Abstract
We examined the health of a control group (18–81 years) in our aging study, which is similar to control groups used in other neuroimaging studies. The current study was motivated by our previous results showing that one third of the elder control group had moderate to severe white matter hyperintensities and/or cortical volume loss which correlated with poor performance on memory tasks. Therefore, we predicted that cardiovascular risk factors (e.g., hypertension, high cholesterol) within the control group would account for significant variance on working memory task performance. Fifty-five participants completed 4 verbal and spatial working memory tasks, neuropsychological exams, diffusion tensor imaging (DTI), and blood tests to assess vascular risk. In addition to using a repeated measures ANOVA design, a cluster analysis was applied to the vascular risk measures as a data reduction step to characterize relationships between conjoint risk factors. The cluster groupings were used to predict working memory performance. The results show that higher levels of systolic blood pressure were associated with: 1) poor spatial working memory accuracy; and 2) lower fractional anisotropy (FA) values in multiple brain regions. In contrast, higher levels of total cholesterol corresponded with increased accuracy in verbal working memory. An association between lower FA values and higher cholesterol levels were identified in different brain regions from those associated with systolic blood pressure. The conjoint risk analysis revealed that Risk Cluster Group 3 (the group with the greatest number of risk factors) displayed: 1) the poorest performance on the spatial working memory tasks; 2) the longest reaction times across both spatial and verbal memory tasks; and 3) the lowest FA values across widespread brain regions. Our results confirm that a considerable range of vascular risk factors are present in a typical control group, even in younger individuals, which have robust effects on brain anatomy and function. These results present a new challenge to neuroimaging studies both for defining a cohort from which to characterize `normative' brain circuitry and for establishing a control group to compare with other clinical populations.
Keywords: Aging, working memory, cholesterol, blood pressure, DTI, vascular risk
Introduction
In our previous study of age-related memory decline, we found that approximately one third of the radiology reports for our normal control group (64–83 years of age) indicated moderate to severe: 1) white matter hyperintensities (WMHs) consistent with chronic microvascular ischemic change and/or 2) cerebral volume loss (Aine et al., 2010). The group with white matter changes performed worse on word recognition tasks than those revealing volume loss. The WMH finding is consistent with reports indicating that 12–94% of MRIs obtained from elderly participants show white matter changes (de Leeuw et al., 2001; Debette and Markus, 2010; Schmidt et al., 1999; Wen and Sachdev, 2004); most of these reports suggest 75% or more of the MRIs reveal WMHs (Manolio et al., 1994). Prevalence of WMHs varies widely across studies depending on age, health of the group examined, severity of WMHs, as well as location and extent of WMHs. A recent study conducted on middle-aged participants (44–48 years) found a 50.9% occurrence of WMHs (Wen et al., 2009).
In general, normal aging is viewed as a process that affects both gray and white matter with an anterior-to-posterior gradient (i.e., prefrontal change occurs first) (Delano-Wood et al., 2012; Head et al., 2005). Therefore, working memory and executive control processes, supported by prefrontal regions, are among the first to decline with age [e.g., (Moscovitch and Winocur, 1995; Tisserand and Jolles, 2003; West, 1996)]. Unfortunately, results from neuroimaging studies examining age-related memory decline have been quite variable in terms of brain activation levels (both under-activation or over-activation in elderly have been reported) [e.g., (Grady and Craik, 2000)] and for task performance (worse performance in elderly or no differences have been reported) [e.g., (Aine et al., 2011; Aine et al., 2006; Daselaar et al., 2003)]. Therefore, our goal is to begin to characterize sources of variability in a control group that are usually left uncontrolled.
WMHs, associated with vascular risk factors such as hypertension and type 2 diabetes, as well as cognitive decline, also progress with an anterior-to-posterior gradient (Artero et al., 2004; Burgmans et al., 2010; DeCarli et al., 1999; Gunning-Dixon and Raz, 2000; Jeerakathil et al., 2004; Kuo and Lipsitz, 2004; Nordahl et al., 2006; Pantoni et al., 2007; Schmidt et al., 2004). For example, a meta-analysis conducted by Gunning-Dixon and Raz (2000), along with other studies (Oosterman et al., 2004; Tullberg et al., 2004), have shown that WMHs are more abundant in frontal regions and are associated with frontal hypometabolism (rCMRglc), prolonged processing times and executive dysfunction. This vascular-related cognitive decline is believed to be due to demyelination or axonal degeneration (Jacobs et al., 2013) in regions connecting frontal cortex and subcortical structures (Kuo and Lipsitz, 2004). Therefore, it was postulated that vascular risk factors underlie at least some of the frontal lobe deficits seen in aging (Aine et al., 2010; Aine et al., 2011). A similar conclusion was reached by Kennedy and Raz (2009) who suggested that: 1) elevated arterial pulse pressure is linked to deterioration of white matter tract integrity in frontal regions and 2) vascular risk may drive the expansion of white matter damage from anterior to posterior regions. In sum, vascular risk factors appear to accelerate age-related decline in brain perfusion which affects cognitive outcomes (Novak and Hajjar, 2010) and the comorbid presence of two or more vascular risk factors can increase the probability of cognitive decline (de la Torre, 2012; Luchsinger et al., 2005).
Here we examine a control group (18–81 years of age) using spatial and verbal working memory tasks, along with measures of vascular risk (e.g., lipid panel, blood pressure measurements), neuropsychological, and neuroimaging measures to characterize their health. This group will be used by us later for comparisons with specific clinical populations. We predicted that health profiles indicative of metabolic syndrome (e.g., hypertension, hyperlipidemia and hyperglycemia) will explain more of the variance on working memory performance than age alone since these vascular risk factors have been independently associated with cognitive decline (e.g., poor executive control and working memory performance) (Awad et al., 2004; Elias et al., 2004; Helzner et al., 2009; Qiu et al., 2005).
Hypertension, Visuospatial Skills and Spatial Memory
Although considerable data is available concerning the negative effects of hypertension on cognition [see review by (Birns and Kalra, 2009)], very few studies have examined whether hypertension selectively affects spatial working memory or if it produces global memory impairment. Elias and colleagues (2004) found that hypertension affected visualization and fluid abilities in particular, compared to verbal skills. This longitudinal study showed that higher levels of systolic or diastolic blood pressure in both young (18–46 years) and older participants (47–83 years) were associated with a significant decline in performance on block design, object assembly, picture completion and arrangement tasks. Similarly, Waldstein and colleagues (2005) found that elders with high systolic blood pressure performed more poorly on the Benton Visual Retention Test. Jennings and colleagues (2006) showed, using positron emission tomography, that hypertensive patients revealed the poorest performance on spatial, compared to verbal memory tasks when using a Sternberg-type task. The above-mentioned studies, in addition to our preliminary data (Aine et al., 2011), led us to hypothesize that hypertension will have a selective negative effect on spatial working memory tasks.
Cholesterol, Verbal Skills and Verbal Memory
In contrast to the negative association seen between hypertension and visuospatial skills or spatial memory, we predict a positive association between verbal working memory performance and cholesterol levels (total cholesterol or TC and low-density lipoprotein or LDL) in our control group. There are very few studies examining this particular relationship. One large study from the Framingham Heart Study found a positive linear association between TC measures and measures of verbal fluency, attention/concentration, and abstract reasoning (Elias et al., 2005). Another study that examined cholesterol levels and cognition in schizophrenia found that higher TC levels were associated with better verbal memory performance across medication groups (Krakowski and Czobor, 2011). Finally, in a population-based cohort of middle-age women, better immediate recall of a word list was positively associated with higher TC and LDL measurements acquired three years earlier (Henderson et al., 2003).
The physiological connection between verbal memory and cholesterol levels is less straightforward than for hypertension and spatial memory. However, brain cholesterol is the main constituent of white matter tracts (Mathew et al., 2011; Uranga and Keller, 2010); 70% of total brain cholesterol is found in the myelin membranes of white matter. Although the exact relationship between plasma cholesterol and brain cholesterol is unknown at this time, considerable indirect evidence suggests that such a relationship exists. It has been shown, for example, that the concentration of sterol circulating in lipoproteins varies markedly during periods of development when brain size, degree of myelination, and brain cholesterol content are also rapidly changing (Dietschy and Turley, 2004). Numerous linkages between white matter structure and verbal function have also been made in developmental studies examining white matter maturation and language development (Fuster, 2003; Nagy et al., 2004; Paus et al., 1999; Peters et al., 2012; Tamnes et al., 2010).
In sum, the overall goal is to demonstrate that vascular risk factors (e.g., high blood pressure, high blood glucose levels), often inadequately documented in aging studies and neuroimaging studies using a control group, contribute to `age-related' decline. These factors must be separated out in order to characterize true age-related effects. Our predictions suggest that vascular pathology in particular contributes most to age-related cognitive decline, which we refer to as `normal aging.' In contrast, a smaller proportion of the elderly population suffers less from vascular-related pathology and correspondingly shows fewer signs of cognitive decline; this group demonstrates `healthy successful aging' (Aine et al., 2011).
To examine effects of vascular risk factors on memory and cognition a cluster analysis was used across age to parse out the vascular risk factors associated with poorer performance, while adjusting for age. The cluster analysis was used as a data reduction technique that permits an evaluation of the joint effects of multiple vascular risk factors. The Framingham Risk Score has also been used to examine conjoint effects of risk factors, which may have subclinical effects if examined separately (Joosten et al., 2013). Although, a cluster analysis approach is in sharp contrast with traditional methods of examining differences between age groups, it does provide a novel view of age- and vascular-related effects on task performance. And finally, we manipulate verbal and spatial memory using stimuli that are identical, similar to a study by Jennings and colleagues (2006), and predict an inverse relationship between hypertension and spatial memory performance and a positive relationship between total cholesterol and verbal memory performance. Although our focus is on normal aging, these results are relevant to all studies using a control group for comparison to disease states or for studies examining normal brain function and structure.
Materials and methods
Participants and Inclusion/Exclusion Criteria
Fifty-five participants (18–81 years) were recruited into three age groups (18–25, 35–45, and ≥ 65 years). Twenty-seven of these participants were male; three young participants and one elder participant were left-handed. Gender representation within each age range was balanced (47% males in the young group; 50% males in the middle age group; and 61% males in the elder group). All participants completed 4 modified Sternberg (working memory) tasks while 53 completed neuropsychological tests to determine general levels of cognitive functioning. The neuropsychological tests administered included the Wechsler Adult Intelligence Scale-IV (WAIS IV), Mini-mental Status Exam (MMSE), California Verbal Learning Test (CVLT), and Rey-Osterreith Complex Figure Test (REY). All participants also underwent a quantitative neurological examination.
Exclusion criteria included history of coronary heart disease, peripheral vascular disease, angina pectoris or cerebrovascular disease. In addition, potential participants were excluded if they showed evidence of: 1) significant chronic neurological disease (e.g., Parkinson's disease, seizure disorder); 2) neuropsychiatric illness (e.g., schizophrenia); 3) severe medical illness (e.g., chronic obstructive pulmonary disease, severe congestive heart failure, liver disease); 4) major depression or any other major DSM-IV axis I disorder, including alcohol and substance abuse within one year of recruitment; and 5) mild cognitive impairment or probable Alzheimer's disease.
The goal was to include elders that were for the most part healthy; therefore, individuals with hypertension or type 2 diabetes were not excluded since they have often been included in studies of aging that rely on self-report. Our approach was to document and quantify the vascular risk of each participant. All measures related to vascular health were obtained by a nurse at the University of New Mexico Clinical and Translational Science Center (CTSC). Since all participants fasted for 12 hours, blood tests and blood pressure measurements were acquired between the hours of 8–10 AM. Only one young male participant elected to have it done in the afternoon. Two seated blood pressure readings (10 minutes apart) were averaged. After blood pressure measurements were obtained, glycated hemoglobin A1C (average blood glucose concentration) and a lipid panel (triglycerides, high density lipoprotein or HDL, LDL, and TC) were collected. See Participant characteristics in Table 1.
Table 1.
Participant characteristics.
| Mean (STD) | Range | N | |
|---|---|---|---|
| Age | 36.9 (20.7) | 18–81 | 55 |
| Edu | 15.2 (1.9) | 12–20 | 55 |
| Sys BP | 120.3 (16.9) | 86–162 | 52 |
| Dia BP | 72.0 (9.6) | 53–95 | 52 |
| A1C | 5.6 (0.4) | 4.7–6.8 | 53 |
| TG | 114.9 (55.3) | 43–288 | 53 |
| TC | 169.8 (33.0) | 108–266 | 53 |
| HDL | 49.2 (16.0) | 16–90 | 53 |
| LDL | 97.6 (28.3) | 47–185 | 53 |
| IQ | 107.4 (12.5) | 71–136 | 53 |
| MMSE | 28.0 (2.2) | 23–30 | 53 |
| REY_I | 21.9 (5.8) | 10–33 | 53 |
| REY_D | 20.5 (7.2) | 0–34 | 53 |
| CVLT5 | 12.1 (2.7) | 6–16 | 53 |
Edu=mean number of years of education completed; Sys BP=Systolic blood pressure; Dia BP=Diastolic blood pressure; A1C=Fasting glycated hemoglobin; TG=Triglycerides; TC=Total cholesterol; HDL=High-density lipoprotein; LDL=Low-density lipoprotein; IQ=Full scale score on WAIS IV; MMSE=Mini-mental status exam; REY_I=Immediate recall on the REY-Osterrieth Complex Figure Test; REY_D=Delayed recall on the REY-Osterrieth Complex Figure Test; CVLT5=Number of words correct on Trial 5 of California Verbal Learning Test.
In the resultant group of participants, two in the middle-age range (35–45 years) reported that they were previously diagnosed as being hypertensive while seven elderly reported being hypertensive. Of the seven hypertensive elderly, two also had been diagnosed with type 2 diabetes. The medicine lists of all participants were reviewed prior to study to help assure that we did not enroll any participants taking psychoactive or other drugs that could affect their cognitive functions (e.g., opioid and seizure medications).
MRI/Diffusion Tensor Imaging (DTI) Data Acquisition and Analysis
The Siemens 3T Tim Trio was used for all T1 structural and T2 scans (MPRAGE, Turbo Spin Echo, FLAIR, and DTI). T1-weighted MPRAGE sequence: 1.0 mm sagittal slices, 7° Flip angle, TR=2530 ms, TE1=1.64 ms, TE2=3.5 ms, TE3=5.36 ms, TE4=7.22 ms, TE5=9.08 ms, FOV was 256 × 256, 6 minutes. T2-weighted Turbo Spin Echo (TSE) sequence: 1.5 mm axial slices, 155° Flip angle, TR=13500 ms, TE1=77 ms, FOV was 220 × 220 with 1.5 mm slice thickness, acquisition time of ~3 minutes. T2-weighted FLAIR sequence: 1.5 mm sagittal slices, TR=6000 ms, TE=412 ms, FOV was 256 × 256, acquisition time of ~5 minutes.
Total number of WMHs and their total volumes were determined from FLAIR MR images using semi-automated JIM software (Xinapse Systems: http://www.xinapse.com/home.php). WMHs were identified based on the intensity contrast of the lesion edges and the surrounding tissue. Lesions were manually outlined in each FLAIR axial slice, by a single investigator, unaware of participants' clinical data, to provide total hemispheric volumes (See Figure 1). These measures (numbers of lesions and total volumes) were considered as independent measures of vascular risk, along with the lipid panel and blood pressure results (described below). To determine the reliability of the manual volume measures, we randomly selected MRIs from 10 participants (determined using a random number generator) and asked a different investigator, also unaware of clinical details, to analyze the MRI data from these participants using Jim software. For inter-rater reliability, there was no bias (difference in median) in number of WMHs or total volumes between the two raters (Wilcoxon Signed Rank, both p > 0.46). The intraclass correlations (ICC) between raters [considered as random, ICC (2,1) in Shrout and Fleiss (1979)] were 0.81 for the numbers of WMHs and 0.97 for total volume (for log transformed total areas, ICC=.71).
Fig. 1.
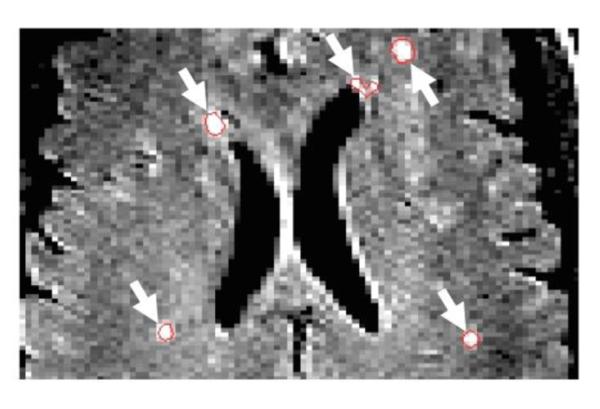
WMHs Identified on FLAIR Axial Slices. Numbers and volumes of WMHs were calculated using JIM software. ROIs (see white arrows) highlight WMHs on an axial FLAIR MR slice.
The DTI sequence had 60 directions, b=800 s/mm2 and 10 measurements of b=0, for 12 minutes of acquisition time. The b=0 measurements were interleaved after every six non-zero b-value measurements. DTI was obtained in the axial direction along the AC-PC line. The FOV was 256 × 256 mm with a 2 mm slice thickness, 72 slices, 128 × 128 matrix size, voxel size = 2 mm × 2 mm × 2 mm, TE=84ms, TR=9000ms, NEX=1, partial Fourier encoding of 3/4, and with a GRAPPA acceleration factor of 2.
Tract-based spatial statistics (TBSS) was used for the analysis (Smith et al., 2006). The analysis was based on the FSL software package. DTI preprocessing consisted of the following steps: 1) gradient directions with more than 10% signal dropouts caused by subject motion were not included in further analysis; 2) motion and eddy current correction (FSL); and 3) correction of gradient directions for any image rotation done during the previous motion correction step. Scalar diffusion parameters such as fractional anisotropy (FA), radial diffusivity (RD) and axial diffusivity (AD) were calculated using dtifit (FSL). The FA image was aligned to a FA template (normalized to the Montreal Neurological Institute brain atlas space) with a nonlinear registration algorithm, FNIRT (FMRIB's Nonlinear Image Registration Tool; FSL). A mean FA image was calculated from the set of spatially normalized images. The TBSS algorithm was applied to the mean FA image to calculate a mean white matter tract skeleton. The FA data of each subject was then projected onto this mean skeleton to obtain a skeletonized image corresponding to each subject.
To examine pathology related effects, mean FA values were calculated from the FA skeleton for the 50 white matter regions defined in the JHU-ICBM 50 region white-matter atlas included in the FSL software package (Mori et al., 2008). The physiological variables were then correlated with the mean FA for each of the 50 regions and the significance was corrected for multiple comparisons (family-wise error rate or FWER).
Spatial/Verbal Working Memory Tasks
Four modified Sternberg tasks (2 spatial and 2 verbal) were used to test working memory performance (Figure 2). Either enhancers were presented in the delay interval of verbal and spatial tasks (VE and SE) or distracters were presented in the delay interval of verbal and spatial tasks (VD and SD). Enhancers contained items that were the same as the to-be-remembered digits or locations of digits while distracters contained items that were different from the to-be-remembered items. The verbal and spatial tasks were blocked and counterbalanced across sessions. Stimuli subtended 4° visual angle in the central visual field. All conditions had two memory set items. Participants were to respond “yes” (right index finger) or “no” (left index finger) if the last display (probe) contained one of the to-be-remembered items (either the red digits themselves or the locations of the red digits). Some of the digits within the matrices were presented backwards to avoid the use of 2-digit numerals. However, the digits to be remembered were always presented normally. The larger grid size was used (16-cell arrays) to discourage participants from using a verbal strategy for remembering locations (e.g., “upper left corner,” “2 o'clock”). Total number of trials for each condition was 120 (e.g., 120 trials of “yes” it matched and 120 trials of “no” it didn't match). Only the “yes” trials were used for the present study. The spatial and verbal tasks took approximately 35 minutes each to complete, which included 3 short rest-breaks.
Fig. 2.
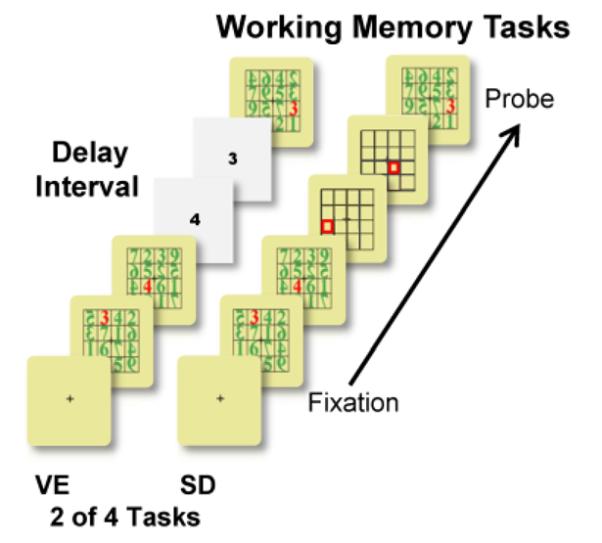
Verbal and Spatial Working Memory Tasks. Four modified Sternberg tasks (2 spatial and 2 verbal) were used to test memory performance across age. Verbal and spatial tasks contained either: 1) enhancers in the delay interval (VE=verbal and SE=spatial tasks); or 2) distracters in the delay interval (VD=verbal and SD=spatial tasks). Two of the four are shown here (VE and SD). Displays appeared sequentially every 1.2 s with a 2 s inter-stimulus interval. Stimulus duration was 266 ms.
Statistics for Behavioral Tasks and Vascular Risk Measures
Age-correction was considered as a pre-processing step. Since age had a statistically significant effect on Total Correct and Reaction Time (RT) (our primary outcome measures), age was adjusted by using a regression model in order to add this residual to the predicted value at age=40. Age 40 is the midpoint of the age range of the middle-age recruitment group; the sample mean was 37 ± 21 (SD). The net result of this operation is the following formula. For y = Total Correct or RT,
Corrected y = observed y + m (40-age), where m is the regression slope.
To examine the interrelationship of risk factors on verbal and spatial task outcomes, an overall MANOVA with task (verbal enhancer, verbal distracter, spatial enhancer, and spatial distracter) as a repeated factor and with systolic blood pressure (BP) and TC (to address our specific hypotheses) as well as other independent risk measures (see list below) as covariates, was conducted. If the overall MANOVA showed significant interactions of task × risk factors on outcome, RM ANOVA analyses were conducted separately for the significant risk factors.
Then we combined the vascular risk factors into a smaller set of “risk clusters” as a data reduction step in order to examine the combined effects of risk factors (e.g., metabolic syndrome), on task performance, where individuals may show elevations for more than one risk factor. Risk cluster groups were formed using average linkage, cluster analysis with Euclidean distances (SAS 9.3). Then a RM ANOVA was conducted with the 4 tasks as a repeated factor and Risk Clusters as a grouping factor. To determine which independent risk factors of the Risk Cluster might explain the effect on task outcomes, the independent risk measures (systolic blood pressure, diastolic blood pressure, A1C, triglycerides, TC, HDL, LDL, number of WMHs, and total volume of WMHs) were added to the model above. This will show whether an individual risk factor is: 1) not important; 2) mediated by the Risk Cluster factor; or 3) an independent “predictor” of task outcomes. All statistics were conducted by a biostatistician using SAS (http://www.sas.com, Cary, NC).
Results
Vascular Risk Factors and Memory Performance
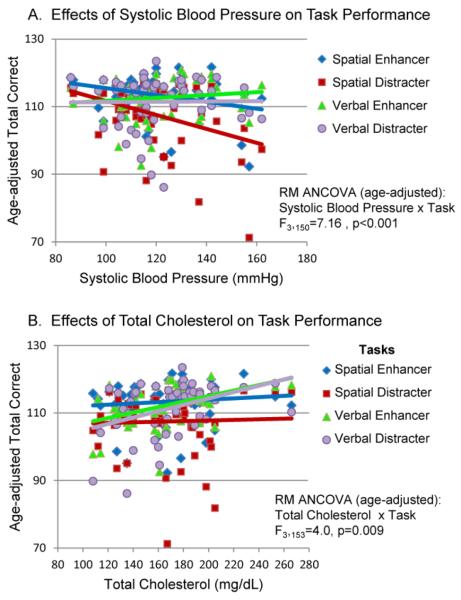
MANOVA showed a significant overall effect of systolic BP (F4,46=2.77, p=.04) and TC (F4,46=3.15, p=.02) with Task × Systolic BP and Task × TC interactions. Per plan, we provided separate analyses for systolic BP and TC since it was predicted that Systolic BP would have a negative effect on spatial working memory performance and that TC would have a positive effect on verbal working memory performance. Figure 3A shows results for age-adjusted task performance [RM ANCOVA with age-adjusted: Systolic BP × Task Interaction, F3,150 =7.16, p<.001]; Systolic BP was associated with poorer accuracy on the spatial working memory tasks, particularly for the spatial distracter task (SD). In contrast, a positive relationship was found between TC and accuracy on verbal working memory tasks [RM ANCOVA with age-adjusted: TC × Task Interaction, F3,153 =4.0, p=0.009]. Accuracy relative to TC results is plotted in Figure 3B where task performance was age-adjusted. RT measures were not significantly different.
Fig. 3.
(A) Age-adjusted Task Performance and Systolic Blood Pressure. When task performance was age-adjusted across 4 working memory tasks an interaction between systolic blood pressure and task was found. Red (SD) and blue (SE) lines reflect significant negative linear relationships between systolic blood pressure and accuracy on the spatial tasks. (B) Age-adjusted verbal working memory performance measures and total cholesterol. Violet (VD) and green (VE) lines reflect significant positive linear relationships between total cholesterol and accuracy (Total correct) on the verbal memory tasks.
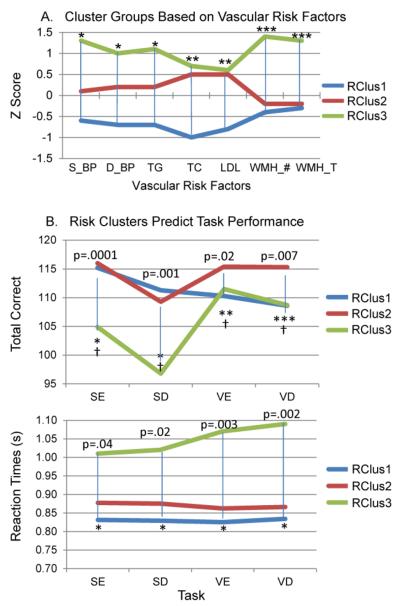
In order to examine combined effects of the vascular risk factors on performance, independent of age, risk cluster groups were formed based on the risk factors (Figure 4A). This seemed especially important since some individuals who did not report a history of hypertension, were “hypertensive” when examining their health status (e.g., measured blood pressure levels), while others had previously unknown high blood glucose levels (A1C >6.0). Intuitive predictions based on Fig. 4A would suggest that: 1) Risk Cluster group 3 (RClus3: green lines) should perform worse on the spatial tasks given the presence of WMHs on their MRIs; and 2) Risk Cluster group 1 (RClus1: blue lines) may perform best overall since they have lower levels of blood pressure, triglycerides, cholesterol, and fewer WMHs. In fact, all members of this cluster group had TC values in the “desirable” range, according to the National Cholesterol Education Program (NCEP), a group who sets guidelines for cholesterol levels (Grundy et al., 2004). However, as Figure 4B shows, while RClus3 did perform more poorly (less accurate on the spatial tasks and longer RTs across all tasks) than the other groups, Risk Cluster group 2 (RClus2: red lines), which shows higher values on TC and LDL measures, had higher accuracy scores than RClus1 for the verbal enhancer/distracter conditions (VE and VD). These effects remained significant when performance was age-adjusted (†). RClus1 and RClus2 did not differ on RT measures. While RClus2 and RClus3 did not differ on TC and LDL levels (although there were more subjects in the NCEP “high” range of TC in RClus3), RClus3 had statistically greater number of WMHs and greater WMH volumes, in addition to higher systolic/diastolic blood pressure and triglycerides. These results help strengthen the positive relationship shown earlier between TC levels and verbal working memory performance (Figure 3B).
Fig. 4.
Cluster Groups based on Vascular Risk Factors Predict Task Performance. (A) All 3 cluster groups differed significantly in terms of blood pressure and triglyceride measures (*). RClus1 (blue lines) differed from RClus2 (red lines) & RClus3 (green lines) on TC and LDL measures (**). RClus3 differed from RClus1 & RClus2 in terms of total number of WMHs and total volume of WMHs (***). A1C and HDL measures were not included since they did not help to differentiate between the cluster groups. (B). RClus3, showing higher values on the risk factors, performed more poorly on spatial tasks (fewer Total correct and longer RTs) and had longer RTs on the verbal tasks. RClus2 performed better on verbal enhancer and distracter tasks (Total correct). For Total Correct: (*)=RClus3 < RClus1 & RClus2; (**)=RClus2 > RClus1; (***)=RClus2 > RClus1 & RClus3. For RT: (*)=RClus3 > RClus 2 & RClus1. Effects were still significant after age-correction: (†)=Age-adjusted differences in task performance (Total correct) at p<.05.
Our Risk Cluster groups were validated in part by showing that individual risk factors are differentiated among the groups (see Fig. 4A). In addition to their “internal validity,” a similar comparison of Total Correct and RT, factors that were not used in cluster formation, between groups establishes “external validity.” Furthermore, we tested whether each pair of our 3 risk clusters obtained by cluster analysis are separated using a Monte Carlo generated null distribution of all squared distances based on the sample size and variance-covariance structure of the cluster groups being compared: Risk Clusters 1 and 3, and 2 and 3 were separated (p<.001); Risk Clusters 1 and 2 were not distinctly separated but formed a continuum where the cluster analysis-derived cutscore defined groups based on high vs. low values along the first principal component.
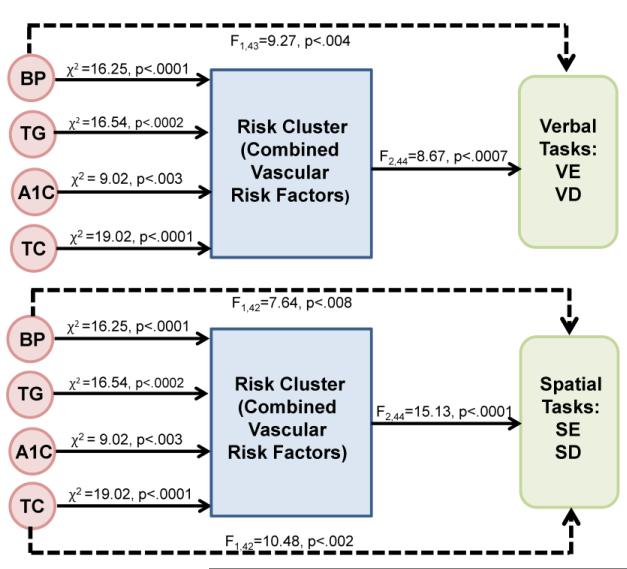
The important goal of establishing the effect of combined risk factors (Risk Cluster) on verbal and spatial memory performance, was accomplished by ANOVA and is represented in the right side of Figure 5 by solid lines (F2,44=8.67, p<.0007 for verbal and F2,44=15.13, p<.0001 for spatial). Since our combined Risk Cluster was defined independently of verbal and spatial performance, the single risk factors were examined for their residual effects on verbal and spatial performance after adjusting for Risk Cluster. In the first phase of screening for candidate risk factors for the verbal tasks, two risk factors (see top portion of Table 2) were found that accounted for most of the variance on verbal task accuracy (systolic blood pressure and TC). However, age was not significant after adjusting for the risk factors in the model. Similarly, when screening for candidate risk factors for the spatial tasks, triglycerides (TG), A1C and TC affected spatial accuracy. Again, age was not significant. Thus, these 4 risk factors were considered in the subsequent analyses for Figure 5. We then verified that each risk factor (e.g., systolic blood pressure, triglycerides) predicts Risk Cluster variables using a logistic regression model. As expected, the risk factors directly to the left of the Risk Cluster box in Figure 5 are verified to be related to the Risk Cluster (all with p ≤ 0.003). In phase 2, the resultant four risk factors (systolic blood pressure, triglycerides, A1C, and TC) were then tested independently for their effect on the verbal and spatial memory outcomes adjusting for Risk Cluster. When Risk Cluster was modeled between the single risk factors and task accuracy, only systolic blood pressure was significant for the verbal tasks while systolic blood pressure and TC were significant for the spatial tasks (see dashed lines in Fig. 5).
Fig. 5.
Path Analysis. Effects of Risk Cluster groups on verbal and spatial task accuracy along with independent and simultaneous effects of risk factors on task accuracy. These 4 risk factors (systolic blood pressure or BP, triglycerides or TG, total cholesterol or TC, and A1C) were entered into a path analysis based on Table 2 results. Solid lines at right of figure represent how well the Risk Cluster groups (combined risk factors for groups 1–3) predict performance on the verbal and spatial working memory tasks. Dashed lines represent how well the 4 risk factors independently predict performance on the verbal and spatial working memory tasks when Risk Cluster was in the model. Solid lines to the left of the Risk Cluster reflect results from a logistic regression (cumulative logit model) representing the 3 Risk Clusters.
Table 2.
Top rows. Phase 1 initial screening for effects of vascular risk factors on working memory performance. Wihout Risk Cluster in the model, risk factors were evaluated using multivariate analysis to see which ones accounted for the most variance on verbal task accuracy (left column) and spatial task accuracy (right column). Four significant risk factors (see **) were evaluated further in a path analysis presented in Figure 5. Bottom rows. Phase 3 univariate analyses were calculated using the 4 resultant risk factors to determine the direct effects of each risk factor on verbal performance (left column) and spatial performance (right column). TC showed a direct effect on verbal memory performance while Sys BP, TG, and A1C each show direct effects on spatial memory performance (see **). Sys BP=Systolic blood pressure, Dia BP=Diastolic blood pressure, TG=Triglycerides, A1C=Average blood glucose across 3 months, TC=Total cholesterol.
| Phase 1 Screening of Risk Factors--Multivariate | |
| Risk Factors--Verbal Task | Risk Factors--Spatial Task |
| Age F1,38=0.73, p=0.4 | Age F1,38=0.05, p=0.8 |
| Sys BP F1,38=5.50, p<0.02** | Sys BP F1,38=3.63, p=0.1 |
| Dia BP F1,38=0.06, p=0.8 | Dia BP F1,38=0.43, p=0.5 |
| TG F1,38=3.61, p=0.06 | TG F1,38=4.56, p<0.04** |
| A1C F1,38=2.30, p=0.1 | A1C F1,38=5.39, p<0.03** |
| TC F1,38= 8.25, p<0.007** | TC F1,38=5.73, p<0.02** |
| WMH_# F1,38=1.53, p=0.2 | WMH_# F1,38=0.21, p=0.6 |
| WMH_T F1,38=1.52, p=0.2 | WMH_T F1,38=0.17, p=0.7 |
| Phase 3 Direct Effect of Risk Factors on Performance--Univariate | |
| Risk Factors--Verbal Task | Risk Factors--Spatial Task |
| Sys BP F1,48=2.71, p=0.1 | Sys BP F1,48=30.61, p<0.0001** |
| TG F1,48=2.55, p=0.1 | TG F1,48=15.23, p<0.0003** |
| A1C F1,48=1.43, p=0.2 | A1C F1,48=11.12, p<0.002** |
| TC F1,48= 10.47, p<0.002** | TC F1,48=0.02, p=0.9 |
In sum, Risk Cluster predicts task accuracy on the verbal tasks, but systolic blood pressure has an independent and simultaneous effect on the verbal tasks; i.e., Risk Cluster did not capture all the variance associated with systolic blood pressure on verbal task accuracy. Systolic blood pressure and TC also have a strong independent effect on spatial task accuracy. It is instructive to do the analysis without Risk Cluster in the model (phase 3), in order to assess direct effects of single risk factors on verbal and spatial accuracy (see bottom portion of Table 2). Only TC had a significant effect on verbal task accuracy. Systolic blood pressure, triglycerides and A1C had a direct and independent effect on spatial task accuracy. Greater TC levels were associated with better verbal memory accuracy while higher systolic blood pressure, blood glucose levels, and triglycerides were associated with poorer accuracy on the spatial memory tasks. Interestingly, when age was entered into these models (univariate analysis), age was significant for the spatial task only (F1,48=17.75, p<.0001). This result suggests that systolic blood pressure, triglycerides and A1C explain the effect of age (i.e., carries the same information as age) for the spatial task only.
Diffusion Tensor Imaging (DTI), Risk Factors and Memory Performance
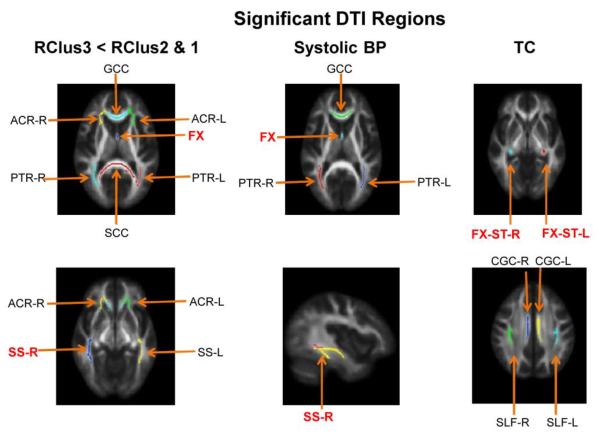
We examined FA values for RClus3, relative to RClus2 and RClus1, and for the 3 risk factors that were shown to be significant, independent of Risk Cluster, in the path analysis shown in Figure 5 (i.e., systolic blood pressure, A1C and TC). The left column of Figure 6 shows regions where FA differed between RClus3 and RClus1/RClus2 (i.e., RClus3 < RClus2 and RClus1), with age correction. These regions survived the FWER multiple comparison tests (48 subjects, 50 FA regions, 3 cluster groups). The resulting FWER F-tests and post-hoc analyses for RClus3<RClus2 & 1 are shown in the two left columns of Table 3. The 2nd column of Figure 6, along with corresponding columns in Table 3, shows regions where lower FA values correlated with higher systolic blood pressure (ANCOVA), with age correction. The next set of columns in Table 3 show results for blood glucose (A1C). Since these regions overlap with systolic blood pressure and many of the regions showing group differences (RClus3<RClus2 &1), they are not shown in Figure 6. The 3rd column of Figure 6 shows white matter tracts where FA was negatively correlated with TC, while Table 3 shows the FWER F-tests along with the ANCOVA results for TC and FA, with age correction. Finally, for comparison, regions showing lower FA values associated with age, without corrections for covariates, are shown in the last two columns of Table 3. In general, white matter regions where FA was negatively associated with systolic blood pressure and A1C are the same, which are a subset of the regions shown for RClus3<RClus2 & 1. Some additional regions are identified for age without correction for covariates. In contrast, white matter regions where FA is negatively associated with TC reveal a different pattern of regions than for RClus3<RClus2 & 1, systolic blood pressure and A1C.
Fig. 6.
DTI results. (A) Left column. Regions showing lower FA values (after FWER multiple comparison tests) for the RClus3 group (relative to RClus2 & 1). Middle column. Regions showing a significant negative correlation between systolic blood pressure and FA, with age correction. Right column. Regions showing a negative correlation between FA and TC, with age correction.
Table 3.
Regions showing lower FA values associated with select risk factors. First 2 columns of results show regions revealing lower FA values between the Risk Cluster groups (FWER results along with post-hoc analyses are shown). For the fornix (FX), for example, RClus3 showed less FA than both RClus1 and RClus2. FWER results and correlations (ANCOVA) between FA values and the other risk factors are shown in the remaining columns. Age was adjusted for Risk Cluster groups, systolic blood pressure, A1C, and TC. In the last two columns, age covariates were not controlled, for comparison.
| FWER Multiple Comparison Tests, ANCOVAs, Post-Hocs for FA Regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Cluster Groups | Systolic BP | A1C | TC | Age | |||||
| FWER | Post-Hoc | FWER | Corr | FWER | Corr | FWER | Corr | FWER | Corr | |
| FX | F=15.01 p<.0001 |
3<2 & 1 | F=20.01 p<.0001 |
r=−0.56 p<.0001 |
F=21.56 p<.0001 |
r=−0.57 p<.0001 |
F=67.98 p<.0001 |
r=−0.78 p<.0001 |
||
| GCC | F=10.74 p<.0002 |
3<2 & 1 | F=12.63 p<.0009 |
r=−0.47 p<.0009 |
F=21.78 p<.0001 |
r=−0.58 p<.0001 |
F=44.36 p<.0001 |
r=−0.71 p<.0001 |
||
| PTR-L | F=10.93 p<.0001 |
3<1 | F=16.96 p<.0002 |
r=−0.53 p<.0002 |
F=12.81 p<.0009 |
r=−0.48 p<.0009 |
F=43.20 p<.0001 |
r=−0.70 p<.0001 |
||
| PTR-R | F=11.53 p<.0001 |
3<1 | F=14.43 p<.0004 |
r=−0.50 p<.0004 |
F=14.16 p<.0005 |
r=−0.49 p<.0005 |
F=68.74 p<.0001 |
r=−0.78 p<.0001 |
||
| SS-R | F=8.49 p<.0008 |
3<1 | F=12.85 p<.0008 |
r=−0.48 p<.0008 |
F=18.20 p<.0001 |
r=−0.54 p<.0001 |
F=31.13 p<.0001 |
r=−0.64 p<.0001 |
||
| SS-L | F=7.91 p<.0001 |
3<2 & 1 | F=16.35 p<.0002 |
r=−0.52 p<.0002 |
F=18.53 p<.0001 |
r=−0.54 p<.0001 |
||||
| CGC-L | F=8.14 p<.007 |
r=−0.40 p<.007 |
||||||||
| CGC-R | F=8.36 p<.006 |
r=−0.40 p<.006 |
||||||||
| SLF-L | F=7.69 p<.008 |
r=−0.39 p<.008 |
||||||||
| SLF-R | F=7.72 p<.008 |
r=−0.39 p<.008 |
||||||||
| FX-ST-L | F=12.78 p<.0009 |
r=−0.47 p<.0009 |
||||||||
| FX-ST-R | F=10.42 p<.002 |
r=−0.44 p<.002 |
F=25.83 p<.0001 |
r=−0.61 p<.0001 |
||||||
| ACR-L | F=9.43 p<.0004 |
3<1 | F=33.69 p<.0001 |
r=−0.66 p<.0001 |
||||||
| ACR-R | F=10.48 p<.0002 |
3<1 | F=31.07 p<.0001 |
r=−0.64 p<.0001 |
||||||
| BCC | F=24.78 p<.0001 |
r=−0.60 p<.0001 |
||||||||
| SCR-R | F=20.71 p<.0001 |
r=−0.57 p<.0001 |
||||||||
| SCR-L | F=18.91 p<.0001 |
r=−0.55 p<.0001 |
||||||||
| SCC | F=8.5 p<.0008 |
3<1 | ||||||||
GCC=genu of the corpus callosum; PTR=posterior thalamic radiation (right and left); SS=sagittal stratum (right and left); CGC=cingulum/cingulate gyrus (right and left); SLF= superior longitudinal fasciculus (right and left); FX-ST=crus of the fornix and stria terminalis (right and left); ACR= anterior corona radiata (right and left); BCC=body of the corpus callosum; SCR=superior corona radiata (right and left); SCC= splenium of the corpus callosum.
Table 4 shows correlations between the FA measures and verbal and spatial performance. Most of the FA values showed significant positive correlations with performance on the delayed recall of the REY Complex Figure test (REY-D) and accuracy on the spatial distracter task (SD-C). Unlike the other regions, the fornix (FX) correlated with RTs (negative correlations) for both spatial and verbal working memory tasks, as well as showing positive correlations with accuracy on the REY Figure (spatial memory), CVLT (verbal memory), and the spatial distracter task (SD-C). The finding of a positive correlation between the CVLT and FA of the crus of the fornix and stria terminalis-right hemisphere (FX-ST-R), along with a positive correlation between TC and accuracy on verbal tasks (VE-C and VD-C), and a negative correlation between TC and FA (regions noted in Table 3) is not contradictory but deserves further study in the future.
Table 4.
Correlations between FA measures and cognitive performance are shown. All of these regions showed significant negative FA correlations for RClus3 (relative to RClus2 & 1), systolic blood pressure, or TC (Fig. 6). FX was correlated with most memory-related task measures (accuracy and RTs). In contrast, FX was not correlated with full scale IQ (FWAIS). Delayed recall on the REY Complex Figure Test (REY-D) was correlated with most of the regions identified. Working memory performance on the Spatial Distracter task (SD-C) was also correlated with most of the FA regions noted in this table. Overall, more FA regions were positively correlated with spatial task accuracy than verbal tasks.
| Spatial Tasks | FX | GCC | ACR-R | ACR-L | SS-R | SSL | SCC | PTR-R | PTR-L | FX-ST-R | FX-ST-L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| REY-D | r=.53** p=.0001 |
r=.30* p=.04 |
r=.34* p=.02 |
r=.40** p=.005 |
r=.40** p=.005 |
r=.31* p=.03 |
r=.42** p=.003 |
r=.48** p=.0005 |
r=.49** p=.0004 |
r=.30* p=.04 |
r=.23 p=.12 |
| SE-C | r=.26 p=.07 |
r=.26 p=.07 |
r=.18 p=.22 |
r=.22 p=.14 |
r=.26 p=.07 |
r=.18 p=.21 |
r=.21 p=.16 |
r=.15 p=.29 |
r=.14 p=.35 |
r=.14 p=.33 |
r=.09 p=.53 |
| SE-RT | r=−.37** p=.01 |
r=−.19 p=.20 |
r=−.16 p=.27 |
r=−.21 p=.15 |
r=−.18 p=.23 |
r=−.06 p=.69 |
r=−.25 p=.09 |
r=−.22 p=.14 |
r=−.25 p=.09 |
r=−.17 p=.26 |
r=−.20 p=.18 |
| SD-C | r=.37** p=.009 |
r=.32* p=.03 |
r=.32* p=.03 |
r=.35** p=.01 |
r=.40** p=.005 |
r=.28* p=.05 |
r=.35** p=.01 |
r=.36** p=.01 |
r=.32* p=.03 |
r=.27 p=.06 |
r=.23 p=.12 |
| SD-RT | r=−.40** p=.005 |
r=−.21 p=.13 |
r=−.20 p=.17 |
r=−.23 p=.11 |
r=−.19 p=.19 |
r=−.07 p=.66 |
r=−.28 p=.06 |
r=−.24 p=.10 |
r=−.25 p=.09 |
r=−.18 p=.21 |
r=−.22 p=.13 |
| Verbal Tasks | |||||||||||
| CVLT | r=.42** p=.003 |
r=.22 p=.13 |
r=.21 p=.15 |
r=.19 p=.19 |
r=.27 p=.07 |
r=.13 p=.39 |
r=.26 p=.07 |
r=.34* p=.02 |
r=.26 p=.08 |
r=.28* p=.05 |
r=.16 p=.28 |
| VE-C | r=.01 p=.92 |
r=−.02 p=.89 |
r=−.08 p=.60 |
r=−.07 p=.61 |
r=.03 p=.82 |
r=−.06 p=.66 |
r=−.15 p=.31 |
r=−.04 p=.80 |
r=−.09 p=.55 |
r=−.08 p=.61 |
r=−.12 p=.41 |
| VE-RT | r=−.30* p=.04 |
r=−.04 p=.81 |
r−.09 p=.55 |
r=−.16 p=.29 |
r=−.18 p=.23 |
r=−.10 p=.50 |
r=−.15 p=.32 |
r=−.23 p=.11 |
r=−.27 p=.06 |
r=−.22 p=.13 |
r=−.23 p=.12 |
| VD-C | r=.10 p=.49 |
r=.10 p=.49 |
r=.04 p=.78 |
r=−.00 p=.99 |
r=.08 p=.59 |
r=.06 p=.69 |
r=.02 p=.91 |
r=.09 p=.55 |
r=−.00 p=.97 |
r=.06 p=.69 |
r=.00 p=.99 |
| VD-RT | r=−.28* p=.05 |
r=−.06 p=.67 |
r=−.10 p=.50 |
r=−.16 p=.27 |
r=−.20 p=.18 |
r=−.12 p=.42 |
r=−.16 p=.28 |
r=−.23 p=.11 |
r=−.27 p=.06 |
r=−.24 p=.10 |
r=−.25 p=.09 |
| FWAIS | r=.18 p=.21 |
r=.01 p=.93 |
r=.03 p=.83 |
r=.12 p=.42 |
r=.19 p=.19 |
r=.05 p=.75 |
r=.20 p=.17 |
r=.20 p=.16 |
r=.23 p=.12 |
r=.03 p=.86 |
r=.00 p=.99 |
SE=Spatial Enhancer Task, VE=Verbal Enhancer Task, SD=Spatial Distracter Task, VD=Verbal Distracter Task. “C”=total correct. CVLT=California Verbal Learning Task. FWAIS=full scale of the WAIS-IV intelligence test.
=p<.05,
=p<.01.
Because we are interested in the relationship between plasma TC and brain myelin, we examined the relationship between FA, RD and AD for the regions showing the largest effect on FA associated with TC (i.e., FX-ST-L and FX-ST-R) to determine whether FA in these regions is associated with a demyelination process or axonal damage. It has been experimentally shown in the mouse and rat models (Janve et al., 2013; Song et al., 2005), human autopsied tissue from prefrontal cortex of older brains (Back et al., 2011), and ex vivo multiple sclerosis spinal cords (Klawiter et al., 2011), that a demyelinating process rather than axonal injury leads to a decrease in FA, an increase in RD, and no change in AD. Figure 7 shows box plots for each of the Risk Cluster groups and for FA, RD and AD. A demyelinating process is one interpretation of the data given the diffusion pattern of results (i.e., lower FA values, higher RD values and no difference in AD). However, one caveat concerns the partial-volume effects of cerebrospinal fluid (CSF) contamination in voxels near the white matter and CSF boundary (Jones and Cercignani, 2010; Metzler-Baddeley et al., 2012), especially in narrow white matter regions such as fornix and genu, regions where we found significant effects. This issue is particularly important since we are comparing results across individuals of different ages (i.e., tissue atrophy associated with normal aging) and our Risk Cluster group 3 has more WMHs, which likely leads to more CSF contamination (Metzler-Baddeley et al., 2012; Vernooij et al., 2008). We have not applied partial volume corrections to these data and thus our suggestion that a demyelinating process may be occurring, is speculative at this point in time. However, we note here that FA is not as susceptible to partial volume effects as are other diffusion parameters (Metzler-Baddeley et al., 2012).
Fig. 7.
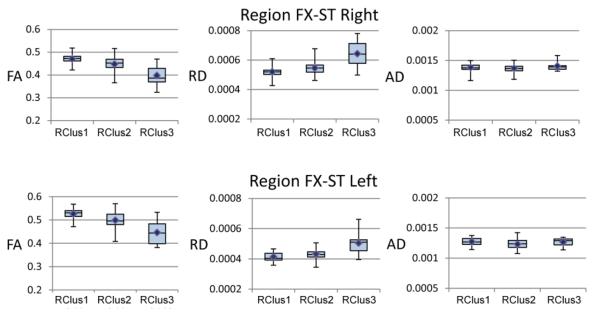
Box plots showing the pattern of effects across Risk Cluster groups for FA, RD and AD and region FX-ST (right and left).
Discussion
Vascular Risk Factors and Memory Performance
As predicted, when age-correction was applied, high systolic blood pressure was associated with lower spatial working memory accuracy. The REY complex figure test, a test of spatial memory, revealed similar results. A recent cross-sectional study by Hale and colleagues (2011) found that performance on spatial tasks (6 spatial tasks) decreased at faster rates as a function of age than performance on verbal tasks (6 verbal tasks) with essentially no difference between simple versus complex tasks. Our results are consistent with their results, however, unlike the present study, these investigators did not examine effects of risk factors such high blood pressure on performance levels, and their participants were screened before recruitment in order to enroll highly functioning individuals (i.e., equivalent to enrolling those with MMSE scores in the 28–30 range).
Elias and colleagues (2004) did examine effects of hypertension on cognitive performance across age and found that higher levels of systolic and diastolic blood pressure for both younger and older age groups were significantly associated with lower scores in visualization/fluid abilities. All participants enrolled in their study were cognitively normal. However, Elias and colleagues did not examine MRI-based measures. Based on our results, along with others, either there is a selective effect of hypertension on spatial working memory (Coltman et al., 2011) or performance decline can be identified earlier using spatial memory tasks (Bisiacchi et al., 2008; Hale et al., 2011; Jenkins et al., 2000; Jennings et al., 2006; Johnson et al., 2009) or both are true. In addition, since the presence of WMHs correlate with frontal lobe pathology initially (i.e., poor executive control and memory performance) and then spreads into parietal regions with time (Artero et al., 2004; Kuo and Lipsitz, 2004; Nordahl et al., 2006), hypertension may account for some of the cognitive decline witnessed in normal aging studies.
Our results also show a positive association between age-adjusted verbal working memory performance (accuracy) and TC level. While the NCEP provides guidelines for cholesterol testing and management (NCEP, 2001) based on the patient's risk profile for coronary heart disease, these guidelines do not appear to map onto levels for optimal cognitive functioning. As mentioned previously, the Elias study (2005) found that individuals with TC levels in the “desirable” range (< 200 mg/dL) according to the NCEP guidelines, performed less well than those rated as “borderline-high” (200–239 mg/dL) and “high” (≥ 240 mg/dL). We also referred to several other studies previously, showing that higher levels of plasma cholesterol were associated with improved verbal memory performance (Henderson et al., 2003; Krakowski and Czobor, 2011; Muldoon et al., 2000).
In order to relate our results to other studies that applied categorical splits to their cholesterol data [e.g., (Muldoon et al., 2000)], we split our TC and LDL data about the median value to examine memory performance differences. Muldoon and colleagues split the final LDL values about the median level of 109 mg/dL, after statin treatment to lower LDL levels, and found that only those individuals in the lower half revealed cognitive decline. In the same manner we divided the cholesterol levels into low and high groups and compared verbal and spatial memory performance between these groups (see Table 5). Low LDL and TC levels were associated with significantly worse accuracy on verbal working memory tasks only. We then regrouped the LDL levels to be consistent with NCEP guidelines (<100 mg/dL is “Optimal” while 100–130 mg/dL is “Near/Above Optimal”). Individuals in the “Optimal” range performed more poorly than individuals in the “Near/Above Optimal” range. There were only 5 cases in the “Borderline High-High” range so statistical significance was not tested. However, mean performance on the verbal enhancer task for these 5 cases was 116.4 while the mean performance on the verbal distracter task was 115.0 (mean LDL=154.2 mg/dL). In all cases, lower levels of cholesterol (TC and LDL) were associated with significantly poorer accuracy on the two verbal working memory tasks. The spatial tasks were not correlated with TC or LDL (as shown in Fig. 3B).
Table 5.
Effects of low cholesterol on verbal memory performance. TC and LDL cholesterol levels were split into two groups (Low and Hi) at the median (TC median=172.5 mg/dL; LDL median=93 mg/dL). Only verbal task accuracy was significantly different between Low and Hi TC or LDL groups (i.e., spatial task accuracy did not differ between groups). In the bottom portion of the table LDL levels were split into groups according to the cholesterol guidelines set by the NCEP. LDL values less than 100 mg/dL is considered “Optimal” while values between 100–129 mg/dL are “Near/Above Optimal.” In general, higher cholesterol levels are associated with better performance on the verbal memory tasks. Mean TC levels for the Low and Hi groups were 143.5 mg/dL and 192.5 mg/dL, respectively. Mean LDL levels for Low and Hi groups were 73.1 mg/dL and 117.0 mg/dL, respectively.
| Verbal Memory Task (Total Correct) | Low TC <172.5 mg/dL |
Hi TC ≥172.5 mg/dL |
|
|---|---|---|---|
| Verbal Enhancer | 110.7 | 115.2 | p<.02 |
| Verbal Distracter | 109.1 | 114.4 | p<.02 |
| Low LDL <93.0 mg/dL |
Hi LDL ≥93.0 mg/dL |
||
| Verbal Enhancer | 110.1 | 115.8 | p<.001 |
| Verbal Distracter | 108.3 | 115.2 | p<.003 |
| LDL <100 mg/dL |
LDL 100–129 mg/dL |
||
| Verbal Enhancer | 111.0 | 114.3 | p<.05 |
| Verbal Distracter | 109.1 | 114.0 | p<.02 |
Recent studies have indicated that brain cholesterol plays an important role in synapse formation, receptor function, myelin formation, and synaptic plasticity [e.g., (Darwish et al., 2010)]. Therefore, some investigators are fearful that restrictions in diet cholesterol at young ages can be deleterious for later brain development and performance (Kolata, 1985). Similarly, some are concerned that lowering cholesterol levels through the use of statins [24–32 million Americans are taking statins for protection against the development of coronary heart disease (Moyer, 2010; Wehrwein, 2011)] may also impair brain structure/function and cognition, particularly in elders (Criqui and Golomb, 2004; Panza et al., 2006; West et al., 2008; Weverling-Rijnsburger et al., 1997). The FDA, for example, recently updated the side effects of statins to include “cognitive (brain-related) impairment such as memory loss, forgetfulness and confusion (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm293330.htm).
Region FX-ST (right and left), which showed a strong negative correlation between FA and TC in Figure 6, showed decreased FA, increased RD and no difference in AD for RClus3 compared to RClus1 and 2 in Figure 7. A possible cause of this particular pattern of effects is that a demyelination process underlies the poorer memory performance of this group. Other clinical studies in schizophrenia (Scheel et al., 2013), multiple sclerosis (Anderson et al., 2011), and normal aging (Bennett et al., 2010; Inano et al., 2011) have found a similar pattern of diffusion parameters and reached similar conclusions. However, in order to be sure that these changes are only due to demyelination, as opposed to being an artifact due to CSF contamination [i.e., partial volume effects (Jones and Cercignani, 2010; Metzler-Baddeley et al., 2012)], a direct MRI measure of myelination is required. It should also be emphasized that our cross-sectional study cannot provide information concerning the direction of causality of observed correlations between DTI indices and risk factors. Therefore, it is equally plausible that “weakened” white matter tracts are more susceptible to risk factors as opposed to risk factors affecting white matter tracts. As reviewed later, the literature suggests that region FX-ST is involved with episodic memory functions, thus demyelination of certain white matter tracts remains as a potential mechanism underlying verbal memory impairment associated with lower cholesterol levels.
WMHs, Risk Factors and Memory Performance
WMHs are a common finding in MR images of older individuals, which increase with age and vascular risk (de Groot et al., 2013; de Leeuw et al., 2001; Jeerakathil et al., 2004), and are associated with cognitive impairment (Gunning-Dixon and Raz, 2000; Kuo and Lipsitz, 2004; Nordahl et al., 2006). Some investigators suggest that WMHs could be used as a MRI biomarker of vascular brain injury (Debette and Markus, 2010; Debette et al., 2011). Recent MR studies using FLAIR and DTI suggest that WMHs are at the apex of white matter alteration (i.e., at the tip of the iceberg--there is more widespread and subtle white matter changes surrounding the apex) (de Groot et al., 2013; Maillard et al., 2011) and that generalized white matter integrity is a function of overall white matter load [(i.e., the greater the load, the more generalized the white matter injury (Maillard et al., 2011)]. Our Risk Cluster group 3 members revealed the greatest numbers and volumes of WMHs, along with higher systolic/diastolic blood pressure, than the other two Risk Cluster Groups (see Fig. 4A). The performance of Risk Cluster group 3 on the spatial working memory tasks (accuracy and RTs) was also worse than the other two groups. These results are consistent with an underlying etiology of chronic ischemia and are associated with vascular risk factors such as hypertension.
DTI, Risk Factors and Memory Performance
The DTI FA results shown in Table 3 for Risk Cluster group 3 (compared to groups 1 and 2), as well as for risk factors systolic blood pressure and A1C, all revealed lower values of FA in the fornix (FX), genu of the corpus callosum (GCC), posterior thalamic radiation (PTR), and sagittal stratum (SS). The genu connects medial and lateral surfaces of the frontal lobes while the fornix provides hippocampal and parahippocampal output to the mammillary bodies (Aggleton et al., 2005; Vann and Aggleton, 2004); it is the largest efferent pathway from the hippocampus (Koenig et al., 2013). The fornix has been specifically associated with episodic memory; this white matter tract connects the hippocampal formation to prefrontal cortex (Metzler-Baddeley et al., 2011; Thomas et al., 2011), indirectly (Poletti and Creswell, 1977). The basal forebrain, which receives projections via the septum, along with the inferior and medial portion of prefrontal cortex (gyrus rectus), has also been associated with episodic memory (Butler et al., 2012; Elderkin-Thompson et al., 2009; Fujii et al., 2002; Phillips et al., 1987). Furthermore, the basal forebrain was the focus of many experimental animal models of Alzheimer's disease (i.e., the cholinergic hypothesis of memory dysfunction) (Voytko, 1996).
The posterior thalamic radiation is a projection fiber tract from the thalamus to occipital and parietal lobes. The sagittal stratum (including the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) connects the occipital lobe to the rest of the brain (Jellison et al., 2004) and FA within this tract has been shown to be negatively affected by increasing systolic blood pressure (Kennedy and Raz, 2009; Salat et al., 2012). Collectively, these frontal, limbic and posterior tracts support memory functions. The anterior cingulum has been associated with both verbal and visual spatial memory (Kantarci et al., 2011; Mielke et al., 2009) as well as the fornix (Mielke et al., 2009; Palacios et al., 2011). The cingulum and fornix are major components of the Papez circuit (Abdul-Rahman et al., 2011; Hong et al., 2012; Shah et al., 2012), a circuit known for the formation of new memories (episodic). Damage to this circuit results in anterograde amnesia (Thomas et al., 2011). Several recent studies suggest that a reduction in FA or increase in mean diffusivity of the fornix correlates with and can longitudinally predict memory decline and progression to Alzheimer's disease (Mielke et al., 2012; Sexton et al., 2010). Others have noted lower values of FA in the sagittal stratum in Alzheimer's disease (Qiu et al., 2010). Studies of memory function in traumatic brain injury and multiple sclerosis also note lower values of FA in the sagittal stratum (Kraus et al., 2007; Yu et al., 2012).
High systolic blood pressure and A1C, indicators of hypertension or type 2 diabetes, respectively, are both associated with lower FA values in the same regions. There is considerable evidence now that elevated systolic blood pressure in midlife increases risk of developing clinically manifested dementia in old age (Freitag et al., 2006; Kivipelto et al., 2001) and that high normal blood pressure in non-demented adults also has a negative effect on cognition (Kennedy and Raz, 2009; Knecht et al., 2009; Knecht et al., 2008). The Risk Cluster group 3 has combined risk factors that resemble metabolic syndrome and, not surprisingly, show more extensively affected white matter regions than for systolic blood pressure and A1C measures alone (i.e., anterior corona radiata and the splenium of the corpus callosum are affected as well). A clinical diagnosis of metabolic syndrome is made when 3 of the following 5 indicators are present: 1) obesity determined by waist circumference; 2) high blood pressure; 3) elevated triglycerides; 4) low HDL and 5) impaired fasting glucose (Grundy, 2001). Therefore, it is quite likely that some members of Risk Cluster group 3 have metabolic syndrome.
Interestingly, in one study, patients with mild cognitive impairment had increased mean diffusivity in the genu and splenium of the corpus callosum, as well as in the anterior corona radiata (Thillainadesan et al., 2012), three of the regions identified in Risk Cluster group 3. The anterior corona radiata connects prefrontal regions with the basal ganglia and spinal cord. However, Head et al. (2005) and Delano-Wood et al. (2012) found anterior-posterior differences in volume and FA (e.g., anterior cingulate and genu versus posterior cingulate and splenium) between non-demented elderly and those with mild cognitive impairment or dementia; age was associated with lower FA values in anterior regions while mild cognitive impairment and Alzheimer's disease were associated with lower FA values in posterior regions. The genu of the corpus callosum and splenium of the corpus callosum together, and related cortical regions (anterior cingulate and posterior cingulate), are often tagged as a network mediating memory functions (Burgess et al., 2000; Kraus et al., 2007; Torta and Cauda, 2011) and have more recently become associated with the default mode network (Raichle and Snyder, 2007). FA in the posterior cingulum is correlated with hippocampal volume and tests of verbal memory (Delano-Wood et al., 2012). Many of the regions associated with the Risk Cluster group 3 were also associated with “age.” These results suggest that single risk factors or their combination (e.g., metabolic syndrome) may be contributing to memory decline seen in aging, mild cognitive impairment and Alzheimer's disease.
A different group of regions was negatively associated with TC. These regions include bilateral superior longitudinal fasciculus (SLF), bilateral cingulum/cingulate gyrus, and bilateral FX-ST (the crus of the fornix and stria terminalis). The SLF is a major tract that connects regions of the temporal (posterior and superior) and parietal lobes with prefrontal cortex (Croxson et al., 2005). This tract has been divided into subcomponents in both nonhuman primates and humans (Catani et al., 2005; Makris et al., 2005; Petrides and Pandya, 1984). The arcuate fasciculus, the pathway connecting Broca's and Wernicke's language areas, is part of the SLF (Catani et al., 2005; Hua et al., 2009; Makris et al., 2005). In addition, anterior and posterior segments of an indirect path connecting language regions, including the angular gyrus, have been documented using DTI. Some investigators link this network and verbal working memory to the articulatory loop (Duffau, 2012; Martino et al., 2013), consistent with notions put forth by Baddeley (2003). For example, electrical stimulation of the left supramarginal gyrus (cortical region) or the posterior part of the lateral portion of the SLF (white matter tract) causes speech production disorders (Duffau, 2012).
Several DTI studies have associated portions of the SLF with verbal processing and verbal memory (Gold et al., 2007; Peters et al., 2012). For example, Karlsgodt and colleagues (2008) found a positive correlation between performance on a Sternberg verbal working memory task and FA in the SLF. Similarly, Peters and colleagues (2012) show significant bilateral increases in FA in the SLF with development, which correlated positively with verbal working memory performance. The cingulum/cingulate gyrus, which also showed lower FA values associated with TC, courses within the cingulate gyrus and arches around the corpus callosum and extends into the parahippocampal gyrus. It follows along the ventral surface of the hippocampus while FX-ST-R (composed of both fornix and stria terminalis fibers) project along the dorsal side of the hippocampus (Wakana et al., 2004). Collectively, these regions comprise part of the limbic system and interconnect portions of the frontal, parietal and temporal lobes (Jellison et al., 2004). The cingulum/cingulate gyrus has also been implicated in the verbal working memory circuit described above (Charlton et al., 2010; Sepulcre et al., 2009).
In sum, the DTI regions revealing lower FA values for our different risk factors have all been implicated in memory functions by others. Differences noted between FA regions associated with systolic blood pressure/A1C measures and the TC measure correspond to a larger anterior-posterior circuit versus one affecting more medial temporal-lateral parietal regions (e.g., temporo-parietal junction), respectively. Others have found that a pattern of lower FA values in frontal regions, typically associated with aging, is also associated with high blood pressure (Delano-Wood et al., 2012; Kennedy and Raz, 2009; Salat et al., 2012). Kennedy and Raz (2009) suggest that vascular risk drives white matter deterioration, even in normotensive individuals, in an anterior-posterior direction. Significant deterioration in posterior regions (i.e., splenium of the corpus callosum) are typically associated with mild cognitive impairment and Alzheimer's disease (Head et al., 2005) and FA of the posterior cingulum has been associated with verbal memory performance (Delano-Wood et al., 2012). Consistent with these results, we suggest that TC may have a selective effect on verbal memory while systolic blood pressure and A1C may have a selective effect on spatial working memory. Individuals diagnosed as having metabolic syndrome (e.g., some Risk Cluster group 3 members) are likely to suffer from a combination of verbal and spatial working memory deficits. Why TC correlated negatively with FA but positively with performance on verbal working memory tasks, remains unclear. Further studies need to be conducted in order to sort out these complex relationships.
Conclusions
Taken together, our results indicate: 1) participants with high blood pressure and high blood glucose levels perform more poorly on spatial working memory tasks than age-equivalent counterparts across the age spectrum; 2) there is a positive correlation between performance on verbal working memory tasks and levels of TC even when performance is age-adjusted; 3) when groups were formed based on low versus high levels of TC or LDL, low cholesterol levels correlated with significantly poorer verbal working memory accuracy; 4) systolic blood pressure and A1C individually show associations with lower FA values in similar regions which are a subset of the regions showing low FA values for Risk Cluster group 3; 5) lower FA values associated with TC are anatomically distinct from regions showing lower FA values associated with systolic blood pressure, A1C and for Risk Cluster group 3 (relative to groups 1 and 2); and 6) our DTI results complement other DTI studies in outlining regions that may underlie verbal and spatial working memory.
These results are encouraging since blood pressure, A1C values, cholesterol levels, and triglycerides are generally modifiable through life-style changes (e.g., diet and exercise). A recent study by Unverzagt and colleagues (2011) also found that systolic blood pressure, in a group that was stroke free, was linearly related to rate of incident cognitive decline. These authors suggest that the vascular risk factors measured by the Framingham Stroke Risk Profile and elevated blood pressure may provide a simple means for identifying individuals at risk for future cognitive decline. Generally, the relationship between cardiovascular fitness and brain health has not been well-studied or widely disseminated to the general public. Many recent studies have shown how physical exercise not only has a neuroprotective effect on brain health but is also associated with increases in gray matter and white matter volumes (Colcombe et al., 2006) and white matter tract integrity [see recent meta-analysis in (Ahlskog et al., 2011)]. Rowe and Kahn (1987) emphasized over twenty years ago that effects of the aging process itself have been exaggerated, while the modifying effects of external factors such as exercise and diet have been underestimated. Similarly, Burke and Barnes (2006) refer to the “myth of brain ageing” as a misconception that dramatic cell loss and morphological changes in neurons occur in normal aging. Indeed, many healthy elderly in our study performed as well as or better than our younger participants. However, the healthy elderly reflecting `healthy successful aging' on one end of the continuum, do not appear to represent the majority of elderly. Instead, elderly with a subset of vascular risk factors appear to better reflect `normal aging,' the middle ground of the continuum. We suggest, therefore, that vascular risk factors confound or help to mask the true effects associated with age-related, cognitive decline (i.e., assuming healthy successful aging).
A finding of great interest and worthy of future study is the positive association between TC/LDL and verbal working memory accuracy. Several population-based studies have already shown that low cholesterol levels (TC and LDL) in late life are associated with poor memory performance, earlier onset of dementia, and higher probability of death from non-cardiovascular diseases (Anstey et al., 2008; Frisardi et al., 2010; West et al., 2008). There is some evidence suggesting that elevated TC in old age is protective (Weverling-Rijnsburger et al., 1997). This is plausible since total serum cholesterol decreases as a risk factor for cardiovascular disease with age and the influence of genetic factors on serum TC and triglycerides reduces with age as well (Heller et al., 1993). Although there is not much information in the literature on the positive association between levels of plasma cholesterol and verbal memory performance, this area should be examined more carefully given the large number of statin users hoping to lower their cholesterol levels as much as possible.
And finally, these results emphasize the important influence of vascular risk factors on memory performance and neuroimaging measures of function and structure. As this study shows, there is considerable variability among constituents of a typical control group regarding their health and many had subclinical or undiagnosed hypertension, hypercholesterolemia, diabetes, and metabolic syndrome, even in younger and middle-aged groups. While all investigators apply inclusion/exclusion criteria for their respective studies, self-report from study participants is never fool-proof. Neuroimaging capabilities have come a long way in terms of their sensitivity to detect small differences between control groups and clinical populations. One goal here was to highlight this sensitivity by demonstrating tremendous individual variation within a single control group. Perhaps it is time to control for additional variance imposed by health status that is extraneous to the study, to reap the full benefit of our powerful neuroimaging tools.
Highlights
Vascular risk factors of our control group (n=55, age 18–81) were examined
High blood pressure correlated negatively with spatial memory accuracy
Total cholesterol correlated positively with verbal memory accuracy
High blood pressure and total cholesterol correlated negatively with DTI measures
Vascular risk factors account for memory decline more than age alone
Acknowledgments
The authors wish to thank Christopher Clifford for his help in establishing inter-rater reliability in analyzing white matter hyperintensities using JIM software. This work was supported by a grant from the National Institute on Aging, award number R01AG029495. This work was also supported in part by: 1) the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1TR000041; 2) National Institute of General Medical Sciences 2P20GM103472-06; 3) the Department of Energy under Award Number DE-FG02-99ER62764 to the Mind Research Network; and 4) the Radiology Department at UNM SOM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes on Aging or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Rahman MF, Qiu A, Sim K. Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One. 2011;6:e18652. doi: 10.1371/journal.pone.0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Bryant JE, Knoefel JE, Adair JC, Hart B, Donahue CH, Montano R, Hayek R, Qualls C, Ranken D, Stephen JM. Different strategies for auditory word recognition in healthy versus normal aging. Neuroimage. 2010;49:3319–3330. doi: 10.1016/j.neuroimage.2009.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Sanfratello L, Adair JC, Knoefel JE, Caprihan A, Stephen JM. Development and decline of memory functions in normal, pathological and healthy successful aging. Brain Topogr. 2011;24:323–339. doi: 10.1007/s10548-011-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualls C, Bockholt J, Best E, Kovacevic S, Cobb W, et al. Aging: Compensation or Maturation? NeuroImage. 2006;32:1891–1904. doi: 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VM, Wheeler-Kingshott CA, Abdel-Aziz K, Miller DH, Toosy A, Thompson AJ, Ciccarelli O. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler. 2011;17:1079–1087. doi: 10.1177/1352458511403528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- Artero S, Tiemeier H, Prins ND, Sabatier R, Breteler MM, Ritchie K. Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J Neurol Neurosurg Psychiatry. 2004;75:1304–1308. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Back SA, Kroenke CD, Sherman LS, Lawrence G, Gong X, Taber EN, Sonnen JA, Larson EB, Montine TJ. White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol. 2011;70:465–476. doi: 10.1002/ana.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birns J, Kalra L. Cognitive function and hypertension. J Hum Hypertens. 2009;23:86–96. doi: 10.1038/jhh.2008.80. [DOI] [PubMed] [Google Scholar]
- Bisiacchi PS, Borella E, Bergamaschi S, Carretti B, Mondini S. Interplay between memory and executive functions in normal and pathological aging. J Clin Exp Neuropsychol. 2008;30:723–733. doi: 10.1080/13803390701689587. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Gronenschild EH, Vuurman EF, Hofman P, Uylings HB, Jolles J, Raz N. Multiple indicators of age-related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage. 2010;49:2083–2093. doi: 10.1016/j.neuroimage.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Butler T, Blackmon K, Zaborszky L, Wang X, DuBois J, Carlson C, Barr WB, French J, Devinsky O, Kuzniecky R, et al. Volume of the human septal forebrain region is a predictor of source memory accuracy. J Int Neuropsychol Soc. 2012;18:157–161. doi: 10.1017/S1355617711001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Lawes IN, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46:474–489. doi: 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Coltman R, Spain A, Tsenkina Y, Fowler JH, Smith J, Scullion G, Allerhand M, Scott F, Kalaria RN, Ihara M, et al. Selective white matter pathology induces a specific impairment in spatial working memory. Neurobiol Aging. 2011;32:2324, e2327–2312. doi: 10.1016/j.neurobiolaging.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Criqui MH, Golomb BA. Low and lowered cholesterol and total mortality. J Am Coll Cardiol. 2004;44:1009–1010. doi: 10.1016/j.jacc.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish DS, Wang D, Konat GW, Schreurs BG. Dietary cholesterol impairs memory and memory increases brain cholesterol and sulfatide levels. Behav Neurosci. 2010;124:115–123. doi: 10.1037/a0018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- de Groot M, Verhaaren BF, de Boer R, Klein S, Hofman A, van der Lugt A, Ikram MA, Niessen WJ, Vernooij MW. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–1042. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Stricker NH, Sorg SF, Nation DA, Jak AJ, Woods SP, Libon DJ, Delis DC, Frank LR, Bondi MW. Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. J Alzheimers Dis. 2012;29:589–603. doi: 10.3233/JAD-2012-102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Duffau H. The “frontal syndrome” revisited: lessons from electrostimulation mapping studies. Cortex. 2012;48:120–131. doi: 10.1016/j.cortex.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Hellemann G, Pham D, Kumar A. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry. 2009;24:459–468. doi: 10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med. 2005;67:24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference? Hypertension. 2004;44:631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006;37:33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- Frisardi V, Panza F, Solfrizzi V, Seripa D, Pilotto A. Plasma lipid disturbances and cognitive decline. J Am Geriatr Soc. 2010;58:2429–2430. doi: 10.1111/j.1532-5415.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- Fujii T, Okuda J, Tsukiura T, Ohtake H, Miura R, Fukatsu R, Suzuki K, Kawashima R, Itoh M, Fukuda H, Yamadori A. The role of the basal forebrain in episodic memory retrieval: a positron emission tomography study. Neuroimage. 2002;15:501–508. doi: 10.1006/nimg.2001.0995. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and Mind: Unifying Cognition. Oxford University Press; New York: 2003. [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grundy SM. United States Cholesterol Guidelines 2001: expanded scope of intensive low-density lipoprotein-lowering therapy. Am J Cardiol. 2001;88:23J–27J. doi: 10.1016/s0002-9149(01)01931-2. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hale S, Rose NS, Myerson J, Strube MJ, Sommers M, Tye-Murray N, Spehar B. The structure of working memory abilities across the adult life span. Psychol Aging. 2011;26:92–110. doi: 10.1037/a0021483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Heller DA, de Faire U, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Guthrie JR, Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. J Neurol Neurosurg Psychiatry. 2003;74:1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Choi BY, Chang CH, Kim SH, Jung YJ, Byun WM, Jang SH. Injuries of the cingulum and fornix after rupture of an anterior communicating artery aneurysm: a diffusion tensor tractography study. Neurosurgery. 2012;70:819–823. doi: 10.1227/NEU.0b013e3182367124. [DOI] [PubMed] [Google Scholar]
- Hua K, Oishi K, Zhang J, Wakana S, Yoshioka T, Zhang W, Akhter KD, Li X, Huang H, Jiang H, et al. Mapping of functional areas in the human cortex based on connectivity through association fibers. Cereb Cortex. 2009;19:1889–1895. doi: 10.1093/cercor/bhn215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. Effects of age and gender on white matter integrity. AJNR Am J Neuroradiol. 2011;32:2103–2109. doi: 10.3174/ajnr.A2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, van der Elst W, Jolles J, Verhey FR, McGlinchey RE, Milberg WP, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janve VA, Zu Z, Yao SY, Li K, Zhang FL, Wilson KJ, Ou X, Does MD, Subramaniam S, Gochberg DF. The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis (MS) lesions. Neuroimage. 2013;74:298–305. doi: 10.1016/j.neuroimage.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Jenkins L, Myerson J, Joerding JA, Hale S. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psychol Aging. 2000;15:157–175. doi: 10.1037//0882-7974.15.1.157. [DOI] [PubMed] [Google Scholar]
- Jennings JR, van der Veen FM, Meltzer CC. Verbal and spatial working memory in older individuals: A positron emission tomography study. Brain Res. 2006;1092:177–189. doi: 10.1016/j.brainres.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23:803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Joosten H, van Eersel ME, Gansevoort RT, Bilo HJ, Slaets JP, Izaks GJ. Cardiovascular risk profile and cognitive function in young, middle-aged, and elderly subjects. Stroke. 2013;44:1543–1549. doi: 10.1161/STROKEAHA.111.000496. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Wersching H, Lohmann H, Berger K, Ringelstein EB. How much does hypertension affect cognition?: explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J Neurol Sci. 2009;283:149–152. doi: 10.1016/j.jns.2009.02.362. [DOI] [PubMed] [Google Scholar]
- Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, Berger K, Ringelstein EB. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51:663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- Koenig KA, Sakaie KE, Lowe MJ, Lin J, Stone L, Bermel RA, Beall EB, Rao SM, Trapp BD, Phillips MD. High spatial and angular resolution diffusion-weighted imaging reveals forniceal damage related to memory impairment. Magn Reson Imaging. 2013;31(5):695–699. doi: 10.1016/j.mri.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata G. Heart panel's conclusions questioned. Science. 1985;227:40–41. doi: 10.1126/science.3880617. [DOI] [PubMed] [Google Scholar]
- Krakowski M, Czobor P. Cholesterol and cognition in schizophrenia: a double-blind study of patients randomized to clozapine, olanzapine and haloperidol. Schizophr Res. 2011;130:27–33. doi: 10.1016/j.schres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, DeCarli C. White matter hyperintensity penumbra. Stroke. 2011;42:1917–1922. doi: 10.1161/STROKEAHA.110.609768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr., Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218:105–121. doi: 10.1007/s00429-012-0386-5. [DOI] [PubMed] [Google Scholar]
- Mathew A, Yoshida Y, Maekawa T, Kumar DS. Alzheimer's disease: cholesterol a menace? Brain Res Bull. 2011;86:1–12. doi: 10.1016/j.brainresbull.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J Neurosci. 2011;31:13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage. 2012;59:1394–1403. doi: 10.1016/j.neuroimage.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Kozauer NA, Chan KC, George M, Toroney J, Zerrate M, Bandeen-Roche K, Wang MC, Vanzijl P, Pekar JJ, et al. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2009;46:47–55. doi: 10.1016/j.neuroimage.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimers Dement. 2012;8:105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Ann N Y Acad Sci. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Moyer MW. Static over statins. Sci Am. 2010;302:26, 28. doi: 10.1038/scientificamerican0410-26. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108:538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- NCEP Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman JM, Sergeant JA, Weinstein HC, Scherder EJ. Timed executive functions and white matter in aging with and without cardiovascular risk factors. Rev Neurosci. 2004;15:439–462. doi: 10.1515/revneuro.2004.15.6.439. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Fernandez-Espejo D, Junque C, Sanchez-Carrion R, Roig T, Tormos JM, Bargallo N, Vendrell P. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol. 2011;11:24. doi: 10.1186/1471-2377-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- Panza F, D'Introno A, Colacicco AM, Capurso C, Pichichero G, Capurso SA, Capurso A, Solfrizzi V. Lipid metabolism in cognitive decline and dementia. Brain Res Rev. 2006;51:275–292. doi: 10.1016/j.brainresrev.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Phillips S, Sangalang V, Sterns G. Basal forebrain infarction. A clinicopathologic correlation. Arch Neurol. 1987;44:1134–1138. doi: 10.1001/archneur.1987.00520230024008. [DOI] [PubMed] [Google Scholar]
- Poletti CE, Creswell G. Fornix system efferent projections in the squirrel monkey: an experimental degeneration study. J Comp Neurol. 1977;175:101–128. doi: 10.1002/cne.901750107. [DOI] [PubMed] [Google Scholar]
- Qiu A, Oishi K, Miller MI, Lyketsos CG, Mori S, Albert M. Surface-based analysis on shape and fractional anisotropy of white matter tracts in Alzheimer's disease. PLoS One. 2010;5:e9811. doi: 10.1371/journal.pone.0009811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, McGlinchey RE, Milberg WP. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59:181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel M, Prokscha T, Bayerl M, Gallinat J, Montag C. Myelination deficits in schizophrenia: evidence from diffusion tensor imaging. Brain Struct Funct. 2013;218:151–156. doi: 10.1007/s00429-012-0389-2. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53:132–139. doi: 10.1212/wnl.53.1.132. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Scheltens P, Erkinjuntti T, Pantoni L, Markus HS, Wallin A, Barkhof F, Fazekas F. White matter lesion progression: a surrogate endpoint for trials in cerebral small-vessel disease. Neurology. 2004;63:139–144. doi: 10.1212/01.wnl.0000132635.75819.e5. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Masdeu JC, Pastor MA, Goni J, Barbosa C, Bejarano B, Villoslada P. Brain pathways of verbal working memory: a lesion-function correlation study. Neuroimage. 2009;47:773–778. doi: 10.1016/j.neuroimage.2009.04.054. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Lonie JA, Bastin ME, Terriere E, O'Carroll RE, Ebmeier KP. MRI correlates of episodic memory in Alzheimer's disease, mild cognitive impairment, and healthy aging. Psychiatry Res. 2010;184:57–62. doi: 10.1016/j.pscychresns.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. 2012;19:289–298. doi: 10.1016/j.jocn.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, MacKay CE, Watkins KE, Ciccarelli O, Cader MZ, Mathews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: A diffusion tensor imaging study. Hum Brain Mapp. 2010;31(10):1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillainadesan S, Wen W, Zhuang L, Crawford J, Kochan N, Reppermund S, Slavin M, Trollor J, Brodaty H, Sachdev P. Changes in mild cognitive impairment and its subtypes as seen on diffusion tensor imaging. Int Psychogeriatr. 2012;24:1483–1493. doi: 10.1017/S1041610212000270. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics. 2011;31:1107–1121. doi: 10.1148/rg.314105729. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–2172. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, Kissela BM, Kelley BJ, Kennedy R, Moy CS, et al. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranga RM, Keller JN. Diet and age interactions with regards to cholesterol regulation and brain pathogenesis. Curr Gerontol Geriatr Res. 2010:219683. doi: 10.1155/2010/219683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: memory or attention? Behav Brain Res. 1996;75:13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Wehrwein P. Harvard Health Letter. Boston: 2011. Statin use is up, cholesterol levels are down: Are Americans' hearts benefiting? [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22:144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44–48. Hum Brain Mapp. 2009;30:1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Beeri MS, Schmeidler J, Hannigan CM, Angelo G, Grossman HT, Rosendorff C, Silverman JM. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am J Geriatr Psychiatry. 2008;16:781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119–1123. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, Maletic-Savatic M, Krupp LB, Wagshul ME. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59:3713–3722. doi: 10.1016/j.neuroimage.2011.10.053. [DOI] [PubMed] [Google Scholar]