeters with the purpose of 3-D radiation dosimetry were first proposed by Maryanski et al in 1994.1 These gel dosimeters were based on the polymerization of acrylamide (AAm) and N,N’-methylen-bisacrylamide crosslinker infused in an aqueous agarose matrix upon irradiation.1 As the concentration of polymerization is proportional to the radiation dose, this can lead to an increase in the transverse relaxation rate of water protons which could be probed by magnetic resonance imaging (MRI).2 Despite the promising results, the clinical use of polymer gel dosimeters has been limited. Firstly, due to the inhibiting role of oxygen in the polymerization process, the gel manufacturing has to be done in a hypoxic environment.3-5 Secondly, the norotoxic and carcinogenic nature of monomers makes the manufacturing process inconvenient.6,7 To solve the problem, in 2001 Fong et al introduced normoxic polymer gel dosimeters which could be manufactured in normal atmospheric condition by adding an antioxidant to the gel structure.8 Although oxygen contamination was ruled out and suppressed in normoxic compositions, the potential health hazards and risks associated with the monomers9,10 still remained as a fundamental complication which restricted clinical application of these types of gel dosimeters. The studies were ongoing using different monomers with the objective of producing more accurate dosimeters that could considerably reduce the safety concerns, and thus would make handling of the dosimeter in clinical environments more convenient.
Acrylamide is a dangerous neurotoxin and a suspected human carcinogen that can be readily absorbed through the skin; as a result, it requires careful handling.11-13 Recently, Senden et al in 2006 have investigated new dosimeter recipes by replacing acrylamide with three different monomers, namely N-isopropyle acrylamide (NIPAM), diacetone acrylamide (DAAM) and N-vinyl formamide (NVF), among which NIPAM has exhibited a better dose response than the others.13 Even though these monomers are similar in their chemical structure to acrylamide, they are much less toxic and more soluble.14
Easier manufacturing and less toxic properties of NIPAM polymer gel, compared to previous types of gel dosimeters, makes it an appropriate candidate for clinical applications. In order to be an effective calibration and verification tool in radiotherapy, reproducibility and stability of dose response are two of the most important characteristics for a dosimetry technique.15 Multiple imaging from one irradiated gel dosimeter (stability of dose response) and reproducibility studies must be conducted in identical conditions. When MRI is used as a mean of reading, some influencing factors like inhomogeneity of magnetic fields (static magnetic field (B0) and radio frequency field (B1)) and temperature changes at the time of scanning can affect the results of gel dosimetry experiments.16 B0 and B1 inhomogeneity may change the dose value in each voxel (depending on the position of voxel in magnetic fields) which will subsequently affect the dose response (R2) of the gel. Relaxation rates (R2) of polymer gel dosimeters are known to decrease with the MR scanning temperature increasing,17 which causes a reduction in both the slope (sensitivity) and intercept of R2–dose response curves.4,18 Since it is assumed that MRI room temperature is kept fixed, most of articles suggest keeping gel dosimeters in MR scanning room for 24 h before imaging in order to have equilibrated temperature in experiments.9 However possible fluctuations in MRI room temperature as a result of ventilation system performance can affect gel dose response in different experiments. Therefore, the aim of this study is to explore the reproducibility and stability of NIPAM polymer gel response together with investigation of magnetic fields homogeneity and temperature changes in the MR scanning room as influencing factors.
Materials and methods
The NIPAM gel was prepared according to the method described by Senden et al in 2006.13 In order to make the required amount of the polymer gel, first a concentration of 5% (by weight) gelatin (300 Bloom, type A, Sigma-Aldrich) was added to 80% of deionized water and left to swell for 10 minutes. It was then heated up to 50°C and stirred with a magnetic stirrer until the gelatin was fully dissolved. The solution was cooled down to 40°C and while being stirred continuously, 3% Bis (99%, Sigma–Aldrich, USA) was added. Once the Bis was dissolved, 3% NIPAM (sigma –Aldrich USA) was added at 37°C and stirred until complete dissolution was achieved. Finally, a solution of antioxidant was prepared with 10 mM of THPC (MERCK-Schuchardt, Germany) and the remaining deionized water, which was added to the gel solution at 35°C. The resulting gel was then transferred to the vials and refrigerated for half an hour to solidify.
To avoid photopolymerisation, the samples were put in a cardboard box before irradiation. The gels were irradiated approximately 2 h after being manufactured, in a rectangular water bath made in-house from Perspex, which was designed to simultaneously expose multiple test tubes to different doses. The samples were irradiated with 9MV X-rays, using a Nepton linear accelerator. The irradiation was directed perpendicular to the length of water bath (Fig. 1). To prevent a dose gradient in the gels, the vials were turned 180° halfway through the irradiation. The irradiated gels were kept at room temperature until the gels were imaged. MR images of the gel were made 24 h after irradiation. The vials were imaged in a rectangular wooden box in which they had been placed in a fixed position, 1 cm from each other. The box was placed in the head-coil of Philips Intra 1.5 T MRI scanner (General electric, USA). T2 weighted images of 5 mm thick slice were taken parallel to the irradiated surface through middle of the gel, using a multi slice spin-echo method, and transverse relaxation rates (R2=T2-1) were then obtained from the signal decay data, using the image processing tool (Jim). For all the measurements, a repetition time (TR) of 4000 ms was used, with 15 time echo ranging from 700 to 1400 ms with increment of 50.
Fig. 1 .

Experimental set up of gel irradiation
Results
Reproducibility of dose response
In order to investigate the absorbed dose response reproducibility, the polymer gel was manufactured as described and the gel vials were irradiated isocentrically to absorb doses of 1 to 10 Gy and one gel vial was kept unirradiated for background measurement. The experiment was repeated three times while keeping the irradiation method, scanning parameters and temperature unchanged.
Fig.2 shows the results obtained from relaxation rate measurements (R2) in three sets of the samples prepared on different days. The data in Fig. 2 shows that the dose response is highly reproducible over the range of the measured dose with the difference of 3%, provided that the chemicals are taken from the same batch.
Fig. 2 .
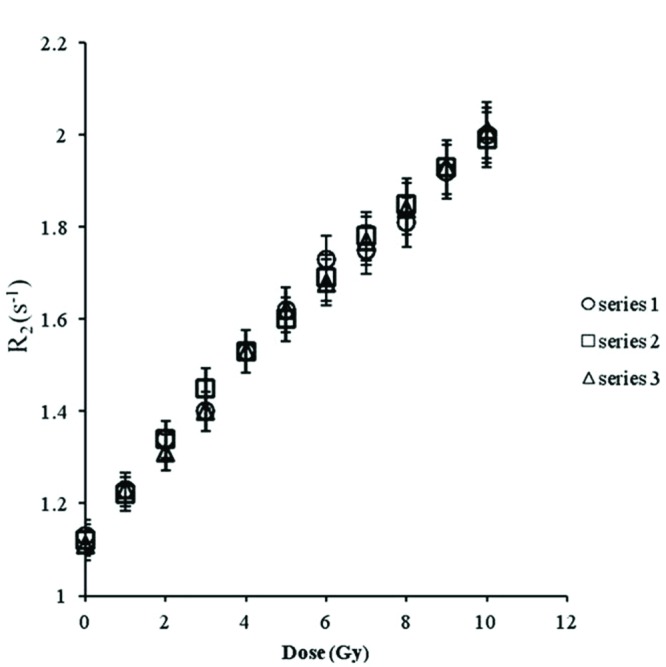
Reproducibility of dose response in same batch of chemical
As a different batch of the chemical may have an effect on the gel’s response,19 two sets of the gel vials were prepared from different batches for the purpose of comparison. The results are shown in Fig. 3. It can be seen that there are no differences in the gel responses within ± 2%.
Fig. 3 .
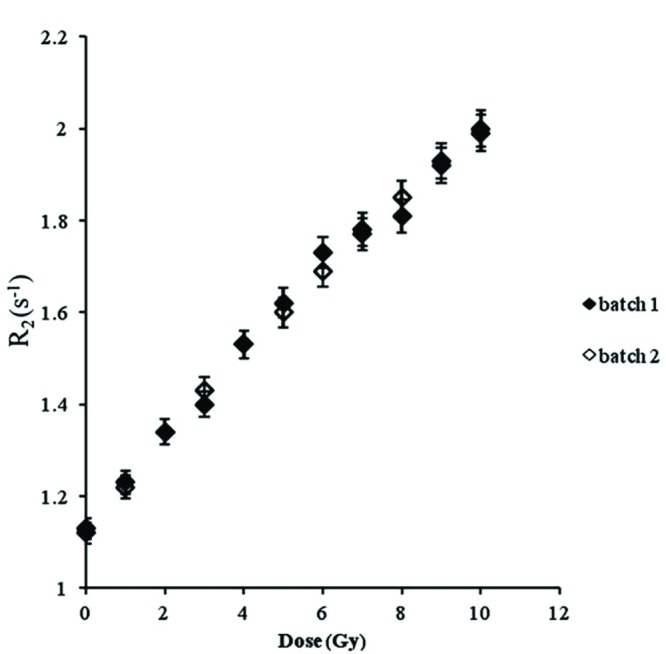
Dose response reproducibility of different batches
Effect of the time of imaging after irradiation
To investigate if the response of the gel was going to be constant with the time after irradiation, the R2 values of a series of the gels were measured for a period of one month, during which five measurements were obtained. Fig. 4 shows the measured R2 for doses of 0 to 10 Gy for five different occasions during a month. It is clear that there is no variation in gel’s dose response with the time after irradiation in the stated measured time.
Fig. 4 .
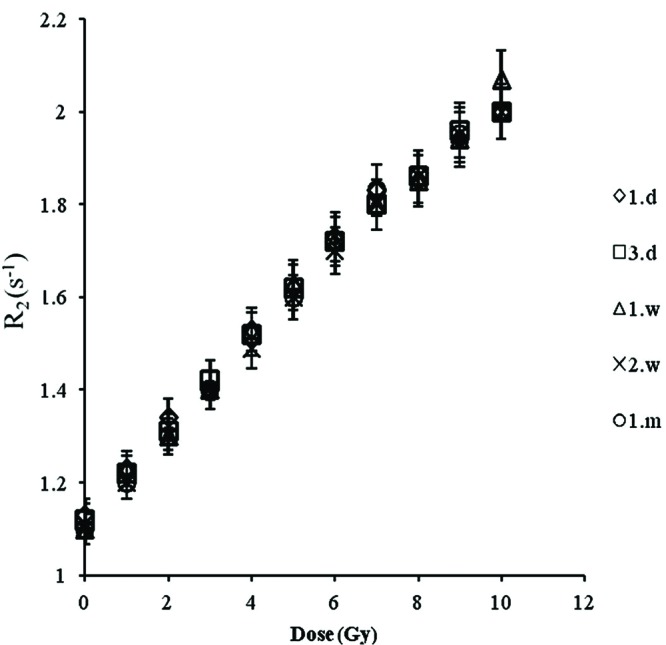
Stability of the gel response with time
Magnetic field homogeneity
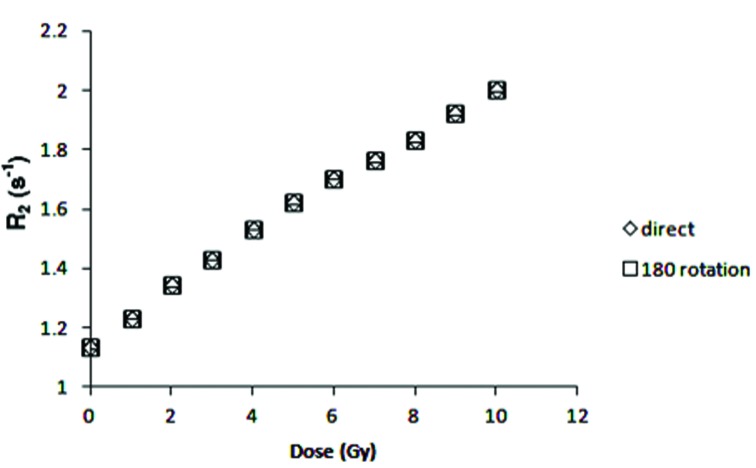
Magnetic fields in MR scanner are not homogeneously distributed, which can affect R2 values.16 To determine if there is any magnetic field inhomogeneity in the position of measurements, first the irradiated samples were scanned in the center of the head coil, using the same pulse sequence and image parameter as described above, then the holder was rotated 180o and the imaging was repeated in the same condition. Fig. 5 shows no differences in R2 values for different positions of vials inside the head coil.
Fig. 5 .
Effect of vial positioning inside the head coil
Scanning room temperature
The first series of reproducibility experiments were performed on the assumption that there was a fixed temperature in the MRI room and the gel dosimeters would be kept in the scanning room for 24 h before imaging to take on the room temperature. However, as Fig. 6 demonstrates, the dose response did not lend itself to reproducibility. One of the intervening factors was thought to be the possible fluctuations in the room temperature which was monitored for 7 days. The room temperature was found to fluctuate up to 5oC in the time recorded (Fig. 7).
Fig. 6 .
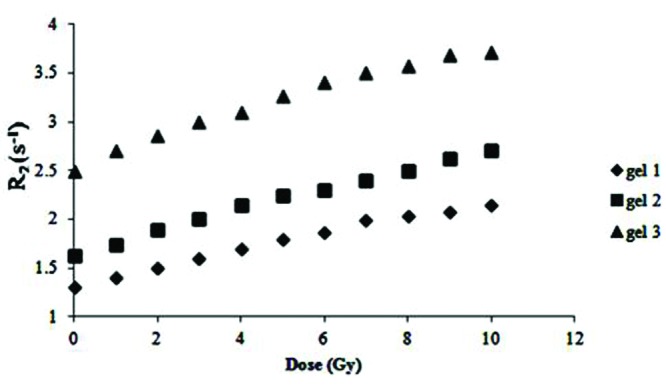
Dose response of NIPAM polymer gel after keeping the vials in MR scanning room
Fig. 7 .
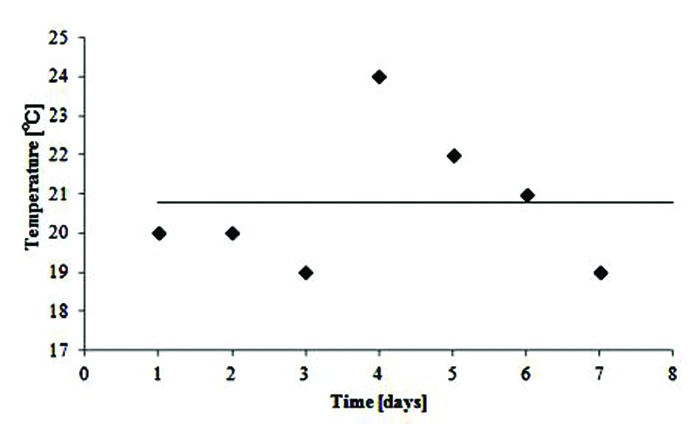
Temperature fluctuations inside MR scanner room
Discussion
The present work involved the investigation of NIPAM polymer gel dosimeter, using MRI. The stability and reproducibility with some of influencing factors related to MR imaging were also determined.
In a fixed temperature (21°C), the response of NIPAM gel was found highly reproducible even for different batches of chemicals. This result complies well with the work carried out by Senden et al (2006) and also a work done by Hsieh et al in 2011.13,20 Even though all types of polymer gel exhibit good reproducibility, using the same batch of chemical, some of them such as BANG have been reported to respond differently to the radiation dose while using chemicals from a different batch.19
The response of the gel was found to be stable 24 h after irradiation. This is in contrast to the results that were achieved by Senden et al in 2006,13 and chang et al in 2011,21 indicating the stability of the dose response after 72 h and Hsieh et al (2011) by reporting termination of the polymerization process 5 h after irradiation.20 The differences in the results make further investigation necessary in this regard.
It is well known that the R2 values of the gel are temperature dependent and in most of articles when MRI is used to read out the gels, it is suggested that the gel phantom be stored in the scanner room to have an equilibrated temperature.9 Then in the first series of experiments for investigating the reproducibility of NIPAM polymer gel dosimeter, the gel vials were kept in MRI room for 24 h before imaging. Since no reproducibility was achieved in data analysis, the attention was directed at room temperature in an attempt to find possible affecting factors. The reason behind this focus was the fact that temperature fluctuations during MRI are known to have a significant influence on the dose response,22 and thus the MRI room temperature was monitored for 7 days. A 5°C fluctuation was found between measurements, while temperature fluctuations of 1°C result in dose deviations of 3-4%.13 To solve the problem, all samples were kept in a water bath at a fixed temperature (21°C) for about 2 h before imaging and a reproducibility experiment was repeated under temperature-controlled conditions. The role attributed to temperature fluctuations has also been recognized by others working in the field. For example, Vandecasteele and De Deene in 2013 reported the maximum temperature range in the scanner room within 1.1°C over a course of seven days.16 Although the measured temperature fluctuations are inconsistent with each other, both of them demonstrate instability of MRI room temperature. The discrepancy between reports could arise from different workloads, poor ventilation systems or different setting of ventilation systems in different conditions.
Inhomogeneity of magnetic fields is one of the influencing factors in MR gel dosimetry experiments. Any deviation in the static magnetic field (B0-field) and radio frequency field (B1-field) can affect the accuracy of the study. Vandecasteele and De Deene explored the influences of B0-field and B1-field spatial inhomogeneities for different geometrically shaped phantoms at different field strengths.16 In the present study, the position of vials was changed inside the MRI head coil in order to see if there was any spatial inhomogeneity in the magnetic fields. The results showed no differences in gel dosimeter responses which suggest the spatial homogeneity of magnetic field in the specified position of measurements.
The rigid background matrix, composed of gelatin or agarose, secures the spatial and temporal stability of gel dosimeters,15 but in normoxic polymer gel, it is not the only role played by these components. In this study, the vials were put in hot water in order to discard irradiated polymer gels and reuse the vials, based on previous experiments with gelatin- and agarose-based dosimeters;19 however, contrary to our expectation, the melting of content was not observed. It might be supposed that the stiffer structure of normoxic polymer gel dosimeters in comparison to previous types could be attributed to the reactions between gelatin and THPC. The experiments showed that in normoxic polymer gels, reactions between gelatin and THPC increase the crosslinking of the gelatin matrix.23 When all essential equipments for entire gel dosimetry process are not assembled in one location, then the more rigid structure of normoxic gel dosimeters provides the opportunity for preparing, irradiating and imaging these gel dosimeters in different places without concern for losing the spatial resolution due to a high temperature or long distance. The heat stability of normoxic polymer gel causes some problems in discarding, because sometimes irradiated gel dosimeters have to be thrown away by its container. Although replacing acrylamaide with N-isopropylacrylamaide resulted in less toxic properties of NIPAM gel, the existence of other toxic ingredients like THPC and unreacted Bis monomers in a stiff irradiated gel dosimeter could still cause some concern regarding discarding the used gels alongside the associated health hazards.15,24
Conclusion
The result of this study showed that in a fixed temperature, the dose response of NIPAM polymer gel dosimeter is reproducible in the same and different batches of chemicals. The gel response was found to be stable for 24 h after irradiation. Fluctuations in MRI room temperature necessitate a stringent attention for controlling the gel temperature especially when calibrating gel is undergoing the image at different times. The new formulation of polymer gel ensures stability of gel spatial resolution and makes it a suitable dosimeter for distant or remote measurements.
Ethical issues
Not applicable.
Competing interests
The authors report no competing interests.
References
- Maryanski M J, Schulz RJ, Ibbott GS, Gatenby JC, Xie J, Horton D. et al. Magnetic resonance imaging of radiation dose distributions using a polymer-gel dosimeter. Phys Med Biol . 1994;39:1437–55. doi: 10.1088/0031-9155/39/9/010. [DOI] [PubMed] [Google Scholar]
- Gore JC, Kang YS, Schulz RJ. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys Med Biol . 1984;29:1189–97. doi: 10.1088/0031-9155/29/10/002. [DOI] [PubMed] [Google Scholar]
- Baldock C, Burford RP, Billingham N, Wagner GS, Patval S, Badawi RD. et al. Experimental procedure for the manufacture and calibration of polyacrylamide gel (PAG) for magnetic resonance imaging (MRI) radiation dosimetry. Phys Med Biol . 1998;43:695–702. doi: 10.1088/0031-9155/43/3/019. [DOI] [PubMed] [Google Scholar]
- De DY, De WC, Van DB, Derycke S, De NW, Achten E. Three-dimensional dosimetry using polymer gel and magnetic resonance imaging applied to the verification of conformal radiation therapy in head-and-neck cancer. Radiother Oncol . 1998;48:283–91. doi: 10.1016/s0167-8140(98)00087-5. [DOI] [PubMed] [Google Scholar]
- Farajollahi AR, Bonnett DE, Ratcliffe AJ, Aukett RJ, Mills JA. An investigation into the use of polymer gel dosimetry in low dose rate brachytherapy. Br J Radiol . 1999;72:1085–92. doi: 10.1259/bjr.72.863.10700826. [DOI] [PubMed] [Google Scholar]
- Hepworth SJ, Leach MO, Doran SJ. Dynamics of polymerization in polyacrylamide gel (PAG) dosimeters: (II) modeling oxygen diffusion. Phys Med Biol . 1999;44:1875–84. doi: 10.1088/0031-9155/44/8/302. [DOI] [PubMed] [Google Scholar]
- Papagiannis P, Pantelis E, Georgiou E, Karaiskos P, Angelopoulos A, Sakelliou L. et al. Polymer gel dosimetry for the TG-43 dosimetric characterization of a new 125I interstitial brachytherapy seed. Phys Med Biol . 2006;51:2101–11. doi: 10.1088/0031-9155/51/8/010. [DOI] [PubMed] [Google Scholar]
- Fong PM, Keil DC, Does MD, Gore JC. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys Med Biol . 2001;46:3105–13. doi: 10.1088/0031-9155/46/12/303. [DOI] [PubMed] [Google Scholar]
- Baldock C, De DY, Doran S, Ibbott G, Jirasek A, Lepage M. et al. Polymer gel dosimetry. Phys Med Biol . 2010;55:R1–63. doi: 10.1088/0031-9155/55/5/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley CA. The development of normoxic polymer gel dosimetry using high resolution MRI [PhD thesis]. [Brisbane]: Queensland University of Technology; 2006. 186 p.
- Ibbott GS. Applications of gel dosimetry. Conference Series . 2004; 3: Conference Series 2004; 3. [Google Scholar]
- MSDS . Material safety sheet acrylamaide. [1.11]. 2-1-2006. Sigma Aldrich.
- Senden RJ, De JP, McAuley KB, Schreiner LJ. Polymer gel dosimeters with reduced toxicity: A preliminary investigation of the NMR and optical dose-response using different monomers. Phys Med Biol . 2006;51:3301–14. doi: 10.1088/0031-9155/51/14/001. [DOI] [PubMed] [Google Scholar]
- Kim BM. Fundamentals of polymer gel dosimeters. Conference Series . 2006;56: Conference Series 2006;56. [Google Scholar]
- Koeva VL. Improved recipes for polymer gel dosimeters containing N-isopropylacrylamide [master’s thesis]. [Ontario]: Queen’s University; 2008. 153 p.
- Vandecasteele J, De DY. On the validity of 3D polymer gel dosimetry: III. MRI-related error sources. Phys Med Biol . 2013;58:63–85. doi: 10.1088/0031-9155/58/1/63. [DOI] [PubMed] [Google Scholar]
- Bloembergen N, Purcell EM, Pound RV. Relaxation effects in nuclear magnetic resonance absorption. Phys Rev . 1948;73:679–712. [Google Scholar]
- Maryanski MJ, Audet C, Gore JC. Effects of crosslinking and temperature on the dose response of a BANG polymer gel dosimeter. Phys Med Biol . 1997;42:303–11. doi: 10.1088/0031-9155/42/2/004. [DOI] [PubMed] [Google Scholar]
- Farajollahi AR. An investigation into the application of polymer gel dosimetry in radiotherapy. Medical Physics . 1999;26:493. [Google Scholar]
- Hsieh B, Chang Y, Han R, Wu J, Hsieh L, Chang C. A study on dose response of NIPAM-based dosimeter used in radiotherapy. J Radioanal Nucl Chem . 2011;290:141–8. [Google Scholar]
- Chang YJ, Hsieh BT, Liang JA. A systematic approach to determine optimal composition of gel used in radiation therapy. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2011;652: 783–5. [Google Scholar]
- De DY, Vergote K, Claeys C, De WC. The fundamental radiation properties of normoxic polymer gel dosimeters: A comparison between a methacrylic acid based gel and acrylamide based gels. Phys Med Biol . 2006;51:653–73. doi: 10.1088/0031-9155/51/3/012. [DOI] [PubMed] [Google Scholar]
- De DY, Hurley C, Venning A, Vergote K, Mather M, Healy BJ. et al. A basic study of some normoxic polymer gel dosimeters. Phys Med Biol . 2002;47:3441–63. doi: 10.1088/0031-9155/47/19/301. [DOI] [PubMed] [Google Scholar]
- Koeva VI, Csaszar ES, Senden RJ, McAuley KB, Schreiner LJ. Polymer Gel dosimeters with increased solubility: A preliminary investigation of the NMR and optical dose-response using different crosslinkers and co-solvents. Macromol Symp . 2008;261:157–66. [Google Scholar]