Abstract
Up to 50% of patients with uveal melanoma develop metastatic disease with poor prognosis. Regional, mainly liver-directed, therapies may induce limited tumor responses but do not improve overall survival. Response rates of metastatic uveal melanoma (MUM) to systemic chemotherapy are poor. Insights into the molecular biology of MUM recently led to investigation of new drugs. In this study, to compare response rates of systemic treatment for MUM we searched Pubmed/Web of Knowledge databases and ASCO website (1980–2013) for “metastatic/uveal/melanoma” and “melanoma/eye.” Forty studies (one case series, three phase I, five pilot, 22 nonrandomized, and two randomized phase II, one randomized phase III study, data of three expanded access programs, three retrospective studies) with 841 evaluable patients were included in the numeric outcome analysis. Complete or partial remissions were observed in 39/841 patients (overall response rate [ORR] 4.6%; 95% confidence intervals [CI] 3.3–6.3%), no responses were observed in 22/40 studies. Progression-free survival ranged from 1.8 to 7.2, median overall survival from 5.2 to 19.0 months as reported in 21/40 and 26/40 studies, respectively. Best responses were seen for chemoimmunotherapy (ORR 10.3%; 95% CI 4.8–18.7%) though mainly in first-line patients. Immunotherapy with ipilimumab, antiangiogenetic approaches, and kinase inhibitors have not yet proven to be superior to chemotherapy. MEK inhibitors are currently investigated in a phase II trial with promising preliminary data. Despite new insights into genetic and molecular background of MUM, satisfying systemic treatment approaches are currently lacking. Study results of innovative treatment strategies are urgently awaited.
Forty clinical studies on metastatic uveal melanoma were reviewed regarding responses to systemic treatments. New insights into genetic and molecular background led to investigation of new substances but promising in vitro data have not yet been translated into satisfying treatment responses; however, preliminary results of ongoing studies are highly encouraging.
Keywords: Clinical trials, drug therapy, metastatic, review, uveal melanoma
Introduction
Ocular melanoma accounts for 3% of all melanoma cases 1. Uveal melanoma (UM) is the most common primary intraocular tumor with an incidence of approximately five cases per million individuals 1. Up to 50% of patients develop metastatic disease with spread of tumor cells to liver (89%), lung (29%), bone (17%), and other organs 1,2. At this stage UM has a poor prognosis with median overall survival (OS) of 4–15 months 3. Survival rates in metastatic UM (MUM) have remained almost unchanged in the past 40 years 1.
As far as MUM is restricted to a limited anatomic region, locoregional treatment modalities can be used to control disease, for example, surgical resection, intraarterial chemotherapy, transarterial percutaneous chemoembolization, selective internal radiation therapy, and radiofrequency ablation 4. Patients in whom surgical resection is feasible show longer OS 5. Liver-directed therapies may induce remission of single metastases but do not prolong OS 4.
MUM is frequently treated with chemotherapeutics like dacarbazine, fotemustine, or gemcitabine/treosulfan although evidence for these regimens is limited. In clinical practice, responses are rarely seen and the impact of systemic chemotherapy on patients' survival is questionable 3.
Our understanding of molecular genetics and intracellular signaling pathways involved in the pathogenesis of MUM has improved over the last decades 6 resulting in the current investigation of targeted therapy approaches. We here review the present status of systemic treatment of MUM and evaluate therapy outcome measured by overall response rate (ORR) (IBM, Ehningen, Germany).
Methods
PubMed search was performed for “metastatic” [and] “uveal” [and] “melanoma” as well as for “melanoma” [and] “eye” [and] “treatment” on 16 May 2013 for the time period between 1980 and May 2013. “Web of Knowledge” and congress abstract search via the American Society of Clinical Oncology homepage was performed (data cut 22 May 2013). The http://ClinicalTrials.gov website was searched for terms “melanoma” and “eye” on 13 May 2013. All retrieved study summaries were screened and compared to published data.
All titles and abstracts in English language were screened for relevant content by the first author (K. B.). The selection process was documented according to PRISMA criteria (Fig. 1) 7. Studies on in vitro data, diagnostics, treatment of the primary tumor, single case reports, and clinical trials on locoregional treatment modalities were excluded. Full text versions of all relevant articles in English language were obtained and their references reviewed for additional relevant reports. Studies with less than four MUM patients, ecological design, without description of objective response assessment and studies not reporting ORR were excluded from meta-analysis (Fig. 1). All remaining studies were reviewed for quality aspects including study design, patient population, histological confirmation of disease, and method of staging/outcome evaluation by first and second author (K. B., A. G.). Patients treated in higher than first-line situations were classified as “non-first-line.”
Figure 1.
Flow of information through the different phases of the review process according to PRISMA statement 7.
Studies were grouped by type of treatment into single-agent or combination chemotherapies, chemoimmunotherapies, immunotherapies, antiangiogenetic therapies, and treatment with kinase inhibitors. In each group, rates of complete (CR) or partial remission (PR) and their exact 95% confidence intervals (95% CI) were computed for each study and overall for the group. In addition, homogeneity of ORR was examined by the exact chi-squared test. In case that homogeneity was rejected, the ORR was computed again, excluding the outlier study that caused heterogeneity. An overall summary analysis was carried out equally for all types of treatment. Statistical analysis was performed using SPSS statistics program version 21.0 (IBM, Ehningen, Germany).
Results
The selection process is outlined in Figure 1. Of 59 retrieved articles including 11 congress abstracts, four were excluded because of small patient numbers (n < 4) 8–10 or ecological design 2. Nine were excluded because ORR was not reported 11–15 or mixture study design did not permit separate analysis of MUM data 16–19. Forty-six studies were included in review discussion, that is, one case series, five pilot, three phase I, one phase I/II, 29 phase II, and one phase III study, data from three expanded access programs, and four retrospective data analyses. In six of the studies response criteria were insufficiently described. The first authors of these reports were contacted by e-mail to comment on response criteria. In case of authors' response studies were included in numeric analysis 20–22 while studies for which response criteria could not be elucidated were excluded 23–25. Two publications were excluded because of presentation-driven interim analyses of incomplete clinical trials, one of them reported within a review publication 26,27 (NCT00338130, NCT01143402). One publication was excluded from numeric analysis because the drug could not be assigned to one treatment subgroup 28.
The numeric analysis included 40 publications with a total of 841 patients (Table 1). Patient numbers ranged from four in a pilot study 29 to 171 patients in a randomized multicenter study 30. Histological confirmation of metastatic disease was reported in 19/40 studies. Immunohistochemical stains of c-kit were performed in one study 31, mutational analysis of c-kit in another study 32 and GNAQ sequencing in a limited number of patients in two studies 33,34. Mean patients' age was 59 years; 546 patients were treated in first-line situation whereas 229 patients had received prior therapies including surgery, liver-directed treatment, chemotherapy, or immunotherapy. Response was evaluated according to WHO response criteria of 1979 35 in 12 and according to RECIST 1.0/1.1 36 in 27 studies.
Study characteristics.
| Author | Year | Drug | Study design | Response assessment | n | First-line | Non-first-line | Mean age | SD | PR/CR | ORR (%) | PFS (mon) | OS (mon) | Histology/genetics |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spagnolo | 2013 | Fotemustine | Retrospective | RECIST | 24 | 24 | 0 | 62 | 9 | 2/0 | 8.3 | unk | 13.9 | no/no |
| Leyvraz | 2012 | Fotemustine (iv vs. ia) | Phase III | RECIST | 83 (a) | 83 | 0 | 59 | unk | 2/unk | 2.4 | 3.7 | unk | yes/no |
| Homsi | 2010 | DHA-paclitaxel | Phase II | RECIST | 22 | 11 | 11 | 56 | 7 | 1/0 | 4.6 | 3.0 | 9.8 | no/no |
| Bedikian | 2008 | Liposomal vincristine | Pilot | WHO | 4 | unk | unk | 56 | unk | 0/1 (b) | 25.0 | unk | unk | yes/no |
| Schmidt-Hieber | 2004 | Bendamustine | Phase II | RECIST | 11 | 0 | 11 | 61 | 0 | 0/0 | 0.0 | unk | unk | yes/no |
| Bedikian | 2003 | Temozolomide | Phase II | WHO | 14 | 9 | 5 | 53 | 2 | 0/0 | 0.0 | 1.8 | 6.7 | no/no |
| Ellerhorst | 2002 | Nitro-camptothecin | Phase II | WHO | 14 | 0 | 14 | 59 | 2 | 0/0 | 0.0 | unk | unk | no/no |
| Atzpodien | 2008 | Cisplatin (iv vs. ia)/gemcitabine/treosulfan | Pilot | WHO | 12 | 1 | 11 | 62 | 6 | 0/0 | 0.0 | unk | 6.0 | no/no |
| O'Neill | 2006 | Dacarbacine/treosulfan | Phase II | RECIST | 14 | 15 | 0 | 64 | 2 | 0/0 | 0.0 | 3.0 | 7.5 | no/no |
| Schmittel (a) | 2005 | Cisplatin/gemcitabine/treosulfan | Phase II | RECIST | 17 | 19 | 0 | 60 | 7 | 0/0 | 0.0 | 3.0 | 7.7 | yes/no |
| Flaherty | 1998 | Diverse chemotherapies | Retrospective pooled analysis | WHO | 64 (c) | unk | unk | 59 | unk | 5/1 | 9.0 | unk | 5.2 | no/no |
| Sacco | 2013 | Dacarbazine | Phase II, randomized | RECIST | 37 | 37 | 0 | unk | 4 | 3/unk | 8.0 | 3.9 | 8.7 | no/no |
| Sunitinib | 37 | 37 | 0 | unk | 9 | 0/0 | 0.0 | 2.8 | 6.4 | |||||
| Schmittel | 2006 | Treosulfan | Phase II, randomized | RECIST | 24 | 17 | 7 | 58 | 3 | 0/0 | 0.0 | 2.0 | unk | yes/no |
| Gemcitabine/treosulfan | 24 | 15 | 9 | 63 | 7 | 0/1 | 4.2 | 3.0 | unk | |||||
| Corrie | 2005 | Gemcitabine/treosulfan | Phase I | RECIST | 5 | 4 | 1 | 50 | 4 | 0/0 | 0.0 | 6.8 | 13.3 | yes/no |
| Schmittel (b) | 2005 | Gemcitabine/treosulfan | Phase II | RECIST | 33 | 28 | 5 | 62 | 14 | 1/0 | 3.0 | 2.5 | 7.5 | yes/no |
| Terheyden | 2004 | Gemcitabine/treosulfan | Phase II | WHO | 20 | 8 | 14 | 62 | 5 | 0/0 | 0.0 | unk | 11.6 | yes/no (d) |
| Keilholz | 2004 | Gemcitabine/treosulfan | Phase I | RECIST1 | 33 | 28 | 5 | 62 | 15 | 1/0 | 3.0 | unk | unk | yes/no |
| Pföhler | 2003 | Gemcitabine/treosulfan | Pilot | WHO | 14 | 13 | 1 | 63 | 8 | 3/1 | 28.6 | 7.1 | 15.3 | no/no |
| Kivelä | 2003 | BOLD/INF-α2b | Phase II | WHO | 22 | 24 | 0 | 61 | 2 | 0/0 | 0.0 | 1.9 | 10.6 | yes/no |
| Pyrhönen | 2002 | BOLD/INF-α2b | Phase II | WHO | 20 | 18 | 4 | 60 | 11 | 0/3 | 15.0 | 4.4 | 12.3 | yes/no |
| Becker | 2002 | fotemustine/INF-α2b/IL-2 | Phase II | WHO | 25 | unk | unk | 56 | unk | 1/1 | 8.0 | unk | 15.0 (e) | no/no |
| Nathan | 1997 | BOLD/INFα-2b | Phase II | WHO | 20 | 23 | 0 | 62 | unk | 4/0 | 20.0 | unk | unk | yes/no |
| Kelderman | 2013 | Ipilimumab | EAP | RECIST, irRC | 22 | 0 | 22 | 54 | 1 | 1/0 | 4.5 | 2.9 | 5.2 | no/no |
| Khattak | 2013 | Ipilimumab | EAP | RECIST | 5 | 0 | 5 | 42 | 2 | 0/0 | 0.0 | unk | 10.3 | no/no |
| Danielli | 2012 | Ipilimumab | EAP | mWHO | 9 | 0 | 13 | 57 | 2 | 0/0 | 0.0 | unk | 6.0 | no/no |
| Khan | 2012 | Ipilimumab | Retrospective | RECIST, irRC | 20 | 0 | 20 | 61 | 7 | 1/0 | 5.0 | unk | unk | no/no |
| Piperno-Neumann | 2013 | Bevacizumab/temozolomide | Phase II | RECIST | 35 | 35 | 0 | 55 | 9 | 0/0 | 0.0 | 3.0 | 12.0 | no/no |
| Guenterberg | 2011 | Bevacizumab/INF-α2b | Phase II | RECIST | 5 | 4 | 1 | 64 | 3 | 0/0 | 0.0 | 4.5 | 10.8 | no/no |
| Tarhini | 2011 | Aflibercept | Phase II | RECIST | 9 | 10 | 0 | 57 | unk | 0/0 | 0.0 | 5.7 | 19.0 | yes/no |
| Zeldis | 2009 | Lenalidomide | Phase II | RECIST | 16 | unk | unk | 53 | 7 | 0/0 | 0.0 | unk | unk | no/no |
| Solti | 2007 | Thalidomide/INF-α2b | Pilot | RECIST | 6 | 0 | 6 | 59 | 1 | 0/0 | 0.0 | 3.6 | 9.0 | no/no |
| Reiriz | 2004 | Thalidomide | Phase II | WHO | 5 | 0 | 5 | 59 | 1 | 0/0 | 0.0 | unk | unk | yes/no |
| Bhatia | 2012 | Carboplatin/paclitaxel/sorafenib | Phase II | RECIST | 24 | 20 | 4 | 61 | 12 | 0/0 | 0.0 | 4.0 | 11.0 | no/no |
| Kaempgen | 2012 | Fotemustine/sorafenib | Case series | Investigator decision1 | 7 | unk | unk | unk | unk | 3/0 | 42.0 | unk | unk | no/no |
| Falchook | 2012 | Trametinib | Phase I | RECIST | 16 | 1 | 15 | 53 | 8 (f) | 0/0 | 0.0 | 1.8 | unk | yes/yes (f) |
| Kirkwood | 2011 | Selumetinib | Phase II | RECIST | 7 | 20 | 0 | 57 | unk | 0/0 | 0.0 | unk | unk | yes/yes (g) |
| Mahipal | 2012 | Sunitinib | Pilot | RECIST | 18 | 3 | 17 | 69 | 12 | 1//0 | 5.0 | 4.2 | 8.2 | no/no |
| Nathan | 2012 | Imatinib | Phase II | RECIST | 25 | 24 | 13 | 63 | unk | 2/0 (h) | 8.0 | 3.0 | 7.4 | yes/yes (h) |
| Hofmann | 2008 | Imatinib | Phase II | RECIST | 9 | 9 | 3 | 63 | 1 | 0/0 | 0.0 | unk | 6.8 | yes/yes (i) |
| Penel | 2008 | Imatinib | Phase II | RECIST | 10 | 6 | 7 | 58 | 1 | 0/0 | 0.0 | unk | 10.8 | yes/no |
a, only iv-group considered; b, CR patient had lung metastases only; c, 5/64 patients received chemotherapy/IL-2; d, not in all patients; e, OS includes intraarterially treated patients; f, no correlation of GNAQ status and response (GNAQ testing in six patients); g, GNAQ mutated in four, wild type in eight patients; h, c-kit exons 11, 13, and 17 analyzed, both PR patients c-kit wild type; i, c-kit immunohistology; bid, twice daily; EAP, expanded access program; iv, intravenous; ia, intraarterial (hepatic); mon, months; n, number of patients; unk, unknown; ORR, overall response rate; CR, rates of complete; PR, partial remission; PFS, progression-free survival; OS, overall survival.
First authors were contacted by email for clarification of objective response assessment.
Response, including CR and PR, was achieved in 39 of 841 patients; ORR was 4.6% (95% CI 3.3–6.3%). No responses were observed in 22/40 studies. Stable (SD) versus progressive disease (PD) was reported over all studies for 184 versus 379 patients (ratio 1:2) while nine studies did not provide information on SD/PD numbers. Median OS was reported in 26/40 studies ranging from 5.2 months in pretreated, predominantly end-stage patients 37 to 19.0 months in selected first-line patients 38. Progression-free survival (PFS) was reported in 21/40 studies ranging from 1.8 to 7.1 months.
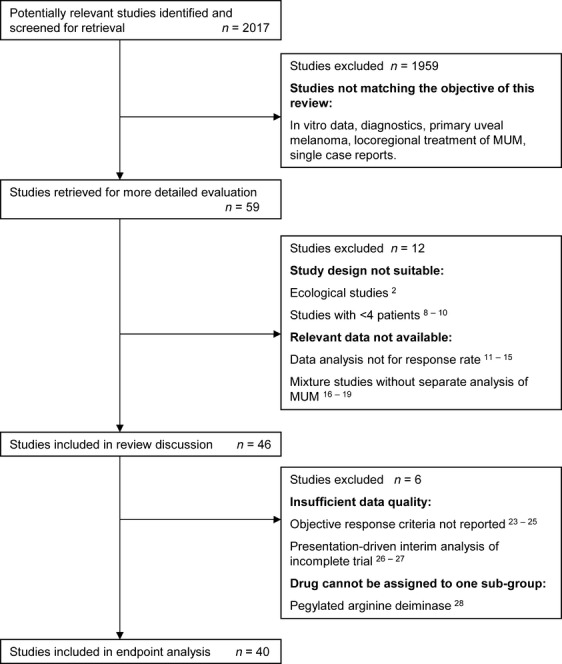
Single-agent chemotherapeutic regimens (dacarbazine 22, fotemustine 30,39, DHA-paclitaxel 40) showed ORR below 10% with the exception of a small pilot study (1 CR/4 patients) 29. Notably, four studies with smaller sample sizes observed no PR/CR (temozolomide 34,41, camptothecin 42, bendamustine 43, treosulfan 44). Testing for equal ORR did not detect substantial heterogeneity (P = 0.56). The estimated ORR was 3.9% (95% CI 1.8–7.2%) (Fig. 2A). Most of the patients were treated in non-first-line situations.
Figure 2.
Response rates for single-agent chemotherapies (A), combination chemotherapies (B), and chemoimmunotherapies and immunotherapy with ipilimumab (C).
The best-investigated combination chemotherapy regimen is gemcitabine/treosulfan, tested in six phase I and II trials (Fig. 2B). An outstanding ORR of 28.6% (one CR, three PR in 14 patients) with OS of 15.3 months, and PFS of 7.1 month 45 could not be reproduced by subsequent studies on gemcitabine/treosulfan with more than 20 patients each and histology-proven disease in 4/5 studies 21,42,44–46. Reports on combination chemotherapies including cisplatin/gemcitabine/treosulfan 46,47, dacarbazine/treosulfan 48, and carboplatin/paclitaxel/sorafenib 49 showed no responses. Analysis of all combination chemotherapies excluding Pföhler et al. 45. for homogeneity reason revealed responses in 9/222 patients (ORR 4.1%; 95% CI 1.9–7.6%).
Chemoimmunotherapy regimens (bleomycin/vincristine/lomustine/dacarbazine [=BOLD]/INF-α2b, fotemustine/INF-α2b/IL-2) were studied in four phase II trials with 20–25 patients each, mainly in first-line situations with histology-proven MUM in 3/4 studies 50–53. The test for equal ORR did not detect substantial heterogeneity (P = 0.16); estimated ORR was 10.3% (95% CI: 4.8–18.7%) (Fig. 2C).
Ipilimumab immunotherapy (3 and 10 mg/kg) was analyzed in three expanded access programs and one retrospective single-center study in non-first-line situations 37,54–56. Two of 56 evaluable patients experienced PR (ORR 3.6%; 95% CI 0.4–12.3%) (Fig. 2C) while 12 patients showed disease stabilization.
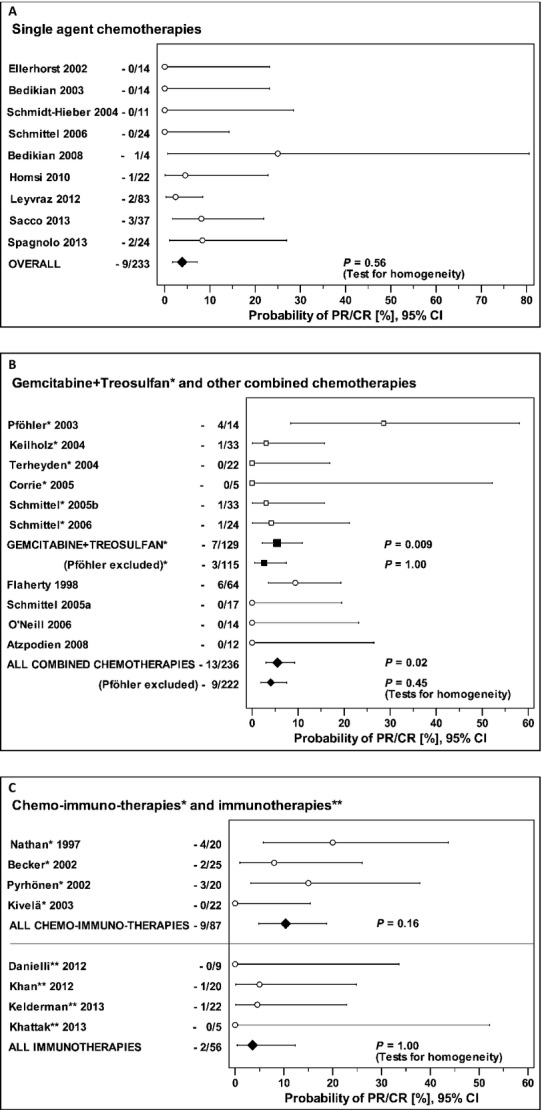
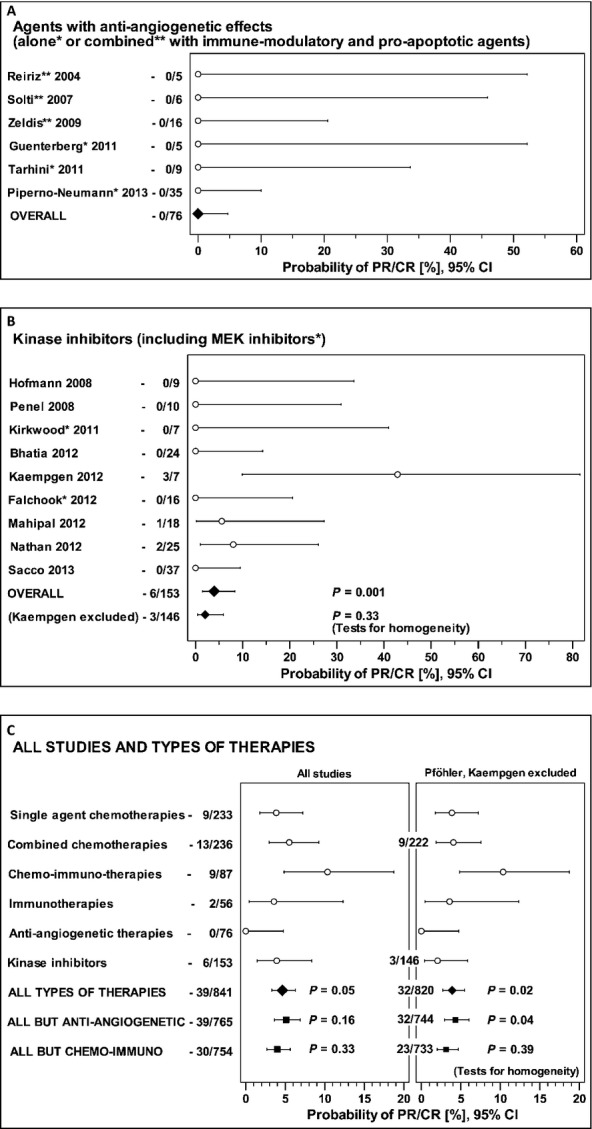
Antiangiogenetic treatment strategies using bevacizumab combined with interferon-α2b 57, temozolomide 58, or the vascular endothelial growth factor (VEGF)-trap aflibercept 38 did not show responses in first-line treatment. The antineoplastic and antiangiogenetic drug thalidomide failed to show responses in second-line situations as single agent 59 and in combination with interferon-α2b 60. Moreover, lenalidomide, which has antiangiogenetic and immunomodulatory properties, did not induce responses 61. Altogether, in 56 evaluable patients ORR was 0% (95% CI 0–4.7%) (Fig. 3A).
Figure 3.
Response rates for agents with antiangiogenetic effect (A), kinase inhibitors (B), and comparison of all treatment modalities (C).
Recent study protocols focus on small molecule kinase inhibitors for targeted therapy of MUM (Fig. 3B). In three studies, imatinib (targets c-kit, platelet-derived growth factor [PDGF]) was applied as first- or second-line treatment (300 or 400 mg bid); 2/3 studies showed no responses 31,62. Two PRs (8%) were observed in one study with 25 patients; both responders presented c-kit wild-type status in the assessed metastases 32. Sunitinib (targets PDGF receptor [PDGFR], VEGF receptor [VEGFR], c-kit, and others) was studied in a pilot trial mainly in second-line situations. One PR (1/18, ORR 5%) and a relatively high proportion of patients in SD status (12/18) were reported 63. Sunitinib was therefore compared to dacarbazine in a randomized phase II trial that revealed no response in the sunitinib (0/37) versus 3 responses in the dacarbazine group (3/37). PFS was not improved in the sunitinib group. Sorafenib (targets RAF, VEGFR, c-kit, PDGFR) was investigated as single agent and in combination with chemotherapy. In a mainly first-line setting sorafenib failed to induce response but 12 of 24 patients showed SD 49. Phase I/II trials on mitogen-activated protein kinase (MEK) inhibitors selumetinib and trametinib that altogether recruited 23 MUM patients showed no responses 33,34. Falchook et al. 33 observed SD in 8/16 second-line patients (50%) with SD achievement not correlating with the mutational status. Overall, kinase inhibitors showed responses in 3/146 patients (ORR 2.1%; (95% CI 0.4–5.9%) (Fig. 3B).
Future perspectives
Advances in knowledge about genetics and signaling pathways led to initiation of clinical trials with innovative therapeutics. Screening the http://ClinicalTrials.gov website for ongoing clinical trials on MUM revealed 15 studies, two of them with randomized design (Table 2). Only two of the phase II studies evaluate chemotherapies (albumin-bound paclitaxel 25, liposomal vincristine 29).
Active/recruiting trials studying treatment approaches for metastatic uveal melanoma as registered on ClinicalTrials.gov.
| ClinicalTrials.gov identifier | Drug | Phase | Planned patients | Status | Sponsor |
|---|---|---|---|---|---|
| NCT01355120 | Ipilimumab (anti-CTLA4 antibody) | II | 41 | Data collection ongoing | University Hospital Essen, Germany |
| NCT01034787 | CP-675,206 (anti-CTLA4 antibody) | II | 32 | Data collection ongoing | Alberta Health Services, Canada |
| NCT01585194 | Ipilimumab (anti-CTLA4 antibody) | II | 141 | Recruiting | MD Anderson Cancer Center, US |
| NCT01587352 | Vorinostat (histone deacetylase inhibitor) | II | 32 | Recruiting | National Cancer Institute, US |
| NCT01413191 | Cixutumumab (anti-IGF-1R antibody) | II | 32 | Data collection ongoing | National Cancer Institute, US |
| NCT01200342 | Genasense/oblimersen (Bcl-2 antisense oligonucleotide) plus carboplatin/paclitaxel | II | 30 | Data collection ongoing | MD Anderson Cancer Center, US |
| NCT00506142 | Marqibo (liposomal vincristine) | II | 50 | Recruiting | Talon Therapeutics, US |
| NCT00738361 | Abraxane (nab-paclitaxel) | II | 25 | Completed, results pending | National Comprehensive Cancer Network, Ohio, US |
| NCT01252251 | Everolimus (mTOR inhibitor) and pasireotide (somatostatin receptor analog) | II | 25 | Recruiting | Memorial Sloan-Kettering Cancer Center, US |
| NCT01200238 | Ganetespib (HSP90 inhibitor) | II | 30 | Recruiting | Dana-Farber Cancer Institute, US |
| NCT01143402 | Selumetinib (MEK inhibitor) versus temozolomide | II | 159 | Recruiting | National Cancer Institute, US |
| NCT01430416 | AEB071 (protein kinase C inhibitor) | I | 65 | Recruiting | Novartis Pharmaceuticals, US |
| NCT01377025 | Sorafenib versus placebo | II | 200 | Recruiting | University Hospital, Essen, Germany |
| NCT01801358 | AEB071 (protein kinase C inhibitor) plus MEK162 | I/II | 90 | Not yet recruiting | Novartis Pharmaceuticals, US |
| NCT01835145 | Cabozantinib versus temozolomide or dacarbazine | II | 69 | Not yet recruiting | National Cancer Institute, US |
The occurrence of UMs in an immunologically privileged site makes immunotherapy a promising treatment approach. Current data on ipilimumab were gained from retrospective analyses only. One was published at the time of manuscript revision and showed, in line with the previously published studies, an ORR of 5.1% (2/39); SD was observed in 44% (week 12) and 25% (week 23) of patients 64. Anti-CTLA4 antibodies are further assessed in three prospective trials. While two of them are expected to report outcomes soon, another large trial on ipilimumab will not be finished before 2017 (Table 2). PD-1 and PD-L1 have become important targets in cutaneous melanoma. To our knowledge, MUM patients have not been included in trials with PD-1 or PD-L1 antibodies yet. However, as PD-L1 expression is found in MUM cells 65 and probably in the tumor environment further investigation of treatment strategies targeting PD-1/PD-L1 in MUM are warranted.
Activating somatic mutations in GNAQ/GNA11, two members of the guanine nucleotide-binding protein family (G-proteins), were found in 83% of UMs 66. Both mutations result in the constitutive activation of the mitogen-activated protein kinase (MAPK) pathway thereby inducing proliferation in the absence of external growth stimuli 67. Hence, blocking this pathway by specific inhibitors may be an effective therapeutic approach for MUM 68–70. Several kinase inhibitors are currently studied in five phase I/II and II studies. A phase II study presently conducted in the US compares selumetinib versus temozolomide/DTIC with a much noticed interim analysis on PFS; ORR was 15% in the selumetinib-group (7/46) compared to 0% in the temozolomide-group (0/46) and 0% in the cross-over group 26. However, tumor regression without reaching RECIST-defined response was seen in 50% in the selumetinib-group versus 11% in the temozolomide group and 23% in the cross-over group. PFS in week 16 was 43.1% for selumetinib versus 8.5% for temozolomide. Interestingly, responses were also seen in GNAQ/GNA11 (Q209, exon 5) wild-type patients. However, retrospective assessment of codon R183, exon 4 revealed a mutation in the patient with objective response according to RECIST. These promising but preliminary data on MEK inhibition had to be excluded from our numeric analysis as final study outcomes should be awaited 26,27.
GNAQ/GNA11 signaling induces activation of phospholipase C (PLC) and protein kinase C (PKC) further downstream of PLC with subsequent MAPK pathway activation 71. There are two trials under way investigating PKC inhibition alone and in combination with MEK inhibition. GNAQ/GNA11 signaling is also linked to the PI3K-AKT pathway in UM, usually in an activating manner resulting in increased cell proliferation and survival 71. Hence inhibition of PI3K or AKT, possibly in combination with MAPK pathway inhibition, appears to be another attractive treatment strategy.
On the basis of promising data on the multikinase inhibitor sorafenib in small case series, a placebo-controlled phase II study is currently conducted in Germany investigating sorafenib versus placebo. Preliminary data on cabozantinib, a c-Met/VEGFR2 inhibitor currently under investigation 12, prompted investigators to initiate a randomized phase II study on cabozantinib versus dacarbacine or temozolomide. Search on the http://ClinicalTrials.gov website additionally revealed results on one terminated, yet unpublished study analyzing the combination of sunitinib/lenalidomide/cyclophosphamide, which showed no response in 12 patients (NCT00482911).
Mutations in BAP1, a deubiquitinating enzyme located on chromosome 3p, are seen in 85% of high-risk (“class-2”) UMs and correlate with development of metastatic disease 72. One substrate of BAP1 is histone H2A; histone-deacetylase inhibitors were shown to reverse the H2A hyperubiquitination caused by BAP1 knock-down in vitro 73 and might therefore be a therapeutic strategy 74. The histone-deacetylase inhibitor vorinostat is currently studied in MUM.
Antiapoptotic bcl-2, which is (over)expressed in more than 95% of UMs 72, provides another potential target. The bcl-2-antisense oligonucleotide oblimersen is currently under investigation. Upregulation of insulin-like growth factor (IGF)-1 and IGF-1R receptor in UM 72 potentially offers the possibility of treatment with the anti-IGF-1R-antibody cixutumumab. Further compounds currently under investigation in phase II studies include the HSP90 inhibitor ganetespib, and the somatostatin receptor analog pasireotide in combination with everolimus. Other treatment approaches such as targeting of somatostatin receptors by octreotid 24 and a phase I/II study on pegylated arginine were disappointing 28.
Altogether, immunotherapeutics and kinase inhibitors are currently the most investigated agents with encouraging interim results on MEK inhibition.
Discussion
Depending on the genetic signatures of the primary tumor 6, up to 50% of UM patients develop metastatic disease. Once metastases occur prognosis is bad and therapeutic options are limited with ORR being considerably low.
The only randomized controlled phase III trial on treatment of MUM (intravenous vs. intraarterial fotemustine) showed improved ORR of liver metastases and prolonged PFS in intraarterially treated patients but similar OS in both groups 30. Response to intravenous fotemustine was as low as 2.4%. Only two phase II studies have up to now been published that were designed as randomized trials with two subgroups 22,44. One of these showed 8% ORR in the dacarbazine group. Relatively high ORRs reported for single-agent or combination chemotherapy in small studies 29 are possibly due to selection bias in small patient numbers. Phase II trials on liposomal vincristine and albumin-bound paclitaxel are ongoing but uncertain to reproduce promising results of previous smaller studies 29,40.
In our pooled analysis, chemoimmunotherapy shows slightly better tumor responses than chemotherapy. This observation has to be interpreted with caution as our analysis considered first-line and higher line studies as well as studies that did not differentiate the outcome of first- and second-line treated patients. Better OS in the chemoimmunotherapy studies might thus partially be due to a first-line treatment situation in the majority of trials.
New insights into tumor biology led to investigation of immunotherapies, antiangiogenetic agents, and targeted therapies. While ipilimumab is effective in metastatic cutaneous melanoma 75, it did not yet appear to be superior to chemotherapy regimens in MUM, possibly because published data have mainly been generated from expanded access programs in non-first-line situations. However, OS of 5.2–10.3 months in pretreated patients might still be promising 37,54,56. Final conclusions can only be drawn from randomized studies, preferably in first-line situations, which are still lacking.
Although VEGF plays a major role in MUM 6, treatment regimens focusing on antiangiogenetic agents did not reveal responses in first-line treatment. Nevertheless, pooled OS of 12.7 months appear promising.
Kinase inhibitors including sorafenib, sunitinib, and imatinib did not show any responses in six of nine studies. Promising results from a small case series on sorafenib combined with fotemustine 20 led to initiation of a large phase II study of sorafenib the results of which are still pending. The ORR for sunitinib was 5% in a pilot trial 63, which, however, could not be confirmed in a subsequent randomized phase II study 22.
GNAQ/GNA11 mutations in over 80% of MUM leading to aberrant activation of the MAPK pathway especially makes MEK an attractive therapeutic target 6. Patients recruited in phase I/II studies, however, did not show objective responses upon MEK inhibitor treatment 33,34. Falchook et al. 33 did not observe a correlation between the mutational status of GNAQ/GNA11 and clinical response to MEK inhibition but the analyzed exons were not specified in the publication. A phase II study is currently conducted on selumetinib with a promising interim analysis but pending final results 26. According to preliminary data, there is no proven correlation of ORR or PFS with GNAQ/GNA11 mutational status. OS was not significantly improved compared to chemotherapy.
Increasing insight into the biology of MUM has not yet translated into higher ORR. Unexpectedly, a correlation of treatment response to mutational/expression status of molecular targets has not been found in smaller trials 32–34 and ongoing clinical studies 26. So far, there is no evidence of a clinically meaningful survival benefit due to novel targeted agents.
With respect to appallingly low ORR, the question is whether disease stabilizations are treatment related or simply reflecting the natural course of disease 13. None of the currently available therapies has shown prolongation of patients' OS. Survival data were reported in 75% of the analyzed studies but cannot be compared due to inhomogeneous patients' characteristics throughout the studies. Only 7/40 publications reported the lengths of metastases-free intervals as primary diagnosis of UM and, if reported, a wide range was seen within and among these studies (0–25 years) 39,49,50,53,63,76,77. As metastases may develop 10 or more years after primary UM, this “dormancy” phenomenon has a high impact on patients' prognosis 78,79. Moreover, other prognostic parameters such as lactate dehydrogenase, sites of metastases, and patients' performance status would need to be equally distributed in the studies to allow comparison of survival data.
According to available study data, survival appears to depend on patient- and tumor-related characteristics rather than on the actual treatment 3; it therefore can only be analyzed in randomized studies recruiting patients with comparable characteristics. Given a poor response rate in most of the studies, determining PFS at a certain time point might be a more suitable endpoint. This would require defined staging intervals, which, however, were inhomogeneous throughout the analyzed studies here and therefore not considered in this review.
Conclusion
This review analyzes data of studies on systemic treatment of MUM published between January 1980 and May 2013. Altogether, published data mainly provided low-level evidence. The limited efficacy of current treatment approaches illustrates the high medical need for more effective treatment options in MUM.
To date, no chemotherapeutic, immunotherapeutic, or targeted drug has shown reproducible ORR >10% or prolonged OS in MUM. Targeted therapeutics as well as immunotherapies might be promising strategies, but need evaluation in prospective trials. Investigation of chemoimmunotherapy-based strategies appeared to become less important, probably due to toxicity profiles although ORR has been superior to all other therapeutic approaches. Most promising preliminary data are available for MEK inhibition. However, these therapeutic regimens should be judged after final data analyses become available. A future goal should be careful design of randomized clinical trials.
Conflict of Interest
K. Buder received educational/travel grants and honoraria for oral presentations from TEVA GmbH, Roche Pharma, and Bristol-Myers Squibb. A. Gesierich received travel grants for congress participation, and was an advisory board member for Bristol-Myers Squibb and Roche Pharma. No conflicts of interest declared for G. Gelbrich. M. Goebeler was an advisory board member for MSD SHARP and DOHME GmbH.
References
- Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch. Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- Augsburger JJ, Correa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am. J. Ophthalmol. 2009;148:119–127. doi: 10.1016/j.ajo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Pflugfelder A, Kochs C, Garbe C, Schadendorf D, Blum A, Capellaro M, et al. S3-guideline – diagnosis, therapy and follow-up of melanoma. J. Dtsch. Dermatol. Ges. 2013;11:563–594. doi: 10.1111/ddg.12044. [DOI] [PubMed] [Google Scholar]
- Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br. J. Ophthalmol. 2009;93:1042–1046. doi: 10.1136/bjo.2008.153684. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Lake SL, Zeschnigk M, Damato BE. Molecular pathology of uveal melanoma. Eye (Lond.) 2013;27:230–242. doi: 10.1038/eye.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J. Clin. Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasiuniene B, Sokolovas V, Brasiunas V, Barakauskiene A, Strupas K. Combined treatment of uveal melanoma liver metastases. Eur. J. Med. Res. 2011;16:71–75. doi: 10.1186/2047-783X-16-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feun LG, Reddy KR, Scagnelli T, Yrizarry JM, Guerra JJ, Russell E, et al. A phase I study of chemoembolization with cisplatin, thiotepa, and lipiodol for primary and metastatic liver cancer. Am. J. Clin. Oncol. 1999;22:375–380. doi: 10.1097/00000421-199908000-00010. [DOI] [PubMed] [Google Scholar]
- Caminal JM, Ribes J, Cleries R, Ibanez N, Arias L, Piulats JM, et al. Relative survival of patients with uveal melanoma managed in a single center. Melanoma Res. 2012;22:271–277. doi: 10.1097/CMR.0b013e328353ef30. [DOI] [PubMed] [Google Scholar]
- Daud A, Kluger HM, Edelman G, Gordon MS, Schimmoller F, Weitzman A, et al. Activity of cabozantinib in metastatic uveal melanoma: updated results from a phase II randomized discontinuation trial (RDT) J. Clin. Oncol. 2013;31:9094. Abstract. [Google Scholar]
- Pons F, Plana M, Caminal JM, Pera J, Fernandes I, Perez J, et al. Metastatic uveal melanoma: is there a role for conventional chemotherapy? A single center study based on 58 patients. Melanoma Res. 2011;21:217–222. doi: 10.1097/CMR.0b013e3283457726. [DOI] [PubMed] [Google Scholar]
- Scheulen ME, Nokay B, Richly H, Hoffmann AC, Kalkmann J, Stattaus J, et al. Register trial of sorafenib (S) for patients (pts) with metastatic uveal melanoma (metUvMel) Eur. J. Cancer. 2011;47:S30. [Google Scholar]
- Valpione S, Aliberti C, Pigozzo J, Midena E, Parrozzani R, Stragliotto S, et al. Metastatic uveal melanoma: a 22 years single center experience. Ann. Oncol. 2012;23:371. [Google Scholar]
- Bedikian AY, Johnson MM, Warneke CL, McIntyre S, Papadopoulos N, Hwu WJ, et al. Systemic therapy for unresectable metastatic melanoma: impact of biochemotherapy on long-term survival. J. Immunotoxicol. 2008;5:201–207. doi: 10.1080/15476910802131519. [DOI] [PubMed] [Google Scholar]
- Borden EC, Jacobs B, Hollovary E, Rybicki L, Elson P, Olencki T, et al. Gene regulatory and clinical effects of interferon beta in patients with metastatic melanoma: a phase II trial. J. Interferon Cytokine Res. 2011;31:433–440. doi: 10.1089/jir.2010.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagan ET, Suman VJ, Dalton RJ, Pitot HC, Long HJ, Veeder MH, et al. Phase III clinical trial of the combination of cisplatin, dacarbazine, and carmustine with or without tamoxifen in patients with advanced malignant melanoma. J. Clin. Oncol. 1999;17:1884–1890. doi: 10.1200/JCO.1999.17.6.1884. [DOI] [PubMed] [Google Scholar]
- Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- Kaempgen E, Schmid M, Erdmann M, Keikavoussi P, Strobel D, Schuler-Thurner B, et al. Predictable clinical responses to sorafenib in stage IV uveal melanoma. J. Clin. Oncol. 2012;30:Abstract e19032. [Google Scholar]
- Keilholz U, Schuster R, Schmittel A, Bechrakis N, Siehl J, Foerster MH, et al. A clinical phase I trial of gemcitabine and treosulfan in uveal melanoma and other solid tumours. Eur. J. Cancer. 2004;40:2047–2052. doi: 10.1016/j.ejca.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Sacco JS, Nathan PD, Danson S, Lorigan P, Nicholson S, Ottensmeier CH, et al. Sunitinib versus dacarbazine as first-line treatment in patients with metastatic uveal melanoma. J. Clin. Oncol. 2013;31:Abstract 9031. [Google Scholar]
- Dorval T, Fridman WH, Mathiot C, Pouillart P. Interleukin-2 therapy for metastatic uveal melanoma. Eur. J. Cancer. 1992;28A:2087. doi: 10.1016/0959-8049(92)90266-5. [DOI] [PubMed] [Google Scholar]
- Valsecchi ME, Coronel M, Intenzo CM, Kim SM, Witkiewicz AK, Sato T. Somatostatin receptor scintigraphy in patients with metastatic uveal melanoma. Melanoma Res. 2013;23:33–39. doi: 10.1097/CMR.0b013e32835b70e9. [DOI] [PubMed] [Google Scholar]
- Guminski AD, Lee A, Lumba S, Maher R. Nab-paclitaxel salvage chemotherapy for metastatic ocular melanoma. J. Clin. Oncol. 2012;30:Abstract e19042. [Google Scholar]
- Carvajal RD, Sosman JA, Quevedo F, Milhem MM, Joshua AM, Kudchadkar RR, et al. Phase II study of selumetinib (sel) versus temozolomide (TMZ) in gnaq/Gna11 (Gq/11) mutant (mut) uveal melanoma (UM) J. Clin. Oncol. 2013;31:Abstract CRA9003. [Google Scholar]
- Romano E, Schwartz GK, Chapman PB, Wolchock JD, Carvajal RD. Treatment implications of the emerging molecular classification system for melanoma. Lancet Oncol. 2011;12:913–922. doi: 10.1016/S1470-2045(10)70274-6. [DOI] [PubMed] [Google Scholar]
- Ott PA, Carvajal RD, Pandit-Taskar N, Jungbluth AA, Hoffman EW, Wu BW, et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest. New Drugs. 2013;31:425–434. doi: 10.1007/s10637-012-9862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedikian AY, Papadopoulos NE, Kim KB, Vardeleon A, Smith T, Lu B, et al. A pilot study with vincristine sulfate liposome infusion in patients with metastatic melanoma. Melanoma Res. 2008;18:400–404. doi: 10.1097/CMR.0b013e328311aaa1. [DOI] [PubMed] [Google Scholar]
- Leyvraz S, Suciu S, Piperno-Neumann S, Baurain JF, Zdzienicki M, Testori A, et al. Randomized phase III trial of intravenous (IV) versus hepatic intra-arterial (HIA) fotemustine in patients with liver metastases from uveal melanoma: final results of the EORTC 18021 study. J. Clin. Oncol. 2012;30:8532. doi: 10.1093/annonc/mdt585. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann UB, Kauczok-Vetter CS, Houben R, Becker JC. Overexpression of the KIT/SCF in uveal melanoma does not translate into clinical efficacy of imatinib mesylate. Clin. Cancer Res. 2009;15:324–329. doi: 10.1158/1078-0432.CCR-08-2243. [DOI] [PubMed] [Google Scholar]
- Nathan PD, Marshall E, Smith CT, Bickerstaff M, Escriu C, Marples M, et al. A Cancer Research UK two-stage multicenter phase II study of imatinib in the treatment of patients with c-kit positive metastatic uveal melanoma (ITEM) J. Clin. Oncol. 2012;30:8523. Abstract. [Google Scholar]
- Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin. Cancer Res. 2012;18:555–567. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Kelderman S, van der Soetekouw MK, van den Kooij AJ, Eertwegh PM, Jansen RL, van den Brom RR, et al. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology. Acta Oncol. 2013 doi: 10.3109/0284186X.2013.786839. doi: 10.3109/0284186X.2013.786839. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tarhini AA, Frankel P, Margolin KA, Christensen S, Ruel C, Shipe-Spotloe J, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage IV melanoma of cutaneous or uveal origin. Clin. Cancer Res. 2011;17:6574–6581. doi: 10.1158/1078-0432.CCR-11-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo F, Grosso M, Picasso V, Tornari E, Pesce M, Queirolo P. Treatment of metastatic uveal melanoma with intravenous fotemustine. Melanoma Res. 2013;23:196–198. doi: 10.1097/CMR.0b013e3283610586. [DOI] [PubMed] [Google Scholar]
- Homsi J, Bedikian AY, Papadopoulos NE, Kim KB, Hwu WJ, Mahoney SL, et al. Phase 2 open-label study of weekly docosahexaenoic acid-paclitaxel in patients with metastatic uveal melanoma. Melanoma Res. 2010;20:507–510. doi: 10.1097/CMR.0b013e3283403ce9. [DOI] [PubMed] [Google Scholar]
- Bedikian AY, Papadopoulos N, Plager C, Eton O, Ring S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003;13:303–306. doi: 10.1097/00008390-200306000-00013. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Bedikian AY, Smith TM, Papadopoulos NE, Plager C, Eton O. Phase II trial of 9-nitrocamptothecin (RFS 2000) for patients with metastatic cutaneous or uveal melanoma. Anticancer Drugs. 2002;13:169–172. doi: 10.1097/00001813-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber M, Schmittel A, Thiel E, Keilholz U. A phase II study of bendamustine chemotherapy as second-line treatment in metastatic uveal melanoma. Melanoma Res. 2004;14:439–442. doi: 10.1097/00008390-200412000-00001. [DOI] [PubMed] [Google Scholar]
- Schmittel A, Schmidt-Hieber M, Martus P, Bechrakis NE, Schuster R, Siehl JM, et al. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann. Oncol. 2006;17:1826–1829. doi: 10.1093/annonc/mdl309. [DOI] [PubMed] [Google Scholar]
- Pfohler C, Cree IA, Ugurel S, Kuwert C, Haass N, Neuber K, et al. Treosulfan and gemcitabine in metastatic uveal melanoma patients: results of a multicenter feasibility study. Anticancer Drugs. 2003;14:337–340. doi: 10.1097/00001813-200306000-00002. [DOI] [PubMed] [Google Scholar]
- Atzpodien J, Terfloth K, Fluck M, Reitz M. Cisplatin, gemcitabine and treosulfan is effective in chemotherapy-pretreated relapsed stage IV uveal melanoma patients. Cancer Chemother. Pharmacol. 2008;62:685–688. doi: 10.1007/s00280-007-0655-9. [DOI] [PubMed] [Google Scholar]
- Schmittel A, Scheulen ME, Bechrakis NE, Strumberg D, Baumgart J, Bornfeld N, et al. Phase II trial of cisplatin, gemcitabine and treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005;15:205–207. doi: 10.1097/00008390-200506000-00010. [DOI] [PubMed] [Google Scholar]
- O'Neill PA, Butt M, Eswar CV, Gillis P, Marshall E. A prospective single arm phase II study of dacarbazine and treosulfan as first-line therapy in metastatic uveal melanoma. Melanoma Res. 2006;16:245–248. doi: 10.1097/01.cmr.0000205017.38859.07. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Moon J, Margolin KA, Weber JS, Lao CD, Othus M, et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS One. 2012;7:e48787. doi: 10.1371/journal.pone.0048787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JC, Terheyden P, Kampgen E, Wagner S, Neumann C, Schadendorf D, et al. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br. J. Cancer. 2002;87:840–845. doi: 10.1038/sj.bjc.6600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela T, Suciu S, Hansson J, Kruit WH, Vuoristo MS, Kloke O, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur. J. Cancer. 2003;39:1115–1120. doi: 10.1016/s0959-8049(03)00132-1. [DOI] [PubMed] [Google Scholar]
- Nathan FE, Berd D, Sato T, Shield JA, Shields CL, De Potter P, et al. BOLD+interferon in the treatment of metastatic uveal melanoma: first report of active systemic therapy. J. Exp. Clin. Cancer Res. 1997;16:201–208. [PubMed] [Google Scholar]
- Pyrhonen S, Hahka-Kemppinen M, Muhonen T, Nikkanen V, Eskelin S, Summanen P, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer. 2002;95:2366–2372. doi: 10.1002/cncr.10996. [DOI] [PubMed] [Google Scholar]
- Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol. Immunother. 2012;61:41–48. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Callahan M, Postow MA, Chapman PB, Schwartz GK, Dickson MA, et al. Ipilimumab in the treatment of uveal melanoma: the Memorial Sloan-Kettering Cancer Center experience. J. Clin. Oncol. 2012;30:8549. Abstract. [Google Scholar]
- Khattak MA, Fisher R, Hughes P, Gore M, Larkin J. Ipilimumab activity in advanced uveal melanoma. Melanoma Res. 2013;23:79–81. doi: 10.1097/CMR.0b013e32835b554f. [DOI] [PubMed] [Google Scholar]
- Guenterberg KD, Grignol VP, Relekar KV, Varker KA, Chen HX, Kendra KL, et al. A pilot study of bevacizumab and interferon-alpha2b in ocular melanoma. Am. J. Clin. Oncol. 2011;34:87–91. doi: 10.1097/COC.0b013e3181d2ed67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno-Neumann S, Servois V, Bidard FC, Mariani P, Plancher C, Diallo A, et al. BEVATEM: phase II study of bevacizumab in combination with temozolomide in patients with first-line metastatic uveal melanoma (MUM): final results. J. Clin. Oncol. 2013;31:9057. Abstract. [Google Scholar]
- Reiriz AB, Richter MF, Fernandes S, Cancela AI, Costa TD, Di Leone LP, et al. Phase II study of thalidomide in patients with metastatic malignant melanoma. Melanoma Res. 2004;14:527–531. doi: 10.1097/00008390-200412000-00014. [DOI] [PubMed] [Google Scholar]
- Solti M, Berd D, Mastrangelo MJ, Sato T. A pilot study of low-dose thalidomide and interferon alpha-2b in patients with metastatic melanoma who failed prior treatment. Melanoma Res. 2007;17:225–231. doi: 10.1097/CMR.0b013e32823ed0d1. [DOI] [PubMed] [Google Scholar]
- Zeldis JB, Heller C, Seidel G, Yuldasheva N, Stirling D, Shutack Y, et al. A randomized phase II trial comparing two doses of lenalidomide for the treatment of stage IV ocular melanoma. J. Clin. Oncol. 2009;27:e20012. Abstract. [Google Scholar]
- Penel N, Delcambre C, Durando X, Clisant S, Hebbar M, Negrier S, et al. O-Mel-Inib: a Cancero-pole Nord-Ouest multicenter phase II trial of high-dose imatinib mesylate in metastatic uveal melanoma. Invest. New Drugs. 2008;26:561–565. doi: 10.1007/s10637-008-9143-2. [DOI] [PubMed] [Google Scholar]
- Mahipal A, Tijani L, Chan K, Laudadio M, Mastrangelo MJ, Sato T. A pilot study of sunitinib malate in patients with metastatic uveal melanoma. Melanoma Res. 2012;22:440–446. doi: 10.1097/CMR.0b013e328358b373. [DOI] [PubMed] [Google Scholar]
- Luke JJ, Callahan MK, Postow MA, Romano E, Ramaiya N, Bluth M, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013 doi: 10.1002/cncr.28282. . doi: 10.1002/cncr.28282. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen PW, Li H, Alizadeh H, Niederkorn JY. PD-L1: PD-1 interaction contributes to the functional suppression of T-cell responses to human uveal melanoma cells in vitro. Invest. Ophthalmol. Vis. Sci. 2008;49:2518–2525. doi: 10.1167/iovs.07-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidervaart W, van Stark F, Nieuwpoort M, Dijkman R, Packer L, Borgstein AM, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. Br. J. Cancer. 2005;92:2032–2038. doi: 10.1038/sj.bjc.6602598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini G, Pratilas CA, Qin LX, Tadi M, Surriga O, Carvajal RD, et al. Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin. Cancer Res. 2012;18:3552–3561. doi: 10.1158/1078-0432.CCR-11-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babchia N, Calipel A, Mouriaux F, Faussat AM, Mascarelli F. The PI3K/Akt and mTOR/P70S6K signaling pathways in human uveal melanoma cells: interaction with B-Raf/ERK. Invest. Ophthalmol. Vis. Sci. 2010;51:421–429. doi: 10.1167/iovs.09-3974. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Chew SA, He B, Riechardt AI, Karadedou T, Kotoula V, et al. Genotype-dependent sensitivity of uveal melanoma cell lines to inhibition of B-Raf, MEK, and Akt kinases: rationale for personalized therapy. Invest. Ophthalmol. Vis. Sci. 2011;52:7248–7255. doi: 10.1167/iovs.11-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Smyth E, Chapman PB, Riechardt AI, Karadedou T, Kotoula V, Wolchok JD, Schwartz GK, Abramson DH, et al. Therapeutic implications of the emerging molecular biology of uveal melanoma. Clin. Cancer Res. 2011;17:2087–2100. doi: 10.1158/1078-0432.CCR-10-3169. [DOI] [PubMed] [Google Scholar]
- Harbour JW. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012;25:171–181. doi: 10.1111/j.1755-148X.2012.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin. Cancer Res. 2012;18:408–416. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW. Genomic, prognostic, and cell-signaling advances in uveal melanoma. Am. Soc. Clin. Oncol. Educ. Book. 2013;2013:388–391. doi: 10.1200/EdBook_AM.2013.33.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty LE, Unger JM, Liu PY, Mertens WC, Sondak VK. Metastatic melanoma from intraocular primary tumors: the Southwest Oncology Group experience in phase II advanced melanoma clinical trials. Am. J. Clin. Oncol. 1998;21:568–572. doi: 10.1097/00000421-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Terheyden P, Brocker EB, Becker JC. Clinical evaluation of in vitro chemosensitivity testing: the example of uveal melanoma. J. Cancer Res. Clin. Oncol. 2004;130:395–399. doi: 10.1007/s00432-004-0569-4. [DOI] [PubMed] [Google Scholar]
- Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Blanco PL, Lim LA, Miyamoto C, Burnier MN. Uveal melanoma dormancy: an acceptable clinical endpoint? Melanoma Res. 2012;22:334–340. doi: 10.1097/CMR.0b013e328357bea8. [DOI] [PubMed] [Google Scholar]


