Abstract
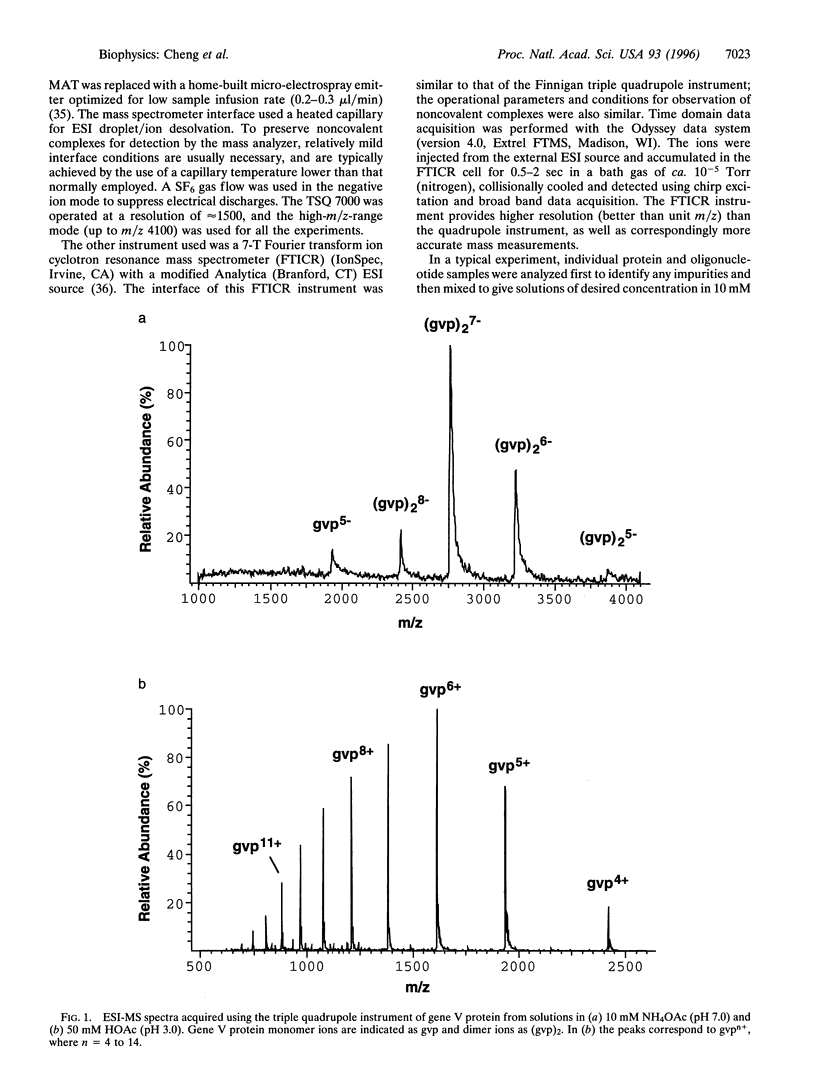
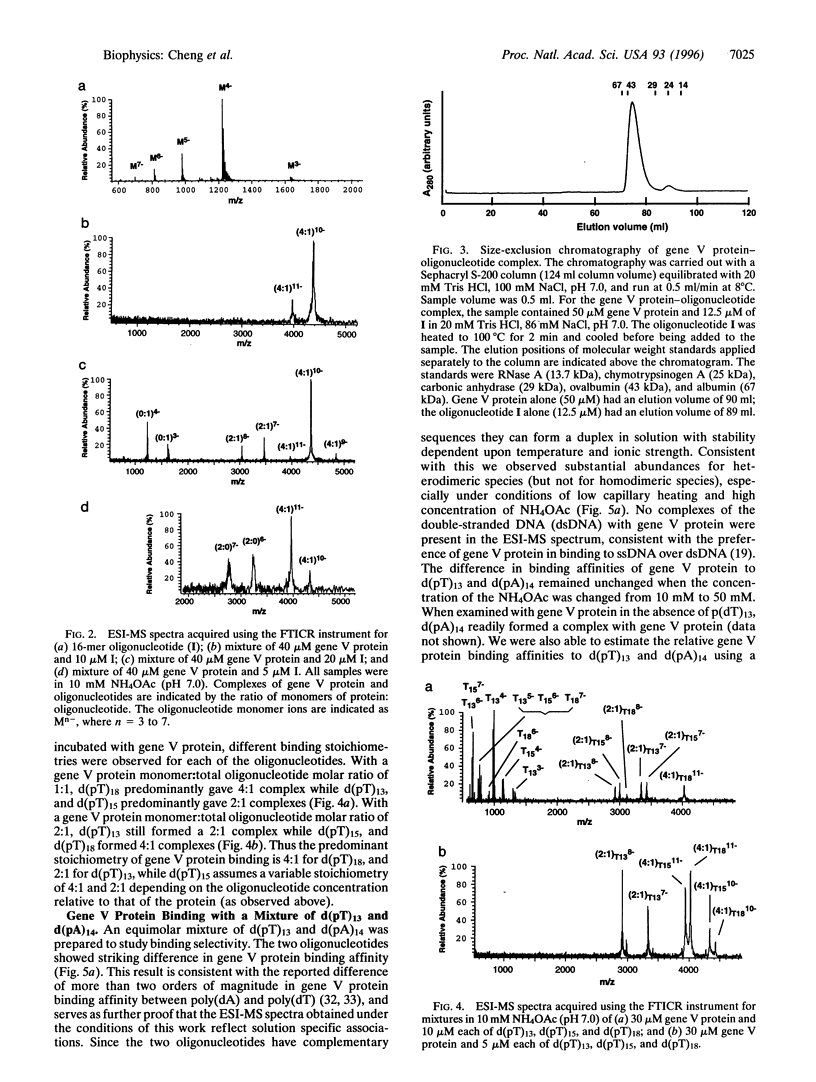
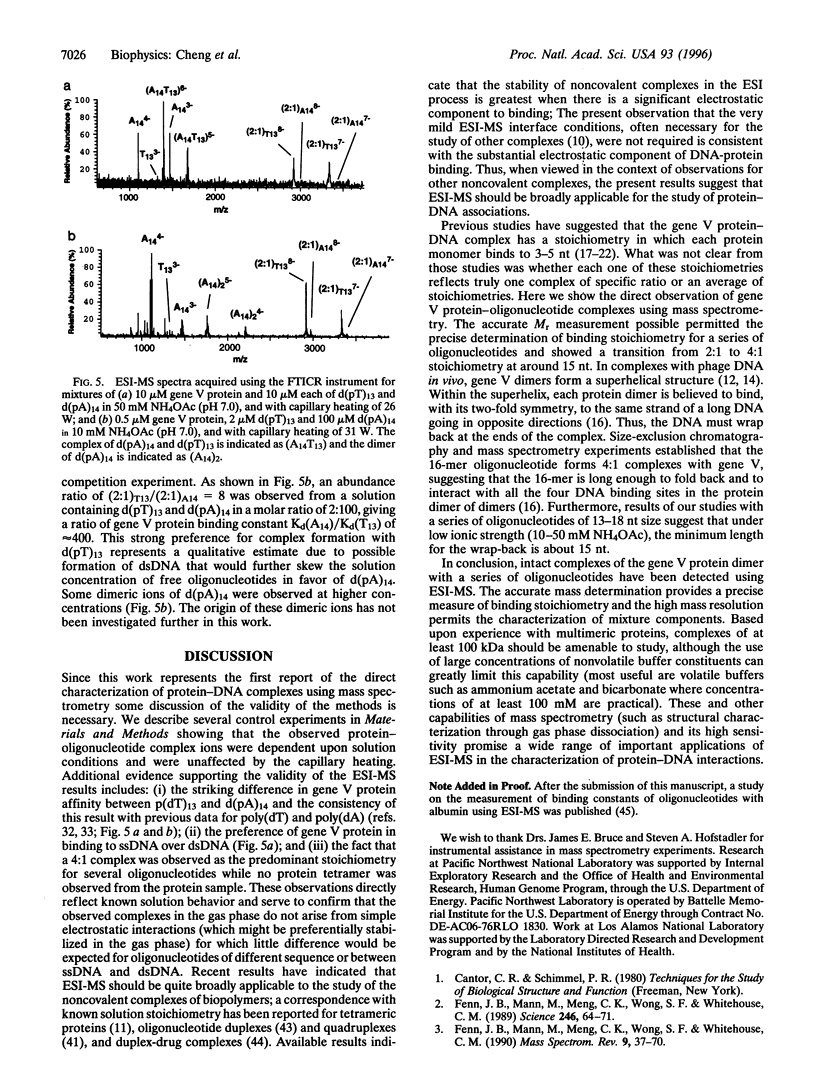
The binding stoichiometry of gene V protein from bacteriophage f1 to several oligonucleotides was studied using electrospray ionization-mass spectrometry (ESI-MS). Using mild mass spectrometer interface conditions that preserve noncovalent associations in solution, gene V protein was observed as dimer ions from a 10 mM NH4OAc solution. Addition of oligonucleotides resulted in formation of protein-oligonucleotide complexes with stoichiometry of approximately four nucleotides (nt) per protein monomer. A 16-mer oligonucleotide gave predominantly a 4:1 (protein monomer: oligonucleotide) complex while oligonucleotides shorter than 15 nt showed stoichiometries of 2:1. Stoichiometries and relative binding constants for a mixture of oligonucleotides were readily measured using mass spectrometry. The binding stoichiometry of the protein with the 16-mer oligonucleotide was measured independently using size-exclusion chromatography and the results were consistent with the mass spectrometric data. These results demonstrate, for the first time, the observation and stoichiometric measurement of protein-oligonucleotide complexes using ESI-MS. The sensitivity and high resolution of ESI-MS should make it a useful too] in the study of protein-DNA interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Alma N. C., Harmsen B. J., de Jong E. A., Ven J., Hilbers C. W. Fluorescence studies of the complex formation between the gene 5 protein of bacteriophage M13 and polynucleotides. J Mol Biol. 1983 Jan 5;163(1):47–62. doi: 10.1016/0022-2836(83)90029-3. [DOI] [PubMed] [Google Scholar]
- Baas P. D. DNA replication of single-stranded Escherichia coli DNA phages. Biochim Biophys Acta. 1985 Jun 24;825(2):111–139. doi: 10.1016/0167-4781(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. Mechanism of DNA binding to the gene 5 protein of bacteriophage fd. Biochemistry. 1984 Jan 17;23(2):340–349. doi: 10.1021/bi00297a025. [DOI] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. Refined structure of the gene 5 DNA binding protein from bacteriophage fd. J Mol Biol. 1983 Sep 15;169(2):565–596. doi: 10.1016/s0022-2836(83)80065-5. [DOI] [PubMed] [Google Scholar]
- Bulsink H., Harmsen B. J., Hilbers C. W. Specificity of the binding of bacteriophage M13 encoded gene-5 protein to DNA and RNA studied by means of fluorescence titrations. J Biomol Struct Dyn. 1985 Oct;3(2):227–247. doi: 10.1080/07391102.1985.10508413. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Neet K. E., Goldthwait D. A. Gene 5 protein of bacteriophage fd: a dimer which interacts co-operatively with DNA. J Mol Biol. 1976 Apr 25;102(4):697–711. doi: 10.1016/0022-2836(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Coleman J. E., Oakley J. L. Physical chemical studies of the structure and function of DNA binding (helix-destabilizing) proteins. CRC Crit Rev Biochem. 1980 Jan;7(3):247–289. doi: 10.3109/10409238009105463. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Folkers P. J., van Duynhoven J. P., Jonker A. J., Harmsen B. J., Konings R. N., Hilbers C. W. Sequence-specific 1H-NMR assignment and secondary structure of the Tyr41----His mutant of the single-stranded DNA binding protein, gene V protein, encoded by the filamentous bacteriophage M13. Eur J Biochem. 1991 Dec 5;202(2):349–360. doi: 10.1111/j.1432-1033.1991.tb16382.x. [DOI] [PubMed] [Google Scholar]
- Fulford W., Model P. Bacteriophage f1 DNA replication genes. II. The roles of gene V protein and gene II protein in complementary strand synthesis. J Mol Biol. 1988 Sep 5;203(1):39–48. doi: 10.1016/0022-2836(88)90089-7. [DOI] [PubMed] [Google Scholar]
- Goodlett D. R., Camp D. G., 2nd, Hardin C. C., Corregan M., Smith R. D. Direct observation of a DNA quadruplex by electrospray ionization mass spectrometry. Biol Mass Spectrom. 1993 Mar;22(3):181–183. doi: 10.1002/bms.1200220307. [DOI] [PubMed] [Google Scholar]
- Gray C. W. Three-dimensional structure of complexes of single-stranded DNA-binding proteins with DNA. IKe and fd gene 5 proteins form left-handed helices with single-stranded DNA. J Mol Biol. 1989 Jul 5;208(1):57–64. doi: 10.1016/0022-2836(89)90087-9. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Gray C. W., Carlson R. D. Neutron scattering data on reconstituted complexes of fd deoxyribonucleic acid and gene 5 protein show that the deoxyribonucleic acid is near the center. Biochemistry. 1982 May 25;21(11):2702–2713. doi: 10.1021/bi00540a020. [DOI] [PubMed] [Google Scholar]
- Kansy J. W., Clack B. A., Gray D. M. The binding of fd gene 5 protein to polydeoxynucleotides: evidence from CD measurements for two binding modes. J Biomol Struct Dyn. 1986 Jun;3(6):1079–1110. doi: 10.1080/07391102.1986.10508487. [DOI] [PubMed] [Google Scholar]
- Liang H., Terwilliger T. C. Reversible denaturation of the gene V protein of bacteriophage f1. Biochemistry. 1991 Mar 19;30(11):2772–2782. doi: 10.1021/bi00225a006. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Model P. Regulation of coliphage f1 single-stranded DNA synthesis by a DNA-binding protein. J Mol Biol. 1973 Aug 5;78(2):285–300. doi: 10.1016/0022-2836(73)90117-4. [DOI] [PubMed] [Google Scholar]
- Michel B., Zinder N. D. Translational repression in bacteriophage f1: characterization of the gene V protein target on the gene II mRNA. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4002–4006. doi: 10.1073/pnas.86.11.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model P., McGill C., Mazur B., Fulford W. D. The replication of bacteriophage f1: gene V protein regulates the synthesis of gene II protein. Cell. 1982 Jun;29(2):329–335. doi: 10.1016/0092-8674(82)90149-0. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Sakairi M., Yergey A. L., Siu K. W., Le Blanc J. C., Guevremont R., Berman S. S. Electrospray mass spectrometry: application of ion evaporation theory to amino acids. Anal Chem. 1991 Jul 15;63(14):1488–1490. doi: 10.1021/ac00014a026. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Skinner M. M., Zhang H., Leschnitzer D. H., Guan Y., Bellamy H., Sweet R. M., Gray C. W., Konings R. N., Wang A. H., Terwilliger T. C. Structure of the gene V protein of bacteriophage f1 determined by multiwavelength x-ray diffraction on the selenomethionyl protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2071–2075. doi: 10.1073/pnas.91.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., Loo J. A., Edmonds C. G., Barinaga C. J., Udseth H. R. New developments in biochemical mass spectrometry: electrospray ionization. Anal Chem. 1990 May 1;62(9):882–899. doi: 10.1021/ac00208a002. [DOI] [PubMed] [Google Scholar]
- Yen T. S., Webster R. E. Translational control of bacteriophage f1 gene II and gene X proteins by gene V protein. Cell. 1982 Jun;29(2):337–345. doi: 10.1016/0092-8674(82)90150-7. [DOI] [PubMed] [Google Scholar]
- Zaman G. J., Kaan A. M., Schoenmakers J. G., Konings R. N. Gene V protein-mediated translational regulation of the synthesis of gene II protein of the filamentous bacteriophage M13: a dispensable function of the filamentous-phage genome. J Bacteriol. 1992 Jan;174(2):595–600. doi: 10.1128/jb.174.2.595-600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



