Introduction
The incidence of kidney cancer has risen steadily over the last few decades with most patients presenting incidentally due to widespread utilization of cross-sectional imaging. The rising incidence is accompanied by stage migration so that the majority of new cancers are small renal masses (SRMs) [1,2]. Extirpative surgery is the gold standard for treatment with partial nephrectomy (PN) favored over radical nephrectomy (RN) due to equivalent oncologic control and potential benefits of maximizing renal function to reduce cardiovascular sequelae [3,4]. Population-based data have supported an overall survival benefit for PN over RN, but the only randomized trial failed to demonstrate a survival benefit for PN [5–7]. Further investigation is needed as the overall rate of surgery, and utilization of PN in particular, has been increasing [8,9].
Increasing rates of detection and renal surgery have not translated into decreased rates of mortality or metastasis, suggesting potential overtreatment [10,11]. A growing interest has emerged to identify patients who can safely defer therapy and undergo active surveillance (AS) to avoid the morbidity of surgical treatment with minimal or no concession of oncologic outcomes. This approach is being increasingly supported by professional organizations for patients with decreased life expectancy or extensive comorbidities. A number of centers have initiated AS protocols to identify criteria for the most appropriate population [12,13].
Studies on AS are scarce with a lack of specific and consistent criteria to follow patients, but several small cohorts have demonstrated encouraging oncologic outcomes and low rates of metastasis with 2.5 years of follow-up [14–16]. The Surveillance, Epidemiology and End Results (SEER) database has been used to examine population-based practice with larger cohorts of patients who undergo nonsurgical management (NSM). The population-based practice of NSM is distinct from evolving AS protocols. However, existing data can help identify deficiencies in care and future areas of attention. One previous analysis has compared NSM to surgical management for early-stage kidney cancer but was without the benefit of Medicare claims data to control for the impact of comorbidities on cause-specific survival [17]. A more recent study used SEER-Medicare linked data, but the primary analysis focused on all renal cell carcinomas <7cm [18].
The goal of the present study is to use SEER cancer registry and Medicare claims linked data to characterize and compare survival among patients who undergo NSM, PN, and RN for T1a kidney cancer in the United States using a propensity-score matched approach and considering other-cause mortality as a competing risk of death.
Methods
After obtaining Institutional Review Board approval, we used linked SEER cancer registry and Medicare claims data from 1995 through 2007 to identify patients >65 years old diagnosed with clinically localized, T1a (≤4cm) renal cortical tumors with no spread to nearby lymph nodes (N0) or metastasis (M0) (classification per the American Joint Committee on Cancer, 2009). Kidney cancer diagnosis codes ICD-0-2, C64.9 and 9th revision ICD-0-9, 189.0 were used as inclusion criteria. Patients lacking Medicare A and/or B coverage or enrolled in a managed care plan during treatment were excluded. Patients were also excluded if they had regional disease (T3-4N0M0, TxN1-2M0), distant metastases (TxNxM1), unknown classification, upper tract transitional cell carcinoma or ureteric, non-cortical renal tumors, multiple procedures, bilateral tumors, a prior diagnosis of another cancer and/or had undergone ablative therapy.
Medicare claims and SEER data have a high agreement (97%) for classifying PN versus RN and high concordance in identifying patients who do not undergo cancer-directed surgery [19,20]. Patients undergoing PN were identified based on CPT codes (50240,50280,50290,50543) or ICD-9-CM codes (55.31,55.39,55.4). Patients undergoing RN were identified based on CPT codes (50220,50225,50230,50545,50546) or ICD-9-CM (55.51,55.52,55.53,55.54). Patients undergoing NSM were defined as lacking a procedural code within six months of diagnosis, which is the time frame SEER collects cancer therapy procedure codes. The same definition for NSM has been used by previous studies with the recognition that a small percentage (<4%) of patients included in the cohort do undergo intervention after six months [8,17,18].
SEER data were used to ascertain patient demographic data including age, sex, race, marital status, urban-rural location categories, tumor-size, baseline end-stage-renal-disease, and year of intervention. Comorbidity data were collected from claims records to calculate Charlson comorbidity index (CCI) using SEER-Medicare provided software on the Medicare Provider Analysis and Review file from the index month to 12 months after the index month [21]. This provided similar CCI scores to using a 12 month window prior to the index month but allowed inclusion of patients 65 years of age and resulted in no difference in adjusted models. Person-time contributed during follow-up was calculated from the date of diagnosis for NSM and the date of surgery for PN and RN until date of death or end of follow-up. The primary outcomes were overall survival (OS) through May 31, 2010 (last month of available Medicare follow-up) and kidney cancer-specific survival (CSS) through December 31, 2007 (last month of SEER follow-up for cause of death codes).
Chi-square (χ2) tests were used to evaluate associations between treatment modality (NSM, PN, or RN), covariates, and observed deaths. Kaplan-Meier survival functions were calculated to obtain survival probabilities at 1, 3, and 5 years, and log-rank tests were used to compare survival curves. Survival analysis was performed in three steps to aid in the adjustment and interpretation of potential confounding between surgical and NSM patients. First, Cox proportional hazards regression was performed, and an adjusted multivariable model developed to compare OS and CSS between groups controlling for demographic covariates and CCI. Next, recognizing a number of causes of death compete with kidney cancer in contributing to mortality, we performed competing risks regression for CSS with other-cause mortality designated as the competing risk according to the method described by Fine and Gray [22]. Stratified analyses were also performed by age, CCI, and tumor size to assess effect modification using Cox regression and interaction terms in logistic regression. Finally, as a check and comparison to our adjusted multivariable model, propensity-scores were calculated and one-to-one nearest-neighbor matching with a 10% caliper was conducted in pairwise fashion followed by Cox regression for the overall cohort and age-stratified cohort. The balancing property was satisfied for comparisons. Propensity-score matching can allow a better estimate of average treatment effect in observational data. Statistical analyses were performed using STATA v.12.0 (STATA Corp, College Station, TX, 2011).
Results
A total of 7177 patients with stage T1aN0M0 kidney cancer were identified who met the inclusion criteria with 754(10.5%), 1849(25.8%), and 4574(63.7%) undergoing NSM, PN, and RN, respectively. Statistically significant differences existed for demographic and comorbidity variables between groups (Supplemental Table). NSM patients were older, more often African-American, unmarried, and had higher CCI relative to surgical patients. Time to surgery was <1 month from diagnosis for 66% of surgical patients, and <1% had surgery in the sixth month. Microscopic confirmation of disease was recorded for 51.6%, 98.1%, and 99.9% of NSM, PN, and RN patients, respectively, with radiologic evidence for the remainder.
A total of 436(57.8%), 389(21.0%), and 1598(34.9%) patients died from any cause after NSM, PN, and RN, respectively, with an overall median follow-up of 56 months (interquartile range 38–85 months). The number of deaths attributed to kidney cancer were 68(9.0%), 39(2.1%), and 227(5.0%) at a mean follow-up of 43 months. The observed OS and CSS rates at 1, 3, and 5-years were generally lower for NSM compared to either surgical intervention (Table 1). Kaplan-Meier survival curves showed worse survival for NSM compared to surgery, and lower survival for RN compared to PN by log-rank test for both OS and CSS (p-value<0.01) (Supplementary Figure). However, the proportion of observed deaths attributed to kidney cancer was comparable between all three groups with an overall average of 20.4% χ2=2.25, p=0.32).
Table 1.
Kaplan-Meier overall and cancer-specific survival probabilities at 1, 3, and 5 years and cumulative incidence of death at 5 years by treatment strategy for early-stage kidney cancer patients 65 years or older, SEER-Medicare 1995–2007.
| Kaplan-Meier Survival Function | Years Postb | Treatment Strategya (N) | ||
|---|---|---|---|---|
| NSM (754) | PN (1849) | RN (4574) | ||
| OS | ||||
| 1 | 88.1% | 98.3% | 96.8% | |
| 3 | 63.3% | 91.8% | 86.7% | |
| 5 | 46.4% | 83.1% | 76.1% | |
| Mean (SDc) follow-up (months) | 44.7 (29.9) | 61.5 (29.9) | 67.9 (37.0) | |
| CSS | ||||
| 1 | 95.9% | 99.6% | 99.2% | |
| 3 | 89.4% | 98.1% | 96.5% | |
| 5 | 82.5% | 96.7% | 93.5% | |
| Mean (SDc) follow-up (years) | 30.0 (26.9) | 37.4 (29.1) | 47.3 (35.3) | |
|
Cumulative Incidence
| ||||
| All-Cause Mortality | 5 | 36.2% | 9.5% | 16.7% |
| Kidney Cancer Mortality | 5 | 8.5% | 1.6% | 3.9% |
NSM: Nonsurgical management; PN: Partial nephrectomy; RN: Radical nephrectomy
Year of diagnosis is considered surgery year for NSM patients
Standard deviation; follow-up in years through 5/31/2010 for OS and 12/31/2007 for CSS
The final adjusted Cox proportional hazards model indicated a significantly lower hazard of death from any cause for PN compared to NSM [Hazard Ratio (95% confidence interval) 0.40(0.34–0.46)] as well as RN compared to NSM [HR0.50(0.45–0.56)] with an additional survival benefit noted for PN over RN [HR0.80(0.71–0.90)] (Table 2). Competing risks regression revealed similar relationships for CSS with a lower rate of death due to kidney cancer for surgery compared to NSM (p<0.01) and notable CSS benefit for PN over RN [HR0.67(0.47–0.95)]. Propensity-score matched analysis confirmed the findings from the above models. Model predicted survival improvement for PN over NSM was 1.0%, 4.1%, and 7.2% at 1, 3, and 5 years, respectively, corresponding to respective numbers needed to treat of 100, 24, and 14 patients with PN to avoid 1 kidney cancer death.
Table 2.
Overall cohort survival analysis utilizing competing risks regression for CSS and propensity score matching and age-stratified analysis for early-stage kidney cancer patients 65 years or older, SEER-Medicare 1995–2007.
| OVERALL COHORT
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivalc | Groupd | Unadjusted | Adjusteda | Propensity Score Matchedb | ||||||
| HR | (95% CI) | p-value | HRa | (95% CI) | p-value | HRb | (95% CI) | p-value | ||
| OS | PN vs NSM | 0.25 | (0.22–0.29) | <0.01 | 0.40 | (0.34–0.46) | <0.01 | 0.36 | (0.30–0.44) | <0.01 |
| RN vs NSM | 0.36 | (0.32–0.40) | <0.01 | 0.50 | (0.45–0.56) | <0.01 | 0.50 | (0.42–0.58) | <0.01 | |
| PN vs RN | 0.70 | (0.63–0.78) | <0.01 | 0.80 | (0.71–0.90) | <0.01 | 0.86 | (0.75–0.99) | 0.04 | |
| CSS | PN vs NSM | 0.24 | (0.16–0.36) | <0.01 | 0.42 | (0.27–0.64) | <0.01 | 0.34 | (0.19–0.59) | <0.01 |
| RN vs NSM | 0.44 | (0.34–0.58) | <0.01 | 0.62 | (0.46–0.85) | <0.01 | 0.60 | (0.40–0.89) | <0.01 | |
| PN vs RN | 0.55 | (0.39–0.77) | <0.01 | 0.67 | (0.47–0.95) | 0.03 | 0.62 | (0.42–0.93) | 0.02 | |
|
| ||||||||||
|
AGE-STRATIFIED ANALYSIS
| ||||||||||
| <75 Years Old | 75–<80 Years Old | ≥80 Years Old | ||||||||
| HRa | (95% CI) | p-value | HRa | (95% CI) | p-value | HRa | (95% CI) | p-value | ||
|
| ||||||||||
| OS | PN vs NSM | 0.44 | (0.35–0.56) | <0.01 | 0.33 | (0.25–0.43) | <0.01 | 0.36 | (0.27–0.48) | <0.01 |
| RN vs NSM | 0.51 | (0.42–0.63) | <0.01 | 0.38 | (0.30–0.48) | <0.01 | 0.55 | (0.46–0.66) | <0.01 | |
| PN vs RN | 0.87 | (0.73–1.02) | 0.09 | 0.86 | (0.70–1.07) | 0.80 | 0.65 | (0.50–0.84) | <0.01 | |
| CSS | PN vs NSM | 0.64 | (0.28–1.46) | 0.29 | 0.27 | (0.12–0.58) | <0.01 | 0.41 | (0.19–0.91) | 0.03 |
| RN vs NSM | 0.86 | (0.42–1.74) | 0.67 | 0.40 | (0.22–0.73) | <0.01 | 0.68 | (0.45–1.04) | 0.08 | |
| PN vs RN | 0.75 | (0.45–1.26) | 0.28 | 0.66 | (0.34–1.30) | 0.23 | 0.60 | (0.29–1.26) | 0.18 | |
Cox regression adjusted for age, sex, race, residence, marital status, Charlson comorbidity index, baseline end-stage renal disease, tumor size, and year of diagnosis; Fine and Gray competing risks regression used to obtain subhazard ratios for CSS; similar results obtained using propensity score matching for age-stratified cohort
One-to-one propensity score matching balancing age, sex, race, residence, marital status, Charlson comorbidity index, baseline end-stage renal disease, and tumor size
OS: Overall survival; CSS: Kidney cancer-specific survival
Charlson comorbidity index
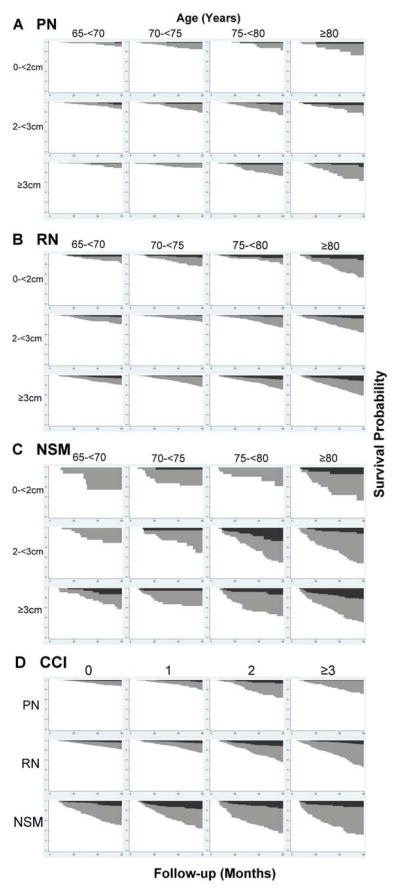
Subgroup analyses revealed notable trends in mortality by tumor size, age group, and CCI (Figure). A greater proportion of kidney cancer deaths occurred with increasing tumor size for NSM patients (5.2%,8.5%, and 10.8% for <2cm,2–<3cm, and ≥3cm, respectively), but it was not significant on multivariable analysis. Other-cause mortality increased substantially with age and CCI for all treatment categories. Observed kidney cancer mortality was generally comparable across strata of CCI for NSM (χ2=3.85, p=0.43). A greater proportion of individuals ≥75 years of age compared to ≤75 years died of kidney cancer (13.2% vs. 4.6%, p<0.01) suggesting potential effect modification by age. Cox regression showed OS still generally favored PN over RN as well as surgery over NSM in the age-stratified analyses (Table 2). However, kidney cancer mortality was not significantly different between any two treatment groups for patients ≤75 years of age as measured by hazard ratios for CSS. The results for patients ≥75 years old were comparable to the overall cohort analysis and driven by the 75–79 year age group.
Figure.
Survival Curves (white) Stratified by Age and Tumor Size for (A) PN, (B) RN, and (C) NSM and Stratified by CCI (D) with overlaid inverted Cumulative Incidence Functions for Kidney Cancer Mortality (black) and Other-Cause Mortality (grey). NSM = nonsurgical management, PN = partial nephrectomy, RN = radical nephrectomy, CCI = Charlson comorbidity index.
Comment
While extirpation is the standard of care for most patients with SRMs, there is a growing need to define the role and criteria for AS in the management of early-stage kidney cancer [17]. The current population-based practice is an important starting point to identify areas for improvement. In our cohort, other-cause mortality was substantial for patients undergoing NSM resulting in a low 5-year OS probability (46.4%). NSM patients had significantly worse CSS compared with either PN or RN, however, the ratio of observed cancer-related to all-cause deaths was consistent among all three treatment groups. An additional key finding of this analysis is the counterintuitive observation that CSS was equivalent among treatment groups for patients <75 years of age, but significantly worse for ≥75 year old patients undergoing NSM. This may indicate that younger patients (<75 years) are well-selected for NSM based on age, comorbidity, and tumor status to optimize CSS, but the selection process for older patients (≥75 years) can be improved before counseling a patient that it is safe to forego surgery. Other factors besides age likely contributed to the decision to defer therapy for patients <75 years. Chief among them are competing risks of death given the poor health status of many of the NSM patients resulting in a poor probability of survival.
We stress that patients who undergo NSM at the population-level may not be representative of patients selected for AS in contemporary protocols. Prior studies suggesting low rates of metastasis and death have been institutional with carefully selected patients undergoing regimented surveillance protocols while the present cohort is a representative population-based sample without defined criteria for AS [12–16,23–25]. On one end of the spectrum, NSM patients may represent a “worst-case scenario” for AS and present a unique opportunity to assess current management in the United States and fuel efforts for improvement. On the other end, a small percentage of the NSM cohort might be diluted by benign disease as not all tumors were biopsied. However, this is likely balanced by possible understaging because patients on NSM may not receive an extensive staging workup, and a small percentage of clinical T1a tumors are upstaged to pathologic T3, which would be excluded in the surgical cohort. Nevertheless, understaging is unlikely to fully account for the 9% of NSM patients dying from kidney cancer during follow-up, which should be a parallel issue for the younger strata. Older patients may also have tumors of a more aggressive biology with the hypothesis being relative immunodeficiency associated with aging facilitates progressive growth of kidney cancer [26]. Regardless of the explanation, the general implication is that greater attention needs to be paid to the risk-stratification (tumor characteristics, comorbidities, etc.) of patients ≥75 years of age as life-expectancy alone is not a valid justification to forego intervention.
Our study revealed an increase in use of NSM from 9% in 1995 to 14% in 2007 suggesting it is being increasingly considered in patients with competing health risks [8,17]. A recent study by Sun et al. reported similar findings for improved OS and CSS for patients undergoing intervention compared to NSM for the cohort of T1 kidney cancer patients as well as the T1a subset, but the difference among patients ≥75 years was not statistically significant [18]. There are a few explanations for the discrepancy. First, their cohort consists of patients from 1988 to 2005 while the present sample is taken from 1995 to 2007, which is important considering changes in the accuracy and coding of SEER data over time. Second, our inclusion criteria were more strict to identify a NSM cohort representative of current population-based practice of <4cm tumors, which differs from Sun et al.’s cohort where, surprisingly, over three times as many patients with <7cm tumors were included in the NSM group (3271) compared to those receiving PN (1051).
Lastly, Sun et al. used an instrumental variable analysis, which they recognize is questionable due to increased variability in the estimated effect and requirement for unverified assumptions. In fact, their test statistics did show worse HRs in the ≥75 year old T1a subset than in the overall cohort comparing surgery to NSM, but the reduced sample size and variability induced by the instrumental variable led to wide confidence intervals. Propensity-score matching provides a check in balancing known covariates between groups when a large selection bias exists and might be a preferred method when a satisfactory instrument cannot be found. The competing risks analysis is a major strength of our approach because traditional techniques cannot account for the exclusion of an outcome of interest (kidney-cancer mortality) due to the occurrence of another outcome (other-cause mortality). Taking the results of Sun et al. together with our results, NSM, as it occurs on the population-level in the United States, appears to be a calculated risk to avoid unnecessary treatment in a larger group of patients. In the absence of a prospective trial, confounders will always exist, and at the very least our analysis demonstrates potential harm from universal application of NSM in the elderly. It should be noted that an unpublished analysis by Huang et al. recently reported contradictory results to both studies under discussion with improved OS and fewer cardiovascular events in the NSM cohort (Abstract 1183, American Urological Association Annual Meeting 2013). The explanation is difficult to surmise with the high comorbidity burden of the NSM cohort and similar data utilized between studies.
Other population-based studies demonstrate an OS benefit for PN as compared to RN – a finding our analysis corroborates. We also found a CSS benefit for PN vs. RN [HR0.67(0.47–0.95)], a controversial finding that varies by methodology of prior studies [5,7,27]. Several explanations for the OS and CSS benefit of PN are possible, the most highly purported being selection bias (as high-risk tumors are more likely to undergo RN), implications of nephron-sparing surgery for cardiovascular risk, and perhaps tumor biology, which SEER-Medicare data does not capture [6,28–30]. Notably, absolute difference in cumulative incidence of kidney cancer death between RN and PN was small (2.3% at 5- years).
There are several important limitations to our study. First, patients were identified retrospectively, and SEER lacks specific disease characteristics or intraoperative findings such as tumor location. Cause of death data form registries is also subject to misattribution. Second, there may be residual confounding due to unknown factors and limitations on a categorical index such as CCI calculated from claims data to fully account for comorbidity risk. Third, Medicare data are restricted to patients ≥65 years of age, and results may not be applicable to patients <65 years old who present with SRMs. Finally, as mentioned the NSM population may be enriched by benign pathology, but this would result in a more conservative estimate of CSS (biased toward the null).
It is important to specifically consider the influence of confounding by indication. The best candidates for NSM may be older individuals with multiple comorbidities, which imposes an inherent bias for comparative effectiveness as these patients experience significant all-cause mortality. However, these competing risks of death may be necessary to reduce the proportion of deaths due to kidney cancer. Younger patients diagnosed with kidney cancer are more likely to undergo intervention, but they may also have lower risk disease if the small renal tumor is caught earlier in the natural history. Unmeasured confounders, as mentioned, also play a role in the selection bias. A urologist’s gestalt about a patient’s life expectancy or suitability for surgery may be difficult to quantify objectively. Lastly, patient preference or inability to access care might lead to a decision to undergo NSM when it would not regularly be advised. Therefore, the results should not be construed to represent a controlled comparison of AS to surgery but as a comparison of the current population-based practice.
These limitations underscore a need for prospective institutional data to capture the rationale for immediate treatment, delayed intervention, and continued non-surgical management. Because a randomized trial to eliminate selection bias is not forthcoming, large retrospective cohort studies may provide the best assessment with clinical relevance and statistical power. Notwithstanding the limitations, our analysis attempts to generalize to the 50 million Medicare beneficiaries in the United States at risk for developing early-stage kidney cancer.
Conclusions
NSM for early-stage kidney cancer was associated with an increased risk of cancer death compared to surgery among Medicare beneficiaries, but there was a much greater risk of death from other causes. No difference in CSS was found by treatment approach for patients <75 years of age, indicating that these patients are well selected for treatment. Improved risk-stratification may be needed for patients ≥75 years of age as a significant proportion may still die of kidney cancer. The growing acceptance and use of observation for SRMs requires continued research into specific comorbidities and competing risks of death to help patients make informed decisions.
Supplementary Material
Supplementary Figure. Kaplan-Meier Survival Curves for (A) Overall and (B) Cancer-Specific Survival. NSM = nonsurgical management, PN = partial nephrectomy, RN = radical nephrectomy.
Supplemental Table. Demographics, comorbidity indices, and tumor characteristics by treatment strategy in early-stage kidney cancer patients 65 years or older, SEER-Medicare 1995-2007.
Abbreviations and Acronyms
- CCI
Charlson comorbidity index
- CSS
cancer-specific survival
- HR
hazard ratio
- NSM
nonsurgical management
- OS
overall survival
- PN
partial nephrectomy
- RN
radical nephrectomy
- SEER
Surveillance, Epidemiology and End Results
- SRM
small renal mass
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathew A, Devesa SS, Fraumeni JF, Jr, et al. Global increases in kidney cancer incidence, 1973–1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Pettus JA, Jang TL, Thompson RH, et al. Effect of baseline glomerular filtration rate on survival in patients undergoing partial or radical nephrectomy for renal cortical tumors. Mayo Clin Proc. 2008;83:1101–1106. doi: 10.4065/83.10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183:1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: Effect on overall and noncancer mortality. Cancer. 2009;115:1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 7.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–553. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Abdollah F, Bianchi M, et al. Treatment management of small renal masses in the 21st century: a paradigm shift. Ann Surg Oncol. 2012;19:2380–2387. doi: 10.1245/s10434-012-2247-0. [DOI] [PubMed] [Google Scholar]
- 9.Patel HD, Mullins JK, Pierorazio PM, et al. Trends in Renal Surgery: Robotic Technology Is Associated with Increased Use of Partial Nephrectomy. J Urol. 2013;189:1229–1235. doi: 10.1016/j.juro.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57–60. [PubMed] [Google Scholar]
- 11.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: A need to reassess treatment effect. JNCI. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 12.Jewett MAS, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: Progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39–44. doi: 10.1016/j.eururo.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Pierorazio PM, Hyams ES, Mullins JK, et al. Active surveillance for small renal masses. Rev Urol. 2012;14:13–19. [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: Meta-analysis and review of the world literature. J Urol. 2006;175:425–431. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 15.Smaldone MC, Uzzo RG. Active surveillance: A potential strategy for select patients with small renal masses. Future Oncol. 2011;7:1133–1147. doi: 10.2217/fon.11.97. [DOI] [PubMed] [Google Scholar]
- 16.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: A systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zini L, Perrotte P, Jeldres C, et al. A population-based comparison of survival after nephrectomy vs nonsurgical management for small renal masses. BJU Int. 2009;103:899–904. doi: 10.1111/j.1464-410X.2008.08247.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, Becker A, Tian Z, et al. Management of Localized Kidney Cancer: Calculating Cancer-specific Mortality and Competing Risks of Death for Surgery and Nonsurgical Management. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.03.034. (published online ahead of print) [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9:92. doi: 10.1186/1472-6963-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper GS, Virnig B, Klabunde CN, et al. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40:IV-43–IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed January 15, 2013];SEER-Medicare: Calculation of Comorbidity Weights. http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Abouassaly R, Lane BR, Novick AC. Active surveillance of renal masses in elderly patients. J Urol. 2008;180:505–509. doi: 10.1016/j.juro.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Rosales JC, Haramis G, Moreno J, et al. Active surveillance for renal cortical neoplasms. J Urol. 2010;183:1698–1702. doi: 10.1016/j.juro.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Youssif TA, Kassouf W, Steinberg J, et al. Active surveillance for selected patients with renal masses: Updated results with long-term follow-up. Cancer. 2007;110:1010–1013. doi: 10.1002/cncr.22871. [DOI] [PubMed] [Google Scholar]
- 26.Scoll BJ, Wong YN, Egleston BL, et al. Age, tumor size and relative survival of patients with localized renal cell carcinoma: a surveillance, epidemiology and end results analysis. J Urol. 2009;181:506–511. doi: 10.1016/j.juro.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol. 2012;188:51–57. doi: 10.1016/j.juro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors- Is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–62. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20:1341–1350. doi: 10.1681/ASN.2008090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Kaplan-Meier Survival Curves for (A) Overall and (B) Cancer-Specific Survival. NSM = nonsurgical management, PN = partial nephrectomy, RN = radical nephrectomy.
Supplemental Table. Demographics, comorbidity indices, and tumor characteristics by treatment strategy in early-stage kidney cancer patients 65 years or older, SEER-Medicare 1995-2007.