ABSTRACT
Polyomaviruses are ubiquitous pathogens that cause severe disease in immunocompromised individuals. JC polyomavirus (JCPyV) is the causative agent of the fatal demyelinating disease progressive multifocal leukoencephalopathy (PML), whereas BK polyomavirus (BKPyV) causes polyomavirus-induced nephropathy and hemorrhagic cystitis. Vaccines or antiviral therapies targeting these viruses do not exist, and treatments focus on reducing the underlying causes of immunosuppression. We demonstrate that retro-2cycl, an inhibitor of ricin and Shiga-like toxins (SLTs), inhibits infection by JCPyV, BKPyV, and simian virus 40. Retro-2cycl inhibits retrograde transport of polyomaviruses to the endoplasmic reticulum, a step necessary for productive infection. Retro-2cycl likely inhibits polyomaviruses in a way similar to its ricin and SLT inhibition, suggesting an overlap in the cellular host factors used by bacterial toxins and polyomaviruses. This work establishes retro-2cycl as a potential antiviral therapy that broadly inhibits polyomaviruses and possibly other pathogens that use retrograde trafficking.
IMPORTANCE
The human polyomaviruses JC polyomavirus (JCPyV) and BK polyomavirus (BKPyV) cause rare but severe diseases in individuals with reduced immune function. During immunosuppression, JCPyV disseminates from the kidney to the central nervous system and destroys oligodendrocytes, resulting in the fatal disease progressive multifocal leukoencephalopathy. Kidney transplant recipients are at increased risk of BKPyV-induced nephropathy, which results in kidney necrosis and loss of the transplanted organ. There are currently no effective therapies for JCPyV and BKPyV. We show that a small molecule named retro-2cycl protects cells from infection with JCPyV and BKPyV by inhibiting intracellular viral transport. Retro-2cycl treatment reduces viral spreading in already established infections and may therefore be able to control infection in affected patients. Further optimization of retro-2cycl may result in the development of an effective antiviral therapy directed toward pathogens that use retrograde trafficking to infect their hosts.
INTRODUCTION
Human polyomaviruses are widespread pathogens that establish persistent lifelong infections in their hosts (1, 2). JC polyomavirus (JCPyV) and BK polyomavirus (BKPyV) establish persistent infections early in life and chronically infect kidney cells, urinary tract cells, tonsillar stromal cells, and bone marrow-derived cells (3–6). The seroprevalences of JCPyV and BKPyV are 50 and 80%, respectively (7). It is likely that JCPyV and BKPyV persistently replicate at low levels, as virus is sporadically detected in the urine of 30% of the individuals tested (8).
Under conditions of immunosuppression, such as AIDS or immunomodulatory therapy, increased replication of JCPyV results in dissemination of the virus to the central nervous system (5). Lytic infection of oligodendrocytes by JCPyV results in the fatal demyelinating disease progressive multifocal leukoencephalopathy (PML) (9). The incidence of PML in AIDS patients is between 3 and 5%, and the incidence in patients receiving immunomodulatory therapies is between 0.2 and 0.4% (5). BKPyV-associated disease is most often seen in the context of renal transplantation, where immunosuppressive therapies result in increased replication of BKPyV in the transplanted kidney, leading to hemorrhagic cystitis and polyomavirus-induced nephropathy (PVN) (10). The incidence of PVN in transplant recipients can be as high as 10%, often resulting in loss of the transplanted kidney (11). There are no effective antiviral therapies to combat polyomavirus infection.
Despite being structurally simple, polyomaviruses use a complex and incompletely understood entry process to effect transport to the nucleus, where viral transcription and DNA replication occur. After binding to cellular receptors on the cell surface, polyomaviruses enter the classical endocytic pathway (12–14). From early or late endosomes, all of the polyomaviruses studied to date undergo transport to the endoplasmic reticulum (ER), where they interact with ER chaperones to partially disassemble their capsid, resulting in retrotranslocation of the virion into the cytosol (15–19). Despite the importance on ER trafficking, the specific host cellular machinery used to promote ER targeting of virions remains unclear.
In this study, we demonstrate that the small molecule 2-{[(5-methyl-2-thienyl)methylene]amino}-N-phenylbenzamide (retro-2cycl) potently inhibits the infection of tissue culture cells by JCPyV, BKPyV, and the closely related primate virus simian virus 40 (SV40). Retro-2cycl was previously identified by high-throughput screening for small molecules that inhibit the intoxication of host cells by ricin, Shiga-like toxins (SLTs), and the cholera toxin B subunit (CTxB) (20). Retro-2cycl inhibits retrograde trafficking from endosomes to the Golgi apparatus, thus preventing the intoxication of host cells. Rather than binding to the toxins, retro-2cycl interferes with retrograde trafficking of cargo by interaction with an unidentified cellular host factor. We demonstrate that retro-2cycl inhibits polyomavirus infection by inhibiting ER transport, suggesting that polyomaviruses, ricin, SLTs, and CTxB share a dependency on similar retro-2cycl sensitive host factors for successful intracellular transport. Recently, we found that retro-2cycl also inhibits the infection of cells by human papillomaviruses (21). Further optimization of this compound may result in the development of effective antiviral compounds that inhibit the infection of cells by viruses requiring ER transport for infection.
RESULTS
Retro-2cycl inhibits polyomavirus infection in a dose-dependent manner.
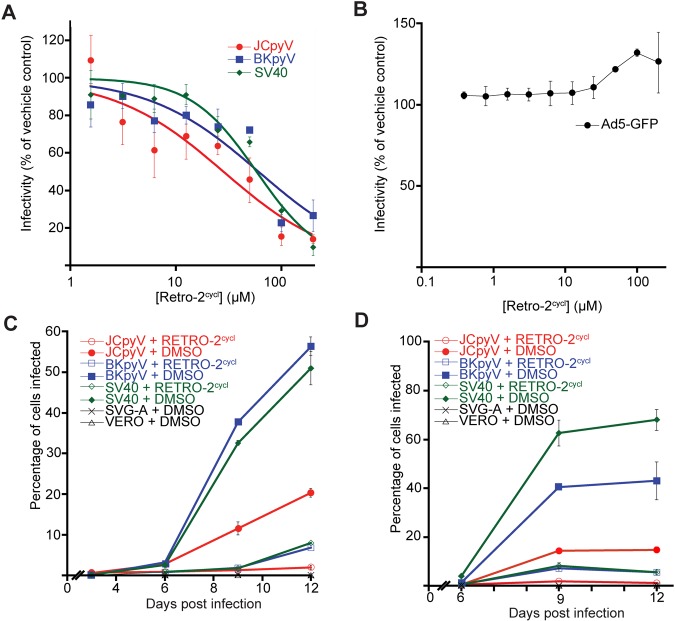
To determine whether retro-2cycl inhibits infection by polyomaviruses, we pretreated permissive cells with retro-2cycl and infected them with JCPyV, BKPyV, or SV40 (20). Retro-2cycl treatment resulted in a dose-dependent decrease in infected cells compared to a vehicle control, with calculated 50% effective concentrations of 28.4, 61.2, and 58.6 µM for JCPyV, BKPyV, and SV40, respectively (Fig. 1A). As a control, we pretreated Vero cells with retro-2cycl and inoculated them with a green fluorescent protein (GFP)-expressing adenovirus (Ad5-GFP), which is known not to require retrograde trafficking (22). Retro-2cycl treatment did not inhibit adenovirus-mediated transduction, suggesting that the effect of retro-2cycl on infection is specific to virions that undergo retrograde trafficking (Fig. 1B). We additionally infected a panel of cell lines with JCPyV pseudovirus expressing a luciferase reporter gene. Retro-2cycl exhibits similar levels of inhibition in the brain-derived SVG-A, HFGT, and POJ19 cell lines, as well as the kidney-derived Vero, Cos-7, 293A, and 293FT cell lines, indicating that retro-2cycl is protective of numerous cell lines derived from multiple organs (see Fig. S1 in the supplemental material). Retro-2cycl exhibited low cellular toxicity at protective levels (see Fig. S2).
FIG 1 .
Retro-2cycl prevents infection with three polyomaviruses. (A) Dose-dependent effect of retro-2cycl treatment on infection. Cells were preincubated with the indicated concentrations of retro-2cycl prior to inoculation with virus. Infections were scored and normalized to a DMSO-treated sample. (B) Retro-2cycl does not block infection with adenovirus pseudovirus. Vero cells were pretreated with equivalent concentrations of retro-2cycl prior to infection with an Ad5-GFP pseudovirus. Cells were scored and normalized to a DMSO-treated sample. (C) Retro-2cycl prevents virus spreading in a multicycle growth assay. Cells were infected with virus for 72 h. Cells were then maintained in 0.1 mM retro-2cycl. Cells were scored for infection every 3 days. (D) Retro-2cycl-treated cultures release less infectious virus into culture medium. Tissue culture medium was harvested every 3 days and used to infect naive cells not treated with retro-2cycl. The data represent the mean of three replicates, and error bars indicate the standard deviation.
Retro-2cycl is able to reduce viral spreading in established tissue culture infections.
Since most individuals are persistently infected with JCPyV or BKPyV prior to immunosuppression, we asked whether retro-2cycl could prevent viral spreading in established tissue culture infections. Cells were infected at a low multiplicity of infectivity (MOI) of 0.01. Following one round of productive infection, 100 µM retro-2cycl was added to these cells and maintained during the course of the experiment. Treatment of cells resulted in a significant reduction of infected cells compared to the vehicle control, and this effect was most striking at 12 days postinfection, where retro-2cycl diminished the spreading of SV40 (84% inhibition), BKPyV (89%), and JCPyV (90.5%) (Fig. 1C). To examine whether the treatment of these cultures with retro-2cycl inhibited virion production, supernatants from each time point were used to reinfect naive cells that were not retro-2cycl treated. Cultures that were previously treated with retro-2cycl produced significantly less infectious virions (Fig. 1D), demonstrating that retro-2cycl decreases the cell-to-cell spreading of polyomaviruses in previously infected cultures.
The bioactive compound is a DHQ derivative of retro-2.
After showing that commercially purchased retro-2 inhibits polyomavirus infection, we chemically synthesized the compound by a previously reported method in order to facilitate subsequent investigations (23). Condensation of 2-aminobenzanilide with 4-methyl-2-thiophencarboxaldehyde in the last step of synthesis yielded a mixture of two products, both having the expected molecular weight of retro-2 (see Fig. S3A in the supplemental material). The two compounds were separated and independently characterized. One product was retro-2, as indicated by a characteristic singlet at 11.0 ppm for the imine proton, as determined by nuclear magnetic resonance analysis (data not shown). The second product was revealed to be a dihydroquinazolinone (DHQ) by X-ray diffraction crystallography and is termed retro-2cycl (Fig. 2A). The spectroscopic data for the compound purchased from ChemBridge were identical to those for the DHQ derivative and not to those of the structure reported for retro-2 (data not shown). To test the inhibitory effects of both compounds on JCPyV infection, SVG-A cells were incubated with 100 µM retro-2 or retro-2cycl and challenged with JCPyV. Surprisingly, both retro-2 and retro-2cycl inhibited polyomavirus infection with similar efficacies (Fig. 2B).
FIG 2 .
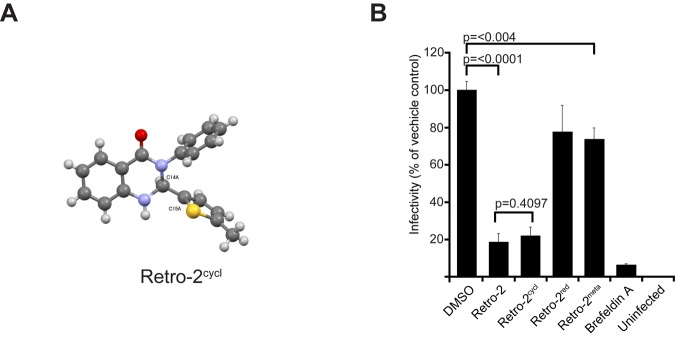
Structure of retro-2cycl and inhibitory activities of retro-2 analogs. (A) The structure of retro-2cycl was solved by X-ray diffraction, and it was verified to be a DHQ. (B) Retro-2cycl protects cells from polyomavirus infection. Cells were pretreated with retro-2, the indicated analog, the vehicle control, or BFA (71 nM, 20 ng/ml) and infected with JCPyV. Infected cells were scored and normalized to the vehicle-treated control. The data represent the average of triplicate samples. Error bars indicate the standard deviation.
Imine species similar in structure to retro-2 are commonly invoked as mechanistic intermediates in the formation of DHQ (24–28), which suggests that the two chemical species could interconvert in the infectivity assay, thus accounting for their similar biological activities. Accordingly, we found that treatment of retro-2 with scandium(III) trifluoromethanesulfonate in methanol resulted in rapid conversion to the DHQ (data not shown). In aqueous medium, retro-2 most likely cyclizes into a DHQ as well. During the preparation of this report, another group reported that retro-2 slowly cyclizes in methanol and named this compound “Retro-2cycl” (29).
It was still unclear whether the biologically active compound was an imine or a DHQ. Treatment of retro-2cycl with sodium cyanoborohydride in methanol slowly produced a reduced species (retro-2red), which indicated that cyclization is reversible and that retro-2 and retro-2cycl exist in equilibrium (see Fig. S3B in the supplemental material). However, despite their structural similarities, retro-2red is significantly less active than retro-2 (Fig. 2B). We also prepared a retro-2 regioisomer wherein the carboximide and imine moieties are meta substituted (retro-2meta), therefore precluding cyclization (see Fig. S3C). This compound was also significantly less active and served as a useful negative control in subsequent experiments. Together, the lack of biological activity intrinsic to retro-2red, as well as retro-2meta, leads us to the conclusion that the chemical species responsible for inhibition of polyomavirus infection is, in fact, the DHQ, retro-2cycl.
Retro-2cycl inhibits polyomavirus infectivity at early time points during infection.
We hypothesized that retro-2cycl inhibited retrograde trafficking of polyomavirus to the ER. SVG-A or Vero cells were synchronously infected with JCPyV, BKPyV, or SV40, and retro-2cycl was added to cells at the indicated time points. The results show that the addition of retro-2cycl at time points up to 4 h postinfection (hpi) significantly reduces infectivity, with a progressive loss of its inhibitory effect from 6 to 18 hpi (Fig. 3A). These kinetics are consistent with previous reports showing that polyomaviruses colocalize with ER markers at 6 to 16 hpi, demonstrating that the protective effect of retro-2cycl is lost following time points consistent with localization to the ER (13–15, 30). To rule out an effect on virus binding, we treated cells with retro-2cycl or retro-2meta and measured the binding of labeled virus or cholera toxin to cells by flow cytometry. Treatment of the cells with retro-2cycl or retro-2meta had no effect on the binding of CTxB or JCPyV to cells but slightly reduced the binding of BKPyV and SV40 (Fig. 3B). The reduction of SV40 binding is not due to a reduction of cell surface receptor expression, as CTxB binding to GM1, also the receptor for SV40, was not reduced (Fig. 3B). Retro-2cycl also does not interact directly with either SV40 or BKPyV, as incubation of retro-2cycl with either virus does not decrease the ability to bind to cells (see Fig. S4 in the supplemental material). Retro-2cycl also had no effect on the endocytosis of JCPyV, BKPyV, SV40, or CTxB (see Fig. S5).
FIG 3 .
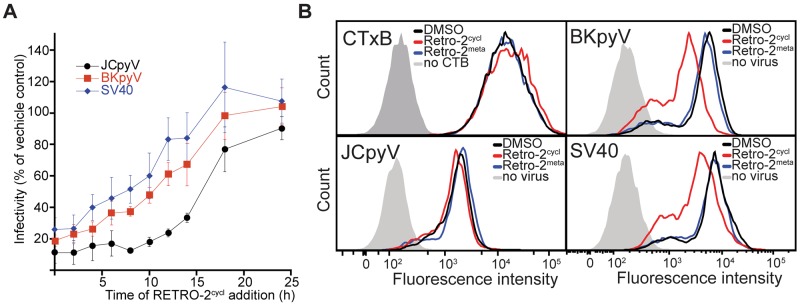
Retro-2cycl inhibits polyomavirus infectivity at early time points during infection. (A) Cells were chilled prior to incubation with JCPyV, BKPyV, or SV40 to synchronize infections, and retro-2cycl (0.1 mM) was added at the indicated time points. After 72 h, infected cells were scored and normalized to the vehicle control. The data represent the mean of three replicates, and error bars indicate the standard deviation. (B) Effect of retro-2cycl on virus binding to cells. Cells were detached and pretreated with the vehicle control or retro-2cycl for 30 min at 37°C. Cells were then inoculated with Alexa Fluor 633-labeled JCPyV, BKPyV, SV40, or CTxB for 1 h on ice. Samples were then washed and read by flow cytometry. CTB, CTxB.
Retro-2cycl reduces retrograde trafficking of polyomaviruses to the ER.
Since retro-2cycl prevents intracellular trafficking of ricin, SLTs, and CTxB, we sought to determine whether treatment of cells with retro-2cycl would interfere with the delivery of virions to the ER. Cells were preincubated with retro-2cycl or a vehicle control and then inoculated with virus. Cells were then fixed at 8 hpi and immunostained for the ER protein PDI (protein disulfide isomerase) and VP1. To determine the colocalization of these two proteins, we used a proximity ligation assay (PLA) that generates a fluorescent signal only when the target proteins are within 40 nm of each other. Colocalization of polyomaviruses and PDI was readily detectable in cells treated with the vehicle control (Fig. 4A and B). In contrast, treatment of cells with brefeldin A (BFA) or retro-2cycl significantly reduces the PLA signal between VP1 and PDI, demonstrating that virion ER transport is inhibited (Fig. 4B). When fluorescently labeled BKPyV is added to cells in the presence of retro-2 cycl, redistribution of virions from a reticular, ER-type pattern toward the cell periphery is observed, further demonstrating that retro-2cycl alters virion transport (see Fig. S6 in the supplemental material).
FIG 4 .
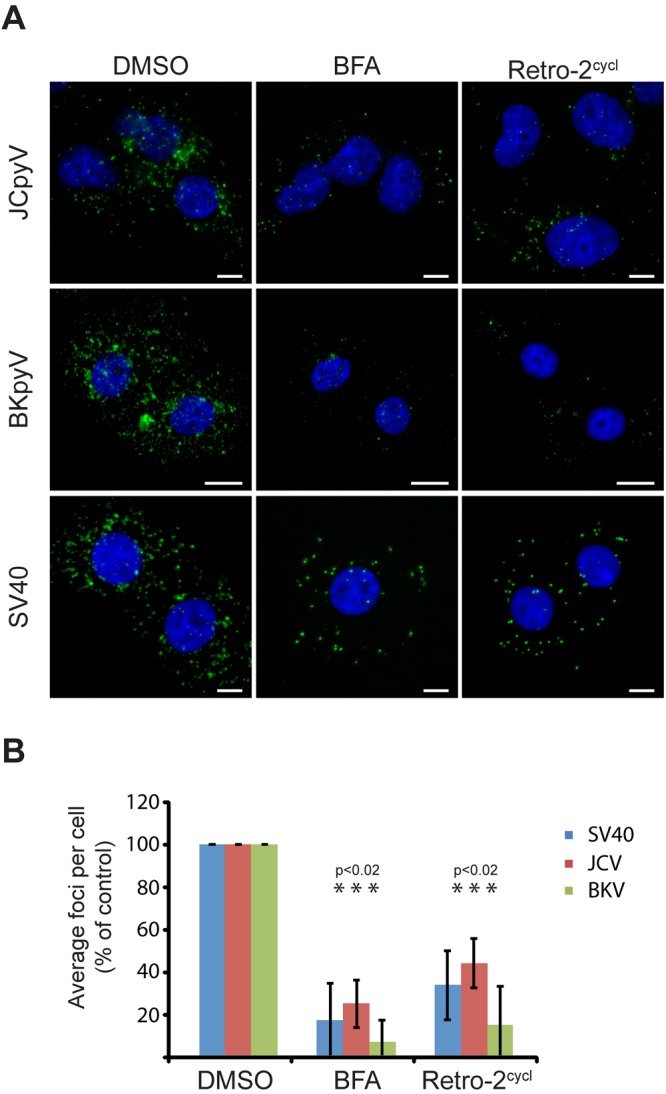
Retro-2cycl inhibits polyomavirus ER trafficking. (A) Colocalization was assessed with a PLA. Error bars denote the standard deviation. (B) Cells were pretreated with the indicated drug (500 ng/ml BFA or 0.1 mM retro-2cycl) for 0.5 h prior to inoculation with JCPyV, BKPyV, or SV40 at an MOI of 100. Cells were incubated for 8 h with the indicated drugs, fixed, and permeabilized. Cells were then stained with a mouse monoclonal antibody to PDI and a rabbit polyclonal antibody to VP1 prior to detection by PLA. Fluorescent foci indicate areas of colocalization. BKV, BKPyV.
Retro-2cycl prevents exposure of the viral minor capsid proteins.
Upon trafficking to the ER, polyomaviruses interact with host cell chaperones to isomerize their interpentameric disulfide bonds and expose the previously sequestered minor capsid protein VP2 (18, 19, 31, 32). Exposure of VP2 can therefore further verify whether ER transport is inhibited in retro-2cycl-treated cells. We inoculated cells with JCPyV, BKPyV, or SV40 in the presence of 100 µM retro-2cycl or the vehicle control. In cells treated with the dimethyl sulfoxide (DMSO) control, discrete puncta corresponding to VP2 were visualized at 10 hpi in a perinuclear region of the cells (Fig. 5A), similar to previously published reports (19). In contrast, treatment of cells with BFA results in a significant reduction in the number of cells with VP2 exposed, demonstrating that ER transport is required to expose VP2. Treatment of cells with retro-2cycl, but not poorly neutralizing retro-2meta, resulted in a significant reduction in the proportion of cells exposing VP2 to levels similar to those of cells treated with BFA (Fig. 5A and C). These puncta colocalize with the ER rather than other organelles such as lysosomes, a result that is consistent with previous studies (Fig. 5B) (19). We also observed a decrease in VP2 and PDI association by PLA after retro-2cycl treatment, further demonstrating that VP2 is exposed in the ER (see Fig. S7 in the supplemental material). Thus, retro-2cycl treatment of cells reduces ER trafficking and, as a consequence, prevents exposure of the minor capsid proteins of JCPyV, BKPyV, and SV40.
FIG 5 .

Retro-2cycl inhibits VP2 exposure of polyomaviruses. (A) VP2 is exposed at late time points during infection. Cells were pretreated with the indicated drugs and then inoculated with JCPyV, BKPyV, or SV40 at an MOI of 10 for 10 h before fixation and staining for VP2. VP2 puncta are green, and nuclei are blue. Scale bars, 10 µm. (B) VP2 is exposed in the ER. Cells were incubated with SV40 for 10 h, fixed, and then stained for VP1 (green), VP2 (red), and PDI (purple), and the nuclei were stained with BOBO-3 (blue). On the right, enlargements of the boxed area of the fluorescence micrograph show individual antibody staining. (C) Quantitation of panel A. Cells from triplicate samples were scored for the presence of VP2. Error bars show the standard deviations.
DISCUSSION
We demonstrate that the small molecule retro-2cycl, a recently described inhibitor of bacterial intoxication, inhibits infection by three polyomaviruses. Retro-2cycl inhibits polyomavirus infection in a manner similar to its effects on ricin and Shiga-like toxins, blocking retrograde transport to the ER or Golgi compartment (20). This effect appears to be specific for viruses that use retrograde transport, as transduction of cells by a GFP-expressing adenovirus is not inhibited by retro-2cycl. This work demonstrates that retro-2cycl is not only an effective antiviral compound but will also aid in the further delineation of the endocytic pathways used by polyomaviruses to target the ER.
A critical step in the infectious entry of polyomaviruses is ER transport, and we show that retro-2cycl significantly reduces the transport of virions to the ER (32, 33). Since virions cannot interact with ER-resident chaperones in retro-2cycl-treated cells, necessary uncoating steps are inhibited, as evidenced by a lack of exposure of the minor capsid protein VP2 in retro-2cycl-treated samples. All of the polyomaviruses studied to date undergo ER transport, and in recent years, nine new human polyomaviruses have been discovered (34). Several of these newly discovered viruses are associated with human diseases, including Merkel cell polyomavirus, which is the causative agent of the fatal cancer Merkel cell carcinoma (35). Since retro-2cycl is protective against JCPyV, BKPyV, and SV40, it is likely that this compound will inhibit the replication of these new polyomaviruses and will be a useful tool in verifying whether these new polyomaviruses target the ER for productive infection. We also found that pretreatment of cells with retro-2cycl had no effect on the binding of JCPyV or CTxB to cells but did slightly reduce the binding of BKPyV and SV40. This is unlikely to be due to reductions in cell surface receptor expression, as the SV40 receptor, GM1, was not reduced, as evidenced by the binding of CTxB. This was also not due to direct binding of retro-2cycl to the virions, as incubation of labeled viruses with retro-2cycl did not reduce binding. Finally, treatment of the cells did not prevent any of these viruses from entering the cell. The major effect is at the level of intracellular trafficking to the ER.
BFA is another small molecule that has been reported to inhibit the ER accumulation of polyomaviruses (30, 31, 36–38). However, BFA is highly cytotoxic to cells, making this molecule less appealing for the development of antiviral or antitoxin therapies (39). Additionally, BFA treatment rapidly alters the morphology of the Golgi apparatus, inhibits endosomal maturation, and inhibits protein secretion, demonstrating that this compound elicits numerous effects besides inhibiting retrograde trafficking (40–43). Conversely, retro-2cycl does not alter cell compartment morphology and is well tolerated when administered to mice (20). Thus, retro-2cycl is likely the first small-molecule inhibitor of polyomavirus infectivity that shows promise as a potential antiviral therapy.
We also show that the biologically active chemical species of retro-2 is a DHQ derivative of retro-2 and not an imine, as was originally reported (20). While we were completing these studies, another group confirmed that retro-2 is converted to a DHQ (29). With the correct structure of the retrograde transport inhibitor now established, we can consider the medicinal chemistry optimization of retro-2cycl as a potential drug lead.
The inhibitory effect of retro-2cycl is strikingly similar to the effect seen on ricin toxin and Shiga-like toxins, where retro-2cycl treatment prevents endosome-to-Golgi apparatus trafficking and, as a consequence, also inhibits ER trafficking (20). This suggests that there may be an overlap of the cellular proteins used by toxins and polyomaviruses to effect ER transport. However, there are likely significant differences in the kinetics or pathways used by polyomaviruses and bacterial toxins to target the ER, since ricin and Shiga-like toxins rapidly traffic to the Golgi apparatus (44), an association that has yet to be identified for any polyomavirus. This suggests that the cellular host factors targeted by retro-2cycl may be involved in multiple retrograde trafficking pathways, that only a small proportion of virions traffic to the Golgi compartment, or that polyomaviruses may rapidly traffic through the Golgi complex prior to ER accumulation. SLTs, CTxB, and some polyomaviruses bind to glycolipids and may therefore provide a rationale for how this compound inhibits trafficking (14, 45–50). However, whereas numerous host cellular transport factors are known to promote endosome-to-Golgi apparatus transport of ricin toxins and SLT, such as the retromer complex, syntaxin 5 and 16, EpsinR, Rab6a, and clathrin, the roles of these factors in polyomavirus entry are not known (51–53, 57, 58). Additionally, transport to late endosomes is known to be important for polyomaviruses, but it is not known whether cellular retrograde transport factors involved in transport from late endosomes, such as Rab9 and Tip47, are important for polyomavirus infectivity. Future work examining the role of these host factors in polyomavirus infection and determining what cellular host factor retro-2cycl binds will aid our understanding of how polyomaviruses and toxins undergo retrograde trafficking.
It is unlikely that retro-2cycl binds to polyomaviruses directly and therefore decreases the likelihood of escape mutations, since infectious mutants would have to use alternate trafficking pathways to enter cells. Since the majority of people are persistently infected with JCPyV and BKPyV (2, 7, 54–56), the ability of retro-2cycl to reduce the spreading of JCPyV, BKPyV, and SV40 in established infections suggests that these compounds may control viral dissemination in previously infected individuals. Further optimization of retro-2cycl may result in effective antiviral therapies to treat or prevent diseases caused by human polyomaviruses or other pathogens that use retrograde trafficking during infection.
MATERIALS AND METHODS
Cells, viruses, plasmids, and antibodies.
For all of the details of the cells, viruses, plasmids, and antibodies used in this study, see Text S1 (Materials and Methods) in the supplemental material.
Retro-2cycl inhibition of infection.
For all of the details of the infection studies described here, including dose-dependent inhibition of infection by retro-2cycl, time course experiments, multicycle growth assays, and cell tropism experiments, see Text S1 (Materials and Methods) in the supplemental material.
Flow cytometry.
For all of the details of the flow cytometry done in this study, including scoring of viral infectivity and binding assays, see Text S1 (Materials and Methods) in the supplemental material.
Viability assays.
For all of the details of the viability assays done in this study, see Text S1 (Materials and Methods) in the supplemental material.
Virus purification and labeling.
For all of the details of the virus purification and labeling done in this study, as well as details of pseudovirus production, see Text S1 (Materials and Methods) in the supplemental material.
Retro compounds.
For all of the details of the synthesis of retro-2cycl and its chemical derivatives, as well as X-ray crystallography and the deposition of X-ray structures, see Text S1 (Materials and Methods) in the supplemental material
Microscopy.
For all of the details of the virus purification and labeling done in this study, as well as details of PLAs, see Text S1 (Materials and Methods) in the supplemental material.
SUPPLEMENTAL MATERIAL
Supplemental materials and methods. Download
Retro-2cycl inhibits JCPyV infection in multiple cell lines. Cells were incubated with 100 µM retro-2cycl, 100 µM retro-2meta, or 71 nM (20 ng/ml) BFA prior to incubation with a JCPyV pseudovirus that produces luciferase in transduced cells. After 72 h, luciferase expression was determined and data were normalized to DMSO-treated control cells. The data represent the mean of three replicates, and error bars represent the standard deviation. Download
Viability of SVG-A and Vero cells after retro-2cycl treatment. SVG-A or Vero cells were incubated with the indicated concentrations of retro-2cycl for 72 h prior to determination of viability by the MTS assay. Values were normalized to DMSO-treated control cells. The data represent the mean of three replicates, and error bars represent the standard deviation. Download
Generation of retro-2 analogs. (A) Condensation of 2-aminobenzanilide with 4-methyl-2-thiophencarboxaldehyde in methanol yields two products termed retro-2 and retro-2cycl. (B) Treatment of retro-2 with sodium cyanoborohydride and acetic acid in methanol results in the formation of retro-2red, which is unable to cyclize. (C) A meta-substituted version of retro-2, termed retro-2meta, is also unable to cyclize. Download
Retro-2 does not directly interfere with virion binding to cells. Alexa Fluor 488-labeled BKPyV (top) or SV40 (bottom) was preincubated with 100 µM retro-2 or the vehicle control for 1 h on ice and added to prechilled cells. Unbound virus was removed by extensive washing, and binding was determined by flow cytometry. Download
Endocytosis is not inhibited by retro-2cycl treatment. Cells were chilled to prevent endocytosis, and Alexa Fluor 488-labeled JCPyV, BKPyV, or SV40 was bound to cells for 1 h on ice. Unbound virus was removed by extensive washing, and samples were heated for 120 min prior to fixation. Cells were imaged by confocal microscopy, and images were captured before (green) and after (red) trypan blue addition to the samples. Trypan blue will quench the fluorescence of solvent-accessible virions, resulting in internalized virions being pseudocolored yellow. This experiment demonstrates that retro-2cycl treatment does not inhibit the endocytosis of virions or toxins. In contrast, chlorpromazine and nystatin inhibited the endocytosis of JCPyV and BKPyV, SV40, and CTxB, respectively. Scale bars, 10 µm. Download
Retro-2cycl treatment results in redistribution of virions toward the cell periphery. Vero cells were pretreated with DMSO, retro-2cycl, or BFA for 0.5 h prior to incubation with BKPyV at an MOI of 10. After 6 h, cells were fixed, permeabilized, and stained with primary antibodies to VP1. Samples were imaged by confocal microscopy, and a single confocal slice at an approximately equivalent distance from the coverslip is shown. Scale bars, 10 µm. Download
Retro-2cycl abolishes the close proximity of PDI and VP2. Vero cells were pretreated with DMSO, retro-2cycl, or BFA for 0.5 h prior to incubation with SV40 at an MOI of 50. After 8 h, cells were fixed, permeabilized, and stained with primary antibodies to VP2 and PDI. The close proximity of these antibodies was then detected by a PLA. In the DMSO-treated samples, SV40 VP2, and PDI are within 40 nm and a green fluorescence signal can be detected. In retro-2cycl- and BFA-treated samples, however, this close proximity is reduced and less fluorescence signal is seen. Cell nuclei were counterstained with Hoechst, and a maximum image projection of serial z stacks is shown. Scale bars, 10 µm. Download
ACKNOWLEDGMENTS
We thank all of the members of the Atwood lab for their helpful comments.
This work was supported by R01CA071878 (W.J.A.), R01NS043097 (W.J.A.), P01NS065719 (W.J.A.), NSF CHE1058041 (P.G.W.), P01CA16038 (D.D.), a Johnson and Johnson Translational Innovation Partnership Award (W.J.A. and J.S.), and Ruth L. Kirschstein National Research Service award F32NS070687 (C.D.S.N.). Confocal microscopy analysis was completed in the Leduc Bioimaging Facility at Brown University and the Cooley lab at Yale University. Flow cytometry was performed in the Genomics Core at Brown, which is supported by P30GM103410 (W.J.A.).
Footnotes
Citation Nelson CDS, Carney DW, Derdowski A, Lipovsky A, Gee GV, O’Hara B, Williard P, DiMaio D, Sello JK, Atwood WJ. 2013. A retrograde trafficking inhibitor of ricin and Shiga-like toxins inhibits infection of cells by human and monkey polyomaviruses. mBio 4(6):e00729-13. doi:10.1128/mBio.00729-13.
REFERENCES
- 1. Jiang M, Abend JR, Johnson SF, Imperiale MJ. 2009. The role of polyomaviruses in human disease. Virology 384:266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kean JM, Rao S, Wang M, Garcea RL. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog. 5:e1000363. 10.1371/journal.ppat.1000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. 1996. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J. Virol. 70:7004–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. 1998. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J. Virol. 72:9918–9923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, Major EO. 2012. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 25:471–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shinohara T, Matsuda M, Cheng SH, Marshall J, Fujita M, Nagashima K. 1993. BK virus infection of the human urinary tract. J. Med. Virol. 41:301–305 [DOI] [PubMed] [Google Scholar]
- 7. Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, Miller E. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71:115–123 [DOI] [PubMed] [Google Scholar]
- 8. Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y. 1990. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J. Infect. Dis. 161:1128–1133 [DOI] [PubMed] [Google Scholar]
- 9. Zurhein G, Chou SM. 1965. Particles resembling papova viruses in human cerebral demyelinating disease. Science 148:1477–1479 [DOI] [PubMed] [Google Scholar]
- 10. Boothpur R, Brennan DC. 2010. Human polyoma viruses and disease with emphasis on clinical BK and JC. J. Clin. Virol. 47:306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G. 1999. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation 67:918–922 [DOI] [PubMed] [Google Scholar]
- 12. Engel S, Heger T, Mancini R, Herzog F, Kartenbeck J, Hayer A, Helenius A. 2011. The role of endosomes in SV40 entry and infection. J. Virol. 85:4198–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Querbes W, O’Hara BA, Williams G, Atwood WJ. 2006. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. J. Virol. 80:9402–9413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian M, Cai D, Verhey KJ, Tsai B. 2009. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog. 5:e1000465. 10.1371/journal.ppat.1000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schelhaas M, Malmström J, Pelkmans L, Haugstetter J, Ellgaard L, Grünewald K, Helenius A. 2007. Simian virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131:516–529 [DOI] [PubMed] [Google Scholar]
- 16. Lilley BN, Gilbert JM, Ploegh HL, Benjamin TL. 2006. Murine polyomavirus requires the endoplasmic reticulum protein derlin-2 to initiate infection. J. Virol. 80:8739–8744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magnuson B, Rainey EK, Benjamin T, Baryshev M, Mkrtchian S, Tsai B. 2005. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell 20:289–300 [DOI] [PubMed] [Google Scholar]
- 18. Rainey-Barger EK, Magnuson B, Tsai B. 2007. A chaperone-activated nonenveloped virus perforates the physiologically relevant endoplasmic reticulum membrane. J. Virol. 81:12996–13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodwin EC, Lipovsky A, Inoue T, Magaldi TG, Edwards AP, Van Goor KE, Paton AW, Paton JC, Atwood WJ, Tsai B, Dimaio D. 2011. BiP and multiple DNAJ molecular chaperones in the endoplasmic reticulum are required for efficient simian virus 40 infection. mBio 2:e00101–11. 10.1128/mBio.00101-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stechmann B, Bai SK, Gobbo E, Lopez R, Merer G, Pinchard S, Panigai L, Tenza D, Raposo G, Beaumelle B, Sauvaire D, Gillet D, Johannes L, Barbier J. 2010. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 141:231–242 [DOI] [PubMed] [Google Scholar]
- 21. Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, Kuruvilla L, Guie MA, Poffenberger AC, Nelson CD, Atwood WJ, Dimaio D. 2013. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc. Natl. Acad. Sci. U. S. A. 110:7452–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meier O, Greber UF. 2004. Adenovirus endocytosis. J. Gene Med. 6(Suppl 1):S152–S163 [DOI] [PubMed] [Google Scholar]
- 23. Gillet DBJ, Johnnes L, Stechmann B, Bai S. WO2009153665A2 World Intellectual Property Organization patent. 2009 Dec 23;
- 24. Narasimhulu M, Lee YR. 2011. Ethylenediamine diacetate-catalyzed three-component reaction for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones and their spirooxindole derivatives. Tetrahedron 67:9627–9634 [Google Scholar]
- 25. Prakash M, Kesavan V. 2012. Highly enantioselective synthesis of 2,3-dihydroquinazolinones through intramolecular amidation of imines. Org. Lett. 14:1896–1899 [DOI] [PubMed] [Google Scholar]
- 26. Sharma M, Pandey S, Chauhan K, Sharma D, Kumar B, Chauhan PM. 2012. Cyanuric chloride catalyzed mild protocol for synthesis of biologically active dihydro/spiro quinazolinones and quinazolinone-glycoconjugates. J. Org. Chem. 77:929–937 [DOI] [PubMed] [Google Scholar]
- 27. Shaterian HR, Oveisi AR, Honarmand M. 2010. Synthesis of 2,3-dihydroquinazoline-4(1H)-ones. Synth. Commun. 40:1231–1242 [Google Scholar]
- 28. Wang M, Zhang TT, Liang Y, Gao JJ. 2011. Strontium chloride-catalyzed one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones in protic media. Chin. Chem. Lett. 22:1423–1426 [Google Scholar]
- 29. Park JG, Kahn JN, Tumer NE, Pang YP. 2012. Chemical structure of retro-2, a compound that protects cells against ribosome-inactivating proteins. Sci. Rep. 2:631. 10.1038/srep00631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson CD, Derdowski A, Maginnis MS, O’Hara BA, Atwood WJ. 2012. The VP1 subunit of JC polyomavirus recapitulates early events in viral trafficking and is a novel tool to study polyomavirus entry. Virology 428:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. 2002. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 76:5156–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai B, Qian M. 2010. Cellular entry of polyomaviruses. Curr. Top. Microbiol. Immunol. 343:177–194 [DOI] [PubMed] [Google Scholar]
- 33. Sapp M, Day PM. 2009. Structure, attachment and entry of polyoma- and papillomaviruses. Virology 384:400–409 [DOI] [PubMed] [Google Scholar]
- 34. DeCaprio JA, Garcea RL. 2013. A cornucopia of human polyomaviruses. Nat. Rev. Microbiol. 11:264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng H, Shuda M, Chang Y, Moore PS. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richards AA, Stang E, Pepperkok R, Parton RG. 2002. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol. Biol. Cell 13:1750–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gilbert J, Benjamin T. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 78:12259–12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barbier J, Bouclier C, Johannes L, Gillet D. 2012. Inhibitors of the cellular trafficking of ricin. Toxins (Basel) 4:15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56:801–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huotari J, Helenius A. 2011. Endosome maturation. EMBO J. 30:3481–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y, Ikehara Y. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 261:11398–11403 [PubMed] [Google Scholar]
- 43. Low SH, Wong SH, Tang BL, Tan P, Subramaniam VN, Hong W. 1991. Inhibition by brefeldin A of protein secretion from the apical cell surface of Madin-Darby canine kidney cells. J. Biol. Chem. 266:17729–17732 [PubMed] [Google Scholar]
- 44. Johannes L, Römer W. 2010. Shiga toxins—from cell biology to biomedical applications. Nat. Rev. Microbiol. 8:105–116 [DOI] [PubMed] [Google Scholar]
- 45. Ewers H, Helenius A. 2011. Lipid-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 3(8):a004721. 10.1101/cshperspect.a004721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Low JA, Magnuson B, Tsai B, Imperiale MJ. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J. Virol. 80:1361–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Campanero-Rhodes MA, Smith A, Chai W, Sonnino S, Mauri L, Childs RA, Zhang Y, Ewers H, Helenius A, Imberty A, Feizi T. 2007. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J. Virol. 81:12846–12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsai B, Gilbert JM, Stehle T, Lencer W, Benjamin TL, Rapoport TA. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorelik L, Reid C, Testa M, Brickelmaier M, Bossolasco S, Pazzi A, Bestetti A, Carmillo P, Wilson E, McAuliffe M, Tonkin C, Carulli JP, Lugovskoy A, Lazzarin A, Sunyaev S, Simon K, Cinque P. 2011. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J. Infect. Dis. 204:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandvig K, Spilsberg B, Lauvrak SU, Torgersen ML, Iversen TG, van Deurs B. 2004. Pathways followed by protein toxins into cells. Int. J. Med. Microbiol. 293:483–490 [DOI] [PubMed] [Google Scholar]
- 51. Johannes L, Popoff V. 2008. Tracing the retrograde route in protein trafficking. Cell 135:1175–1187 [DOI] [PubMed] [Google Scholar]
- 52. Popoff V, Mardones GA, Tenza D, Rojas R, Lamaze C, Bonifacino JS, Raposo G, Johannes L. 2007. The retromer complex and clathrin define an early endosomal retrograde exit site. J. Cell Sci. 120:2022–2031 [DOI] [PubMed] [Google Scholar]
- 53. Bonifacino JS, Hurley JH. 2008. Retromer. Curr. Opin. Cell Biol. 20:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chesters PM, Heritage J, McCance DJ. 1983. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 147:676–684 [DOI] [PubMed] [Google Scholar]
- 55. Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199:837–846 [DOI] [PubMed] [Google Scholar]
- 56. Knowles WA. 2006. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv. Exp. Med. Biol. 577:19–45 [DOI] [PubMed] [Google Scholar]
- 57. Mallard F, Tang B, Galli T, Tenza Dl, Saint-Pol AS, Yuem X, Antony C, Hong W, Goud B, Johannes L. 2002. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amessou M, Fradagrada A, Falguières T, Lord JM, Smith DC, Roberts LM, Lamaze C, Johannes L. 2007. Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J. Cell Sci. 120:1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials and methods. Download
Retro-2cycl inhibits JCPyV infection in multiple cell lines. Cells were incubated with 100 µM retro-2cycl, 100 µM retro-2meta, or 71 nM (20 ng/ml) BFA prior to incubation with a JCPyV pseudovirus that produces luciferase in transduced cells. After 72 h, luciferase expression was determined and data were normalized to DMSO-treated control cells. The data represent the mean of three replicates, and error bars represent the standard deviation. Download
Viability of SVG-A and Vero cells after retro-2cycl treatment. SVG-A or Vero cells were incubated with the indicated concentrations of retro-2cycl for 72 h prior to determination of viability by the MTS assay. Values were normalized to DMSO-treated control cells. The data represent the mean of three replicates, and error bars represent the standard deviation. Download
Generation of retro-2 analogs. (A) Condensation of 2-aminobenzanilide with 4-methyl-2-thiophencarboxaldehyde in methanol yields two products termed retro-2 and retro-2cycl. (B) Treatment of retro-2 with sodium cyanoborohydride and acetic acid in methanol results in the formation of retro-2red, which is unable to cyclize. (C) A meta-substituted version of retro-2, termed retro-2meta, is also unable to cyclize. Download
Retro-2 does not directly interfere with virion binding to cells. Alexa Fluor 488-labeled BKPyV (top) or SV40 (bottom) was preincubated with 100 µM retro-2 or the vehicle control for 1 h on ice and added to prechilled cells. Unbound virus was removed by extensive washing, and binding was determined by flow cytometry. Download
Endocytosis is not inhibited by retro-2cycl treatment. Cells were chilled to prevent endocytosis, and Alexa Fluor 488-labeled JCPyV, BKPyV, or SV40 was bound to cells for 1 h on ice. Unbound virus was removed by extensive washing, and samples were heated for 120 min prior to fixation. Cells were imaged by confocal microscopy, and images were captured before (green) and after (red) trypan blue addition to the samples. Trypan blue will quench the fluorescence of solvent-accessible virions, resulting in internalized virions being pseudocolored yellow. This experiment demonstrates that retro-2cycl treatment does not inhibit the endocytosis of virions or toxins. In contrast, chlorpromazine and nystatin inhibited the endocytosis of JCPyV and BKPyV, SV40, and CTxB, respectively. Scale bars, 10 µm. Download
Retro-2cycl treatment results in redistribution of virions toward the cell periphery. Vero cells were pretreated with DMSO, retro-2cycl, or BFA for 0.5 h prior to incubation with BKPyV at an MOI of 10. After 6 h, cells were fixed, permeabilized, and stained with primary antibodies to VP1. Samples were imaged by confocal microscopy, and a single confocal slice at an approximately equivalent distance from the coverslip is shown. Scale bars, 10 µm. Download
Retro-2cycl abolishes the close proximity of PDI and VP2. Vero cells were pretreated with DMSO, retro-2cycl, or BFA for 0.5 h prior to incubation with SV40 at an MOI of 50. After 8 h, cells were fixed, permeabilized, and stained with primary antibodies to VP2 and PDI. The close proximity of these antibodies was then detected by a PLA. In the DMSO-treated samples, SV40 VP2, and PDI are within 40 nm and a green fluorescence signal can be detected. In retro-2cycl- and BFA-treated samples, however, this close proximity is reduced and less fluorescence signal is seen. Cell nuclei were counterstained with Hoechst, and a maximum image projection of serial z stacks is shown. Scale bars, 10 µm. Download